Challenges and Opportunities of the Dynamic Operation of PEM Water Electrolyzers
Abstract
1. Introduction
2. What Is the Purpose of Dynamic Operation
- The PEMWE system is only used for scheduling.
- All PV energy available for electrolysis is used for H2 production, calculated with the FIT price.
- All PV energy available for electrolysis is used for H2 production, calculated with the open-market price.
- The PEMWE system operates using grid electricity, while PV production is fed to the grid (FIT and free-market prices).
- The PEMWE system is used for scheduling and also utilizes available PV production, calculated with the open-market price.
- The PEMWE system is used for scheduling and available PV production is used for H2 production, calculated with the open-market price; in addition, energy is provided from the grid to maximize electrolyzer utilization.
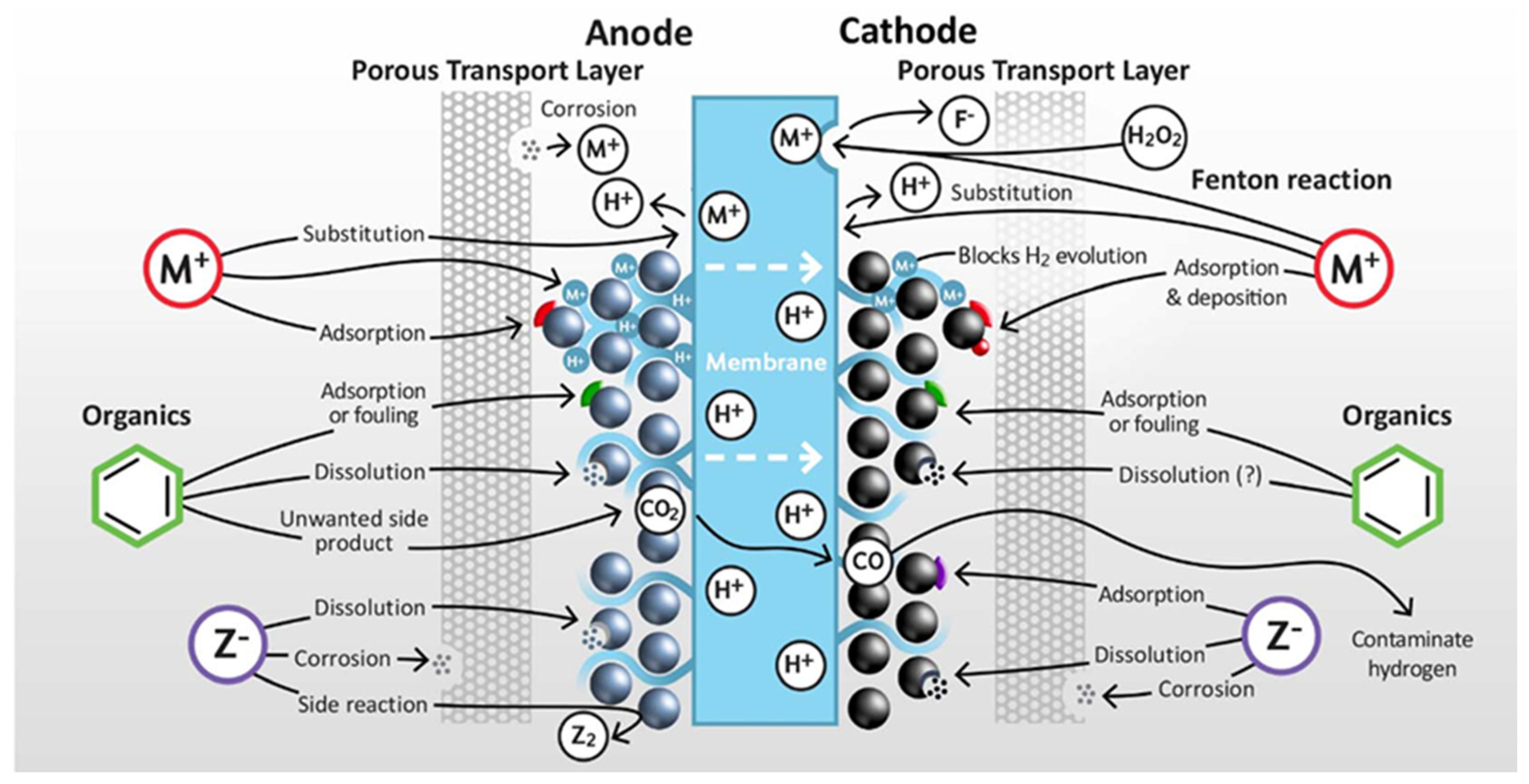
3. Dynamic Operation of PEM Water Electrolysis Systems
- H2 and O2 can both penetrate through the membrane; hence, the product streams are not 100% pure.
- The transport of different gases through the membrane strongly depends on the process parameters, such as temperature, pressure, and current density.
- H+ transport always occurs together with water transport; therefore, a large amount of water is dragged to the cathode of the electrolyzer.
- Metal ions, originating from the corrosion of the catalysts, the cell hardware, and/or the system itself, can reach the membrane and pass to the cathode. The subsequent deposition of these decreases the electrochemical activity and increases the cell voltage.
- Such metal ions can (quasi-) irreversibly bind to the charged groups in the membrane, hence decreasing its conductivity and increasing the cell voltage.
- During operation, a highly acidic condition develops in the electrolyzer.
- The transport of dissolved metal ions decreases the transference number of H+ ions, hence changing the local pH at the electrodes.
- When starting the stack after leaving it under open-circuit conditions, the surface of the catalysts and the cell hardware oxidize or reduce.
- The bubble formation rate and the size of the forming bubbles depend on the experimental parameters. This affects their removal efficiency.
- Local heating might occur due to a partially blocked electrode surface, structural imperfections, or other reasons. This can lead to local catalyst and membrane overuse and subsequent membrane thinning.
- When stopping the process, the remnant gases in the electrode compartments can induce a reverse, fuel cell-like operation.
4. Grid-Independent/Intermittent Operation of PEM Water Electrolysis Systems
4.1. Regular Starts/Stops
4.2. Product Crossover and Purity
4.3. Temperature Fluctuations
4.4. Bubble Management Under Dynamic Load
4.5. The Effect of Dynamic Operation on the Balance-of-Plants Components (BoP)
5. Do Accelerated Stress Tests Provide Relevant Information Regarding Electrolyzer Aging?
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hirth, L.; Ziegenhagen, I. Balancing Power and Variable Renewables: Three Links. Renew. Sustain. Energy Rev. 2015, 50, 1035–1051. [Google Scholar] [CrossRef]
- Al-Shetwi, A.Q.; Hannan, M.A.; Jern, K.P.; Mansur, M.; Mahlia, T.M.I. Grid-Connected Renewable Energy Sources: Review of the Recent Integration Requirements and Control Methods. J. Clean. Prod. 2020, 253, 119831. [Google Scholar] [CrossRef]
- Tan, K.M.; Babu, T.S.; Ramachandaramurthy, V.K.; Kasinathan, P.; Solanki, S.G.; Raveendran, S.K. Empowering Smart Grid: A Comprehensive Review of Energy Storage Technology and Application with Renewable Energy Integration. J. Energy Storage 2021, 39, 102591. [Google Scholar] [CrossRef]
- Hug, W.; Divisek, J.; Mergel, J.; Seeger, W.; Steeb, H. Highly Efficient Advanced Alkaline Electrolyzer for Solar Operation. Int. J. Hydrogen Energy 1992, 17, 699–705. [Google Scholar] [CrossRef]
- Kojima, H.; Nagasawa, K.; Todoroki, N.; Ito, Y.; Matsui, T.; Nakajima, R. Influence of Renewable Energy Power Fluctuations on Water Electrolysis for Green Hydrogen Production. Int. J. Hydrogen Energy 2023, 48, 4572–4593. [Google Scholar] [CrossRef]
- Sayed-Ahmed, H.; Toldy, I.; Santasalo-Aarnio, A. Dynamic Operation of Proton Exchange Membrane Electrolyzers—Critical Review. Renew. Sustain. Energy Rev. 2024, 189, 113883. [Google Scholar] [CrossRef]
- Ouabi, H.; Lajouad, R.; Kissaoui, M.; El Magri, A. Hydrogen Production by Water Electrolysis Driven by a Photovoltaic Source: A Review. e-Prime Adv. Electr. Eng. Electron. Energy 2024, 8, 100608. [Google Scholar] [CrossRef]
- Nnabuife, S.G.; Hamzat, A.K.; Whidborne, J.; Kuang, B.; Jenkins, K.W. Integration of Renewable Energy Sources in Tandem with Electrolysis: A Technology Review for Green Hydrogen Production. Int. J. Hydrogen Energy 2024, 107, 218–240. [Google Scholar] [CrossRef]
- Zsiboracs, H.; Vincze, A.; Pinter, G.; Baranyai, N.H. The Accuracy of PV Power Plant Scheduling in Europe: An Overview of ENTSO-E Countries. IEEE Access 2023, 11, 74953–74979. [Google Scholar] [CrossRef]
- Zsiborács, H.; Pintér, G.; Vincze, A.; Birkner, Z.; Baranyai, N.H. Grid Balancing Challenges Illustrated by Two European Examples: Interactions of Electric Grids, Photovoltaic Power Generation, Energy Storage and Power Generation Forecasting. Energy Rep. 2021, 7, 3805–3818. [Google Scholar] [CrossRef]
- Kılıç, U.; Kekezoğlu, B. A Review of Solar Photovoltaic Incentives and Policy: Selected Countries and Turkey. Ain Shams Eng. J. 2022, 13, 101669. [Google Scholar] [CrossRef]
- Wallnöfer-Ogris, E.; Grimmer, I.; Ranz, M.; Höglinger, M.; Kartusch, S.; Rauh, J.; Macherhammer, M.G.; Grabner, B.; Trattner, A. A Review on Understanding and Identifying Degradation Mechanisms in PEM Water Electrolysis Cells: Insights for Stack Application, Development, and Research. Int. J. Hydrogen Energy 2024, 65, 381–397. [Google Scholar] [CrossRef]
- Feng, Q.; Yuan, X.Z.; Liu, G.; Wei, B.; Zhang, Z.; Li, H.; Wang, H. A Review of Proton Exchange Membrane Water Electrolysis on Degradation Mechanisms and Mitigation Strategies. J. Power Sources 2017, 366, 33–55. [Google Scholar] [CrossRef]
- Frensch, S.H.; Fouda-Onana, F.; Serre, G.; Thoby, D.; Araya, S.S.; Kær, S.K. Influence of the Operation Mode on PEM Water Electrolysis Degradation. Int. J. Hydrogen Energy 2019, 44, 29889–29898. [Google Scholar] [CrossRef]
- Khatib, F.N.; Wilberforce, T.; Ijaodola, O.; Ogungbemi, E.; El-Hassan, Z.; Durrant, A.; Thompson, J.; Olabi, A.G. Material Degradation of Components in Polymer Electrolyte Membrane (PEM)Electrolytic Cell and Mitigation Mechanisms: A Review. Renew. Sustain. Energy Rev. 2019, 111, 1–14. [Google Scholar] [CrossRef]
- Nguyen, E.; Olivier, P.; Pera, M.-C.; Pahon, E.; Roche, R. Impacts of Intermittency on Low-Temperature Electrolysis Technologies: A Comprehensive Review. Int. J. Hydrogen Energy 2024, 70, 474–492. [Google Scholar] [CrossRef]
- Becker, H.; Murawski, J.; Shinde, D.V.; Stephens, I.E.L.; Hinds, G.; Smith, G. Impact of Impurities on Water Electrolysis: A Review. Sustain. Energy Fuels 2023, 7, 1565–1603. [Google Scholar] [CrossRef]
- Janjua, M.; Leroy, R. Electrocatalyst Performance in Industrial Water Electrolysers. Int. J. Hydrogen Energy 1985, 10, 11–19. [Google Scholar] [CrossRef]
- Guruprasad, N.; van der Schaaf, J.; de Groot, M.T. Unraveling the Impact of Reverse Currents on Electrode Stability in Anion Exchange Membrane Water Electrolysis. J. Power Sources 2024, 613, 234877. [Google Scholar] [CrossRef]
- Schalenbach, M.; Kasian, O.; Ledendecker, M.; Speck, F.D.; Mingers, A.M.; Mayrhofer, K.J.J.; Cherevko, S. The Electrochemical Dissolution of Noble Metals in Alkaline Media. Electrocatalysis 2018, 9, 153–161. [Google Scholar] [CrossRef]
- Cherevko, S.; Kulyk, N.; Mayrhofer, K.J.J. Durability of Platinum-Based Fuel Cell Electrocatalysts: Dissolution of Bulk and Nanoscale Platinum. Nano Energy 2016, 29, 275–298. [Google Scholar] [CrossRef]
- Knöppel, J.; Möckl, M.; Escalera-López, D.; Stojanovski, K.; Bierling, M.; Böhm, T.; Thiele, S.; Rzepka, M.; Cherevko, S. On the Limitations in Assessing Stability of Oxygen Evolution Catalysts Using Aqueous Model Electrochemical Cells. Nat. Commun. 2021, 12, 2231. [Google Scholar] [CrossRef]
- Weiß, A.; Siebel, A.; Bernt, M.; Shen, T.-H.; Tileli, V.; Gasteiger, H.A. Impact of Intermittent Operation on Lifetime and Performance of a PEM Water Electrolyzer. J. Electrochem. Soc. 2019, 166, F487–F497. [Google Scholar] [CrossRef]
- Trinke, P.; Haug, P.; Brauns, J.; Bensmann, B.; Hanke-Rauschenbach, R.; Turek, T. Hydrogen Crossover in PEM and Alkaline Water Electrolysis: Mechanisms, Direct Comparison and Mitigation Strategies. J. Electrochem. Soc. 2018, 165, F502–F513. [Google Scholar] [CrossRef]
- Omrani, R.; Shabani, B. Hydrogen Crossover in Proton Exchange Membrane Electrolysers: The Effect of Current Density, Pressure, Temperature, and Compression. Electrochim. Acta 2021, 377, 138085. [Google Scholar] [CrossRef]
- Schalenbach, M.; Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. Pressurized PEM Water Electrolysis: Efficiency and Gas Crossover. Int. J. Hydrogen Energy 2013, 38, 14921–14933. [Google Scholar] [CrossRef]
- Rauls, E.; Hehemann, M.; Keller, R.; Scheepers, F.; Müller, M.; Stolten, D. Favorable Start-Up Behavior of Polymer Electrolyte Membrane Water Electrolyzers. Appl. Energy 2023, 330, 120350. [Google Scholar] [CrossRef]
- Cherevko, S.; Geiger, S.; Kasian, O.; Mingers, A.; Mayrhofer, K.J.J. Oxygen Evolution Activity and Stability of Iridium in Acidic Media. Part 2.—Electrochemically Grown Hydrous Iridium Oxide. J. Electroanal. Chem. 2016, 774, 102–110. [Google Scholar] [CrossRef]
- Yuan, S.; Zhao, C.; Cai, X.; An, L.; Shen, S.; Yan, X.; Zhang, J. Bubble Evolution and Transport in PEM Water Electrolysis: Mechanism, Impact, and Management. Prog. Energy Combust. Sci. 2023, 96, 101075. [Google Scholar] [CrossRef]
- Lee, C.H.; Lee, J.K.; Zhao, B.; Fahy, K.F.; Bazylak, A. Transient Gas Distribution in Porous Transport Layers of Polymer Electrolyte Membrane Electrolyzers. J. Electrochem. Soc. 2020, 167, 024508. [Google Scholar] [CrossRef]
- Staerz, A.F.; van Leeuwen, M.; Priamushko, T.; Saatkamp, T.; Endrődi, B.; Plankensteiner, N.; Jobbagy, M.; Pahlavan, S.; Blom, M.J.W.; Janáky, C.; et al. Effects of Iron Species on Low Temperature CO2 Electrolyzers. Angew. Chem. Int. Ed. 2024, 63, e202306503. [Google Scholar] [CrossRef] [PubMed]
- Aßmann, P.; Gago, A.S.; Gazdzicki, P.; Friedrich, K.A.; Wark, M. Toward Developing Accelerated Stress Tests for Proton Exchange Membrane Electrolyzers. Curr. Opin. Electrochem. 2020, 21, 225–233. [Google Scholar] [CrossRef]
- Kuhnert, E.; Hacker, V.; Bodner, M. A Review of Accelerated Stress Tests for Enhancing MEA Durability in PEM Water Electrolysis Cells. Int. J. Energy Res. 2023, 2023, 3183108. [Google Scholar] [CrossRef]
- Kuhnert, E.; Mayer, K.; Heidinger, M.; Rienessel, C.; Hacker, V.; Bodner, M. Impact of Intermittent Operation on Photovoltaic-PEM Electrolyzer Systems: A Degradation Study Based on Accelerated Stress Testing. Int. J. Hydrogen Energy 2024, 55, 683–695. [Google Scholar] [CrossRef]
- Choi, B.B.; Jo, J.H.; Yoo, Y.S.; Jeon, S.Y.; Lee, T.; Choi, Y.J.; Chung, D.Y.; Lee, E.J.; Yoo, S.J. Accelerated Stress Test of Polymer Electrolyte Membrane Water Electrolyzer via Solar Power Generation Condition. Sustain. Energy Technol. Assess. 2023, 57, 103238. [Google Scholar] [CrossRef]



| PV Regulation | PV not FIT | PV FIT | Grid | PV Regulation + not FIT | PV Regulation + not FIT + Grid | |
|---|---|---|---|---|---|---|
| Capacity Factor | 12% | 40% | 40% | 98% | 40% | 98% |
| Hydrogen production (kg/year) | 19.096 | 63.07 | 63.07 | 154.5 | 63.072 | 154.526 |
| Cost of hydrogen (EUR/kg) | 35.89 | 13.04 | 16.06 | 10.4 | 12.78 | 10.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Endrődi, B.; Trapp, C.A.; Szén, I.; Bakos, I.; Lukovics, M.; Janáky, C. Challenges and Opportunities of the Dynamic Operation of PEM Water Electrolyzers. Energies 2025, 18, 2154. https://doi.org/10.3390/en18092154
Endrődi B, Trapp CA, Szén I, Bakos I, Lukovics M, Janáky C. Challenges and Opportunities of the Dynamic Operation of PEM Water Electrolyzers. Energies. 2025; 18(9):2154. https://doi.org/10.3390/en18092154
Chicago/Turabian StyleEndrődi, Balázs, Cintia Alexandra Trapp, István Szén, Imre Bakos, Miklós Lukovics, and Csaba Janáky. 2025. "Challenges and Opportunities of the Dynamic Operation of PEM Water Electrolyzers" Energies 18, no. 9: 2154. https://doi.org/10.3390/en18092154
APA StyleEndrődi, B., Trapp, C. A., Szén, I., Bakos, I., Lukovics, M., & Janáky, C. (2025). Challenges and Opportunities of the Dynamic Operation of PEM Water Electrolyzers. Energies, 18(9), 2154. https://doi.org/10.3390/en18092154





