Sustainable Energy Storage Systems: Polypyrrole-Filled Polyimide-Modified Carbon Nanotube Sheets with Remarkable Energy Density
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Reagent
Apparatus and Instrumentation
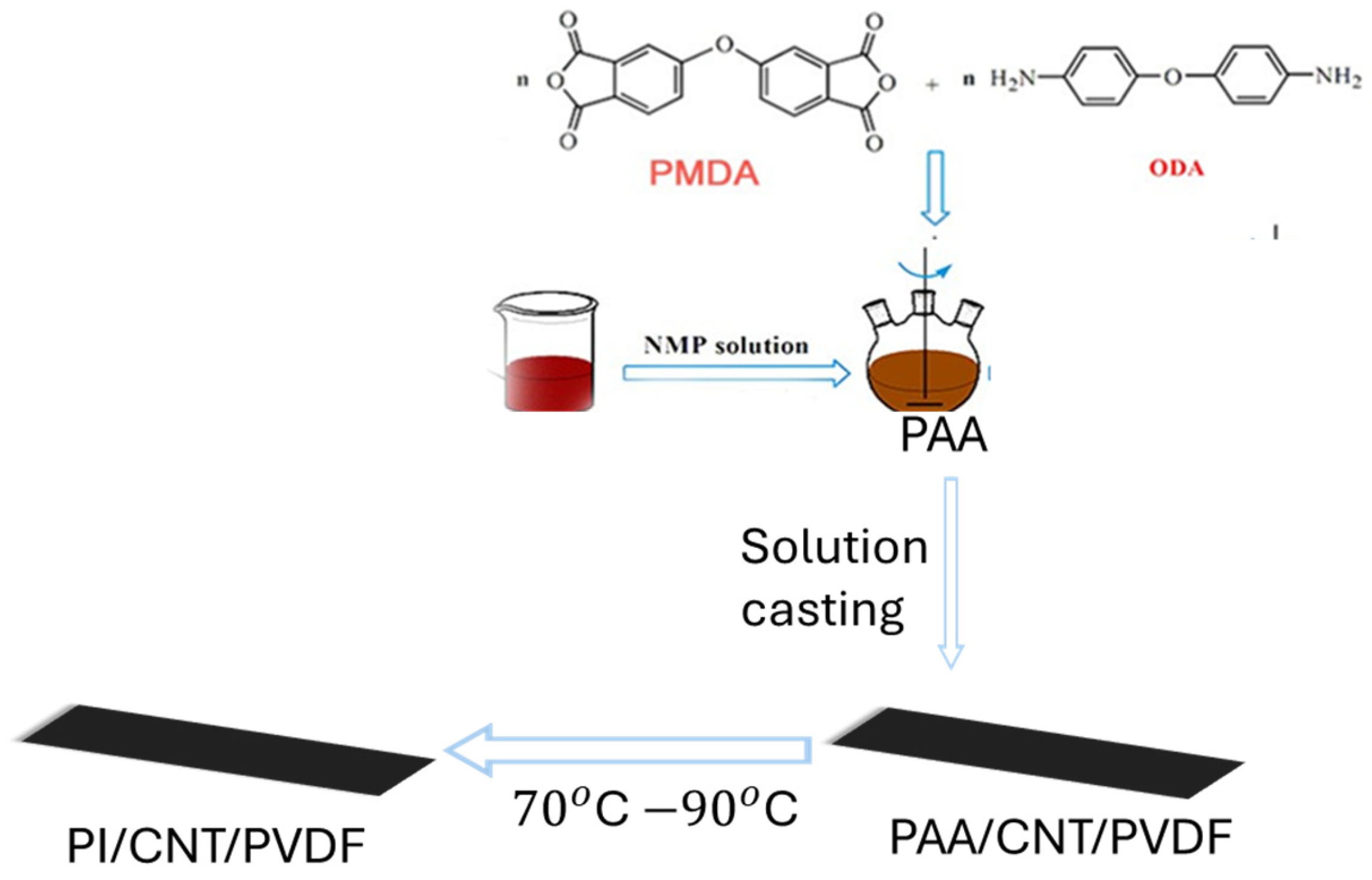
2.2. Synthesis of Polyimide and Processing of Hybrid Nanocomposites
3. Characterization
3.1. Scanning Electron Microscopy (SEM)
3.2. X-Ray Diffraction (XRD)
3.3. Electrochemical Deposition of Polypyrrole
3.4. Differential Scanning Calorimetry
3.5. Electrochemical Characterization (EC)
3.6. Electrochemical Characterization
4. Results and Discussion
4.1. X-Ray Diffraction (XRD)
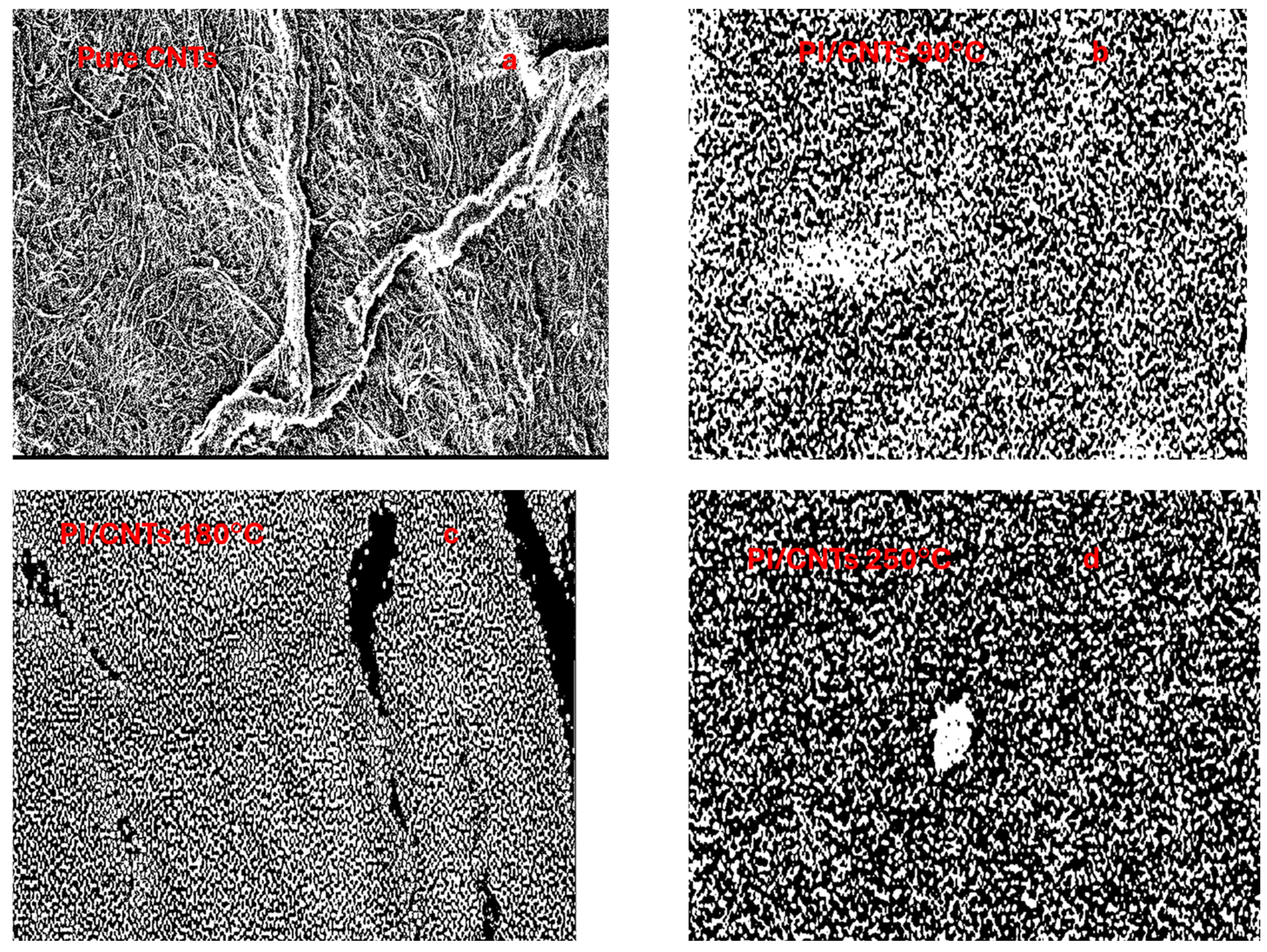
4.2. Scanning Electron Microscopy (SEM)
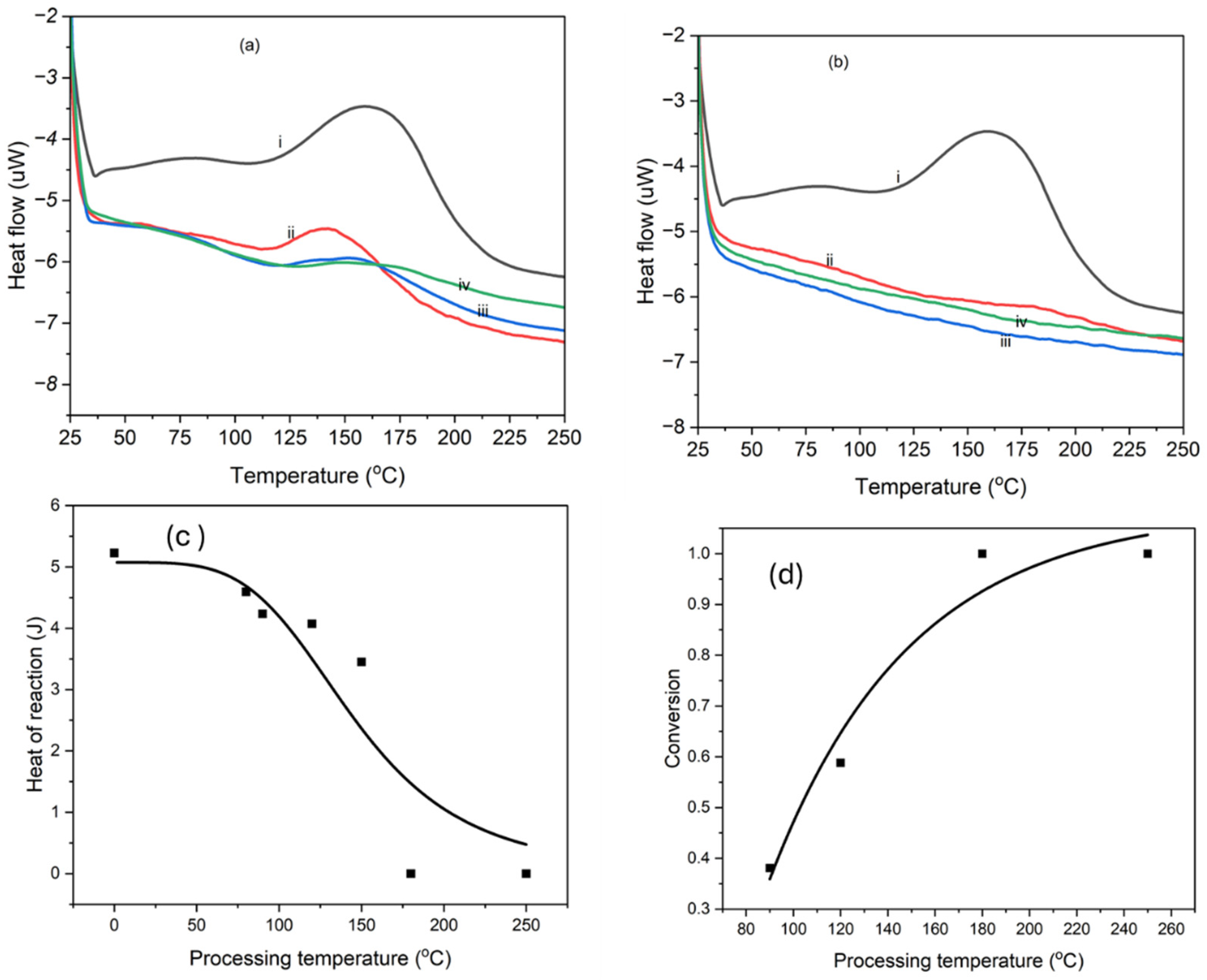
4.3. Thermal Analysis and Imidization
4.4. Electrodeposition of Polypyrrole
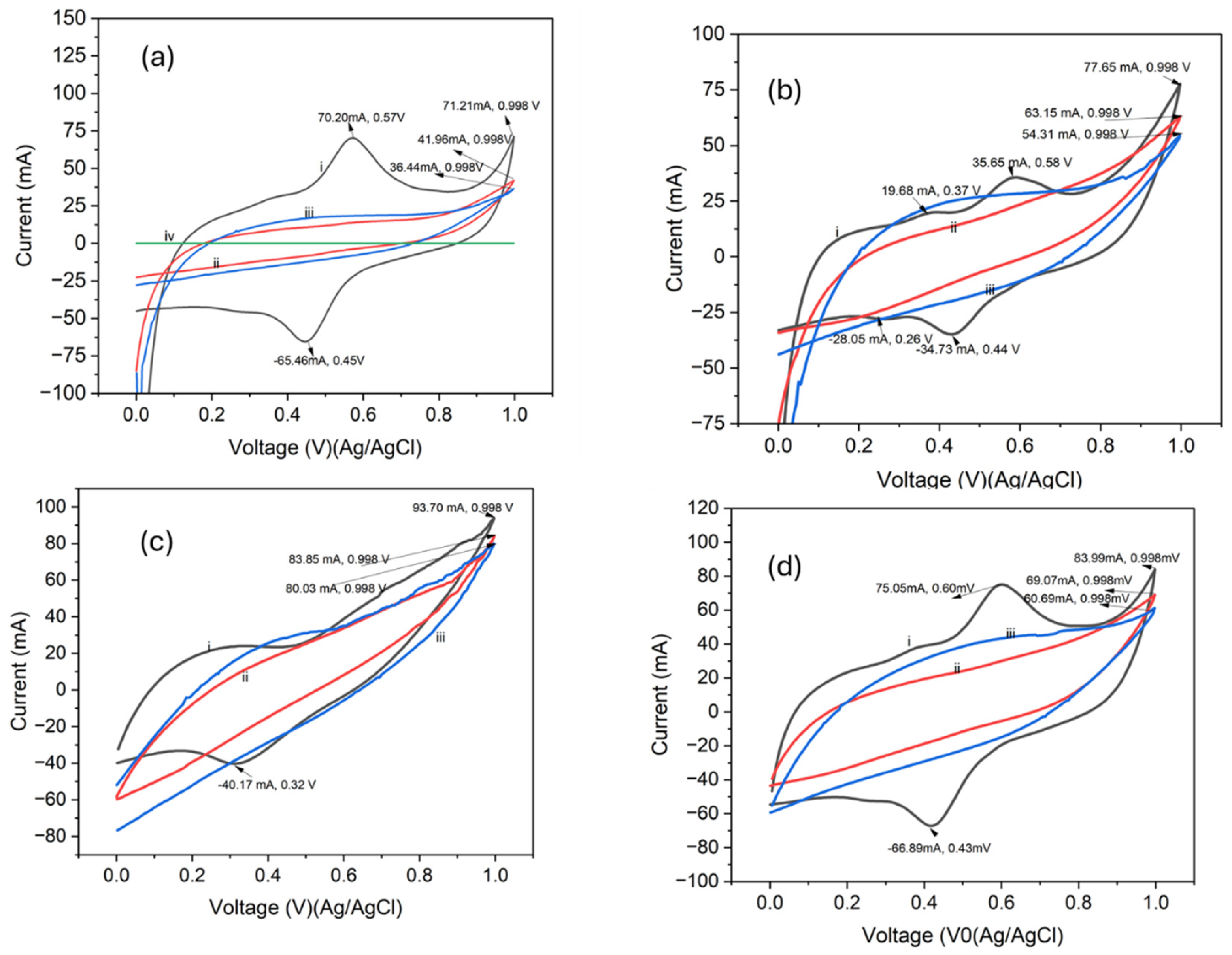
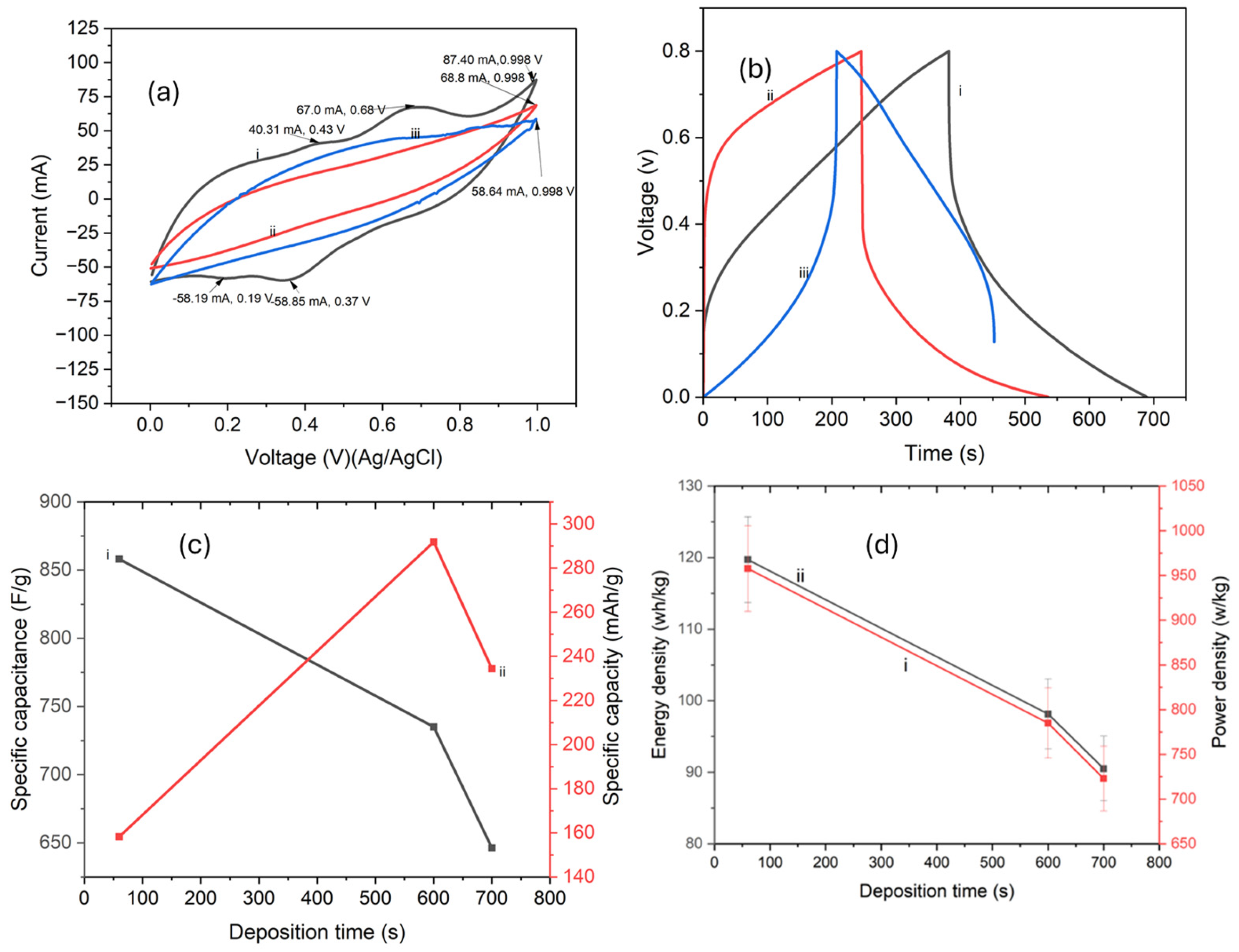
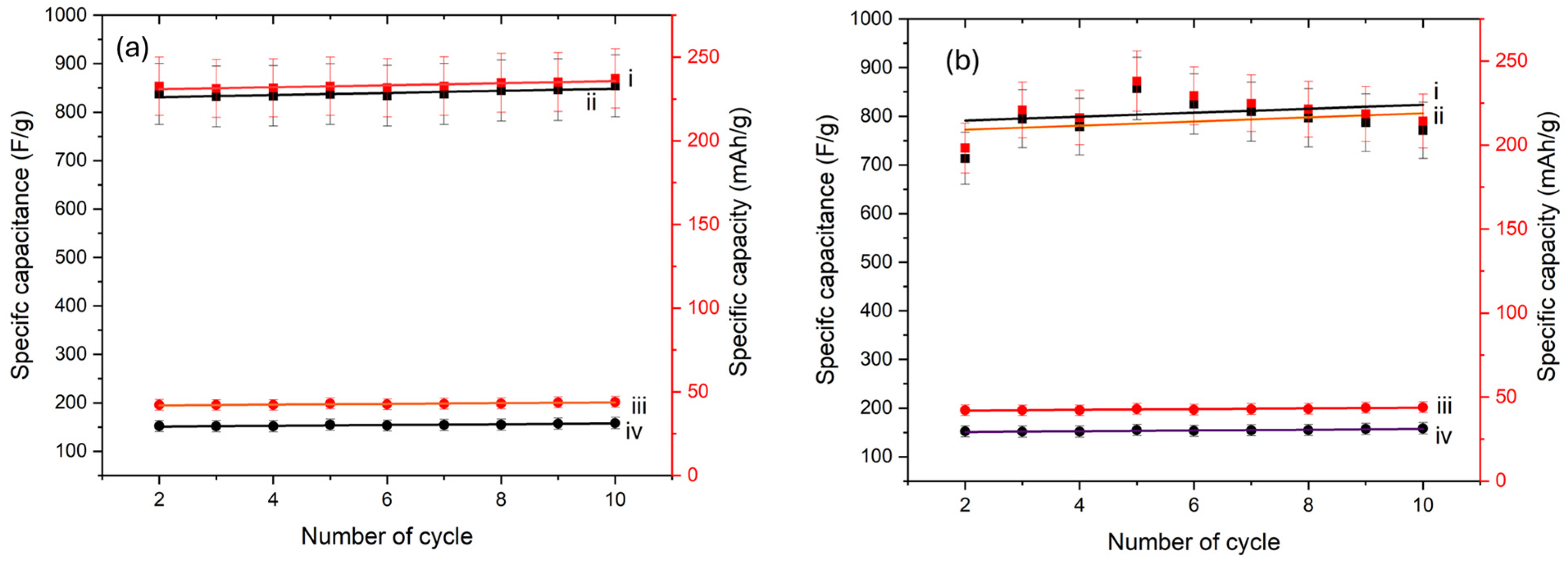
4.5. Electrochemical Properties
5. Effect of Time and Temperature on the Hybrid Nanocomposite
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, F.; Wang, H.; Wu, M.; Nan, J.; Li, T.; Cao, S.A. Electrochemical properties of poly (anthraquinonyl imide) s as high-capacity organic cathode materials for Li-ion batteries. Mater. Chem. Phys. 2018, 214, 120–125. [Google Scholar] [CrossRef]
- Sui, D.; Xu, L.; Zhang, H.; Sun, Z.; Kan, B.; Ma, Y.; Chen, Y. A 3D cross-linked graphene-based honeycomb carbon composite with excellent confinement effect of organic cathode material for lithium-ion batteries. Carbon 2020, 157, 656–662. [Google Scholar] [CrossRef]
- Shi, C.; Takeuchi, S.; Alexander, G.V.; Hamann, T.; O’Neill, J.; Dura, J.A.; Wachsman, E.D. High sulfur loading and capacity retention in bilayer garnet sulfurized-polyacrylonitrile/lithium-metal batteries with gel polymer electrolytes. Adv. Energy Mater. 2023, 13, 2301656. [Google Scholar] [CrossRef]
- Lyu, H.; Sun, X.G.; Dai, S. Organic Cathode Materials for Lithium-Ion Batteries: Past, Present, and Future. Adv. Energy Sustain. Res. 2021, 2, 2000044. [Google Scholar] [CrossRef]
- Shi, T.; Li, G.; Han, Y.; Gao, Y.; Wang, F.; Hu, Z.; Cai, T.; Chu, J.; Song, Z. Oxidized indanthrone as a cost-effective and high-performance organic cathode material for rechargeable lithium batteries. Energy Storage Mater. 2022, 50, 265–273. [Google Scholar] [CrossRef]
- Andezai, A.; Iroh, J.O. Overview of Organic Cathode Materials in Lithium-Ion Batteries and Supercapacitors. Energies 2025, 18, 582. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, L.; Xu, H.; Song, Y.; He, X. Polyimides as Promising Materials for Lithium-Ion Batteries: A Review. Nano-Micro Lett. 2023, 15, 135. [Google Scholar] [CrossRef]
- Liu, S.; Yang, H.; Sui, L.; Jiang, S.; Hou, H. Self-Adhesive Polyimide (PI)@Reduced Graphene Oxide (RGO)/PI@Carbon Nanotube (CNT) Hierarchically Porous Electrodes: Maximizing the Utilization of Electroactive Materials for Organic Li-Ion Batteries. Energy Technol. 2020, 8, 2000397. [Google Scholar] [CrossRef]
- Rathod, V.T.; Kumar, J.S.; Jain, A. Polymer and ceramic nanocomposites for aerospace applications. Appl. Nanosci. 2017, 7, 519–548. [Google Scholar] [CrossRef]
- Barbosa, J.C.; Fidalgo-Marijuan, A.; Dias, J.C.; Gonçalves, R.; Salado, M.; Costa, C.M.; Lanceros-Méndez, S. Molecular design of functional polymers for organic radical batteries. Energy Storage Mater. 2023, 60, 102841. [Google Scholar] [CrossRef]
- Guo, W.; Liu, C.; Sun, X.; Yang, Z.; Kia, H.G.; Peng, H. Aligned carbon nanotube/polymer composite fibers with improved mechanical strength and electrical conductivity. J. Mater. Chem. 2012, 22, 903–908. [Google Scholar] [CrossRef]
- Wang, Q.; Dai, J.; Li, W.; Wei, Z.; Jiang, J. The effects of CNT alignment on electrical conductivity and mechanical properties of SWNT/epoxy nanocomposites. Compos. Sci. Technol. 2008, 68, 1644–1648. [Google Scholar] [CrossRef]
- Ma, P.C.; Liu, M.Y.; Zhang, H.; Wang, S.Q.; Wang, R.; Wang, K.; Wong, Y.K.; Tang, B.Z.; Hong, S.H.; Paik, K.W.; et al. Enhanced electrical conductivity of nanocomposites containing hybrid fillers of carbon nanotubes and carbon black. ACS Appl. Mater. Interfaces 2009, 1, 1090–1096. [Google Scholar] [CrossRef] [PubMed]
- Shchegolkov, A.V.; Babaev, A.A.; Shchegolkov, A.V.; Chumak, M.A. Synthesis of Carbon Nanotubes by Microwave Method: Mathematical Modeling and Practical Implementation. Theor. Found. Chem. Eng. 2024, 58, 929–935. [Google Scholar] [CrossRef]
- Sezer Hicyilmaz, A.; Celik Bedeloglu, A. Applications of polyimide coatings: A review. SN Appl. Sci. 2021, 3, 363. [Google Scholar] [CrossRef]
- Wang, H.; Lin, J.; Shen, Z.X. Polyaniline (PANi) based electrode materials for energy storage and conversion. J. Sci. Adv. Mater. Devices 2016, 1, 225–255. [Google Scholar] [CrossRef]
- Chougule, M.A.; Pawar, S.G.; Godse, P.R.; Mulik, R.N.; Sen, S.; Patil, V.B. Synthesis and characterization of polypyrrole (PPy) thin films. Soft Nanosci. Lett. 2011, 1, 6–10. [Google Scholar] [CrossRef]
- Ramanavičius, A.; Ramanavičienė, A.; Malinauskas, A. Electrochemical sensors based on conducting polymer—Polypyrrole. Electrochim. Acta 2006, 51, 6025–6037. [Google Scholar] [CrossRef]
- Gooneratne, R.; Iroh, J.O. Polypyrrole Modified Carbon Nanotube/Polyimide Electrode Materials for Supercapacitors and Lithium-ion Batteries. Energies 2022, 15, 9509. [Google Scholar] [CrossRef]
- An, H.; Wang, Y.; Wang, X.; Zheng, L.; Wang, X.; Yi, L.; Bai, L.; Zhang, X. Polypyrrole/carbon aerogel composite materials for supercapacitor. J. Power Sources 2010, 195, 6964–6969. [Google Scholar] [CrossRef]
- Mi, H.; Zhang, X.; Xu, Y.; Xiao, F. Synthesis, characterization, and electrochemical behavior of polypyrrole/carbon nanotube composites using organometallic-functionalized carbon nanotubes. Appl. Surf. Sci. 2010, 256, 2284–2288. [Google Scholar] [CrossRef]
- Wang, G.X.; Yang, L.; Chen, Y.; Wang, J.Z.; Bewlay, S.; Liu, H.K. An investigation of polypyrrole-LiFePO4 composite cathode materials for lithium-ion batteries. Electrochim. Acta 2005, 50, 4649–4654. [Google Scholar] [CrossRef]
- Wang, J.; Chen, J.; Konstantinov, K.; Zhao, L.; Ng, S.H.; Wang, G.X.; Guo, Z.P.; Liu, H.K. Sulfur-polypyrrole composite positive electrode materials for rechargeable lithium batteries. Electrochim. Acta 2006, 51, 4634–4638. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, H.; Cui, J.; Yao, S.; Shen, X.; Park, T.J.; Kim, J.K. Recent advances in emerging nonaqueous K-ion batteries: From mechanistic insights to practical applications. Energy Storage Mater. 2021, 39, 305–346. [Google Scholar] [CrossRef]
- Li, K.; Wang, Y.; Gao, B.; Lv, X.; Si, Z.; Wang, H.G. Conjugated microporous polyarylimides immobilization on carbon nanotubes with improved utilization of carbonyls as cathode materials for lithium/sodium-ion batteries. J. Colloid Interface Sci. 2021, 601, 446–453. [Google Scholar] [CrossRef]
- Xu, F.; Wang, H.; Lin, J.; Luo, X.; Cao, S.A.; Yang, H. Poly (anthraquinonyl imide) as a high capacity organic cathode material for Na-ion batteries. J. Mater. Chem. A 2016, 4, 11491–11497. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Wang, C.; Hu, Y.; Lin, H.; Kong, W.; Ma, J.; Jin, Z. π-Conjugated polyimide-based organic cathodes with extremely long cycling life for rechargeable magnesium batteries. Energy Storage Mater. 2020, 26, 494–502. [Google Scholar] [CrossRef]
- Luo, C.; Ji, X.; Hou, S.; Eidson, N.; Fan, X.; Liang, Y.; Deng, T.; Jiang, J.; Wang, C. Azo compounds derived from electrochemical reduction of nitro compounds for high performance Li-ion batteries. Adv. Mater. 2018, 30, 1706498. [Google Scholar] [CrossRef]
- Long, Z.; Shi, C.; Wu, C.; Yuan, L.; Qiao, H.; Wang, K. Heterostructure Fe2O3 nanorods@imine-based covalent organic framework for long cycling and high-rate lithium storage. Nanoscale 2022, 14, 1906–1920. [Google Scholar] [CrossRef]
- Andezai, A.; Iroh, J.O. Influence of the Processing Conditions on the Rheology and Heat of Decomposition of Solution Processed Hybrid Nanocomposites and Implication to Sustainable Energy Storage. Energies 2024, 17, 3930. [Google Scholar] [CrossRef]
- Bong, S.K.; Sang, H.B.; Park, Y.H.; Kim, J.H. Polyimide/Carbon Nanotubes Composite Films: A Potential for FPCB. In Proceedings of the 2006 International Conference on Nanoscience and Nanotechnology, Brisbane, QLD, Australia, 3–7 July 2006; pp. 407–410. [Google Scholar] [CrossRef]
- Seetala, N.; Hendon, C.; Tull-Walker, N.; Van Behr, J.; Hester, B.; Lebron-Colon, M.; Meador, M. Synthesis and characterization of polyimide-carbon nanotube composites. World J. Eng. 2014, 11, 193–198. [Google Scholar] [CrossRef]
- Harraz, F.A. Electrochemical Polymerization of Pyrrole into Nanostructured p-Type Porous Silicon. J. Electrochem. Soc. 2006, 153, C349. [Google Scholar] [CrossRef]
- Li, J.; Yin, D.; Qin, Y. Carbon materials: Structures, properties, synthesis and applications. Manuf. Rev. 2023, 10, 13. [Google Scholar] [CrossRef]
- Sankaran, K.J.; Haenen, K. Properties of Carbon Bulk Materials: Graphite and Diamond. In Synthesis and Applications of Nanocarbons; Wiley: Hoboken, NJ, USA, 2020; pp. 1–23. [Google Scholar] [CrossRef]
- Min, J.H.; Patel, M.; Koh, W.G. Incorporation of conductive materials into hydrogels for tissue engineering applications. Polymers 2018, 10, 1078. [Google Scholar] [CrossRef]
- Ansaldo, A.; Bondavalli, P.; Bellani, S.; Del Rio Castillo, A.E.; Prato, M.; Pellegrini, V.; Pognon, G.; Bonaccorso, F. High-Power Graphene–Carbon Nanotube Hybrid Supercapacitors. ChemNanoMat 2017, 3, 436–446. [Google Scholar] [CrossRef]
- Bandaru, P.R. Electrical properties and applications of carbon nanotube structures. J. Nanosci. Nanotechnol. 2007, 7, 1239–1267. [Google Scholar] [CrossRef]
- DiLeo, R.A.; Frisco, S.; Ganter, M.J.; Rogers, R.E.; Raffaelle, R.P.; Landi, B.J. Hybrid germanium nanoparticle–single-wall carbon nanotube free-standing anodes for lithium ion batteries. J. Phys. Chem. C 2011, 115, 22609–22614. [Google Scholar] [CrossRef]
- Irwin, P.C.; Cao, Y.; Bansal, A.; Schadler, L.S. Thermal and Mechanical Properties of Polyimide Nanocomposites. In Proceedings of the 2003 Annual Report Conference on Electrical Insulation and Dielectric Phenomena, Albuquerque, NM, USA, 19–22 October 2003; pp. 120–123. [Google Scholar]
- Liaw, D.J.; Wang, K.L.; Huang, Y.C.; Lee, K.R.; Lai, J.Y.; Ha, C.S. Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Georgiev, A.; Dimov, D.; Spassova, E.; Assa, J.; Dineff, P.; Danev, G. Chemical and Physical Properties of Polyimides: Biomedical and Engineering Applications; InTech: London, UK, 2012. [Google Scholar]
- Jiang, X.; Bin, Y.; Matsuo, M. Electrical and mechanical properties of polyimide–carbon nanotubes composites fabricated by in situ polymerization. Polymer 2005, 46, 7418–7424. [Google Scholar] [CrossRef]
- Zhang, X.; Bai. Surface electric properties of polypyrrole in aqueous solutions. Langmuir 2003, 19, 10703–10709. [Google Scholar] [CrossRef]
- Patois, T.; Lakard, B.; Monney, S.; Roizard, X.; Fievet, P. Characterization of the surface properties of polypyrrole films: Influence of electrodeposition parameters. Synth. Met. 2011, 161, 2498–2505. [Google Scholar] [CrossRef]
- Wolfart, F.; Dubal, D.P.; Vidotti, M.; Holze, R.; Gómez-Romero, P. Electrochemical supercapacitive properties of polypyrrole thin films: Influence of the electropolymerization methods. J. Solid State Electrochem. 2016, 20, 901–910. [Google Scholar] [CrossRef]
- Andezai, A.; Iroh, J.O. Polypyrrole Hybrid Nanocomposite Electrode Materials with Outstanding Specific Capacitance. Energies 2025, 18, 1304. [Google Scholar] [CrossRef]









| Property | Standalone CNT Sheets | Graphene Sheets | Activated Carbon | Carbon Fibers |
|---|---|---|---|---|
| Electrical Conductivity | ~103–104 S/cm (highly conductive). | ~104–105 S/cm (extremely high for high-quality graphene). | ~10–100 S/cm (moderate conductivity). | ~102–103 S/cm (depends on fiber alignment and purity). |
| Tensile Strength | ~150–300 MPa (moderate strength). | ~100–150 MPa (weaker but improves when stacked). | ~20–80 MPa (low due to porous structure). | ~1–5 GPa (exceptionally high for advanced fibers). |
| Young’s Modulus | ~10–20 GPa (high stiffness). | ~1–5 GPa (lower stiffness due to 2D nature). | ~0.5–2 GPa (weak mechanical stability). | ~70–300 GPa (extremely high for structural uses). |
| Thermal Conductivity | ~1000–2000 W/m·K (exceptionally high). | ~5000 W/m·K (highest known for pure graphene). | ~1–10 W/m·K (low due to high porosity). | ~100–600 W/m·K (moderate, improves with alignment). |
| Porosity | ~10–50 nm (moderate porosity, defined by CNT bundle packing). | ~Few nanometers (depends on stacking, typically low). | ~0.1–1 μm (high porosity due to activated structure). | Negligible (dense and aligned structure). |
| Density | ~1.3–1.5 g/cm3 (lightweight). | ~1–2 g/cm3 (depends on number of layers and defects). | ~0.5–0.9 g/cm3 (very lightweight). | ~1.8–2.0 g/cm3 (heavier due to structural density). |
| Specific Capacitance | ~10–50 F/g (limited to double-layer capacitance). | ~50–200 F/g (depends on surface area and electrolyte). | ~100–300 F/g (high due to extensive surface area). | ~10–50 F/g (low due to dense structure). |
| Thermal Stability | Stable up to ~600–800 °C in inert environments. | Stable up to ~400 °C in air, higher in inert atmospheres. | Stable up to ~600 °C (depends on activation process). | Stable up to ~1000 °C in inert conditions. |
| Flexibility | High; bendable and stretchable. | High for single-layer graphene; reduced for stacked layers. | Low; brittle and prone to cracking. | Low; rigid and prone to fracture under bending. |
| Scalability | Challenging; uniformity over large areas is difficult. | Difficult; large-scale production of defect-free sheets is hard. | High; widely available and inexpensive. | Moderate; high-quality fibers are expensive to produce. |
| Properties | Polypyrrole (PPy) | Polyimide (PI) |
|---|---|---|
| Electrical Conductivity | ~10–100 S/cm (doped with appropriate agents like p-toluene sulfonic acid) | ~10−12 S/cm (intrinsic); can be increased with conductive additives like CNTs. |
| Redox Activity | Exhibits pseudocapacitance; specific capacitance values range from 200–600 F/g, depending on structure and doping. | No intrinsic redox activity; primarily used as a structural matrix. |
| Mechanical Strength | Moderate; tensile strength ranges from 10–50 MPa, brittle in pure form. | High; tensile strength ranges from 80–200 MPa, depending on processing and reinforcement. |
| Thermal Stability | Degrades above 150–200 °C, depending on polymerization and doping conditions. | High stability: thermal degradation starts at ~400 °C, suitable for high-temperature applications. |
| Porosity Contribution | Can form layers with pores in the range of 10–50 nm (dependent on deposition conditions). | Porosity is tunable; partial imidization at 90 °C results in a porous structure, while full imidization at 250 °C reduces porosity. |
| Chemical Stability | Sensitive to over-oxidation in electrolytes; stability depends on the potential range. | Excellent chemical stability; resistant to solvents, acids, and bases. |
| Young’s Modulus | ~1–2 GPa (moderate, brittle polymer). | ~2–8 GPa (high, depends on reinforcement and processing conditions). |
| Density | ~1.5 g/cm3 (bulk material). | ~1.4 g/cm3 (varies slightly with processing). |
| Specific Capacitance | Ranges from 200–600 F/g (varies with doping and structure). | Not applicable; PI does not contribute directly to capacitance. |
| Scalability | Easily deposited via chemical or electrochemical polymerization. | Scalable synthesis through imidization of polyamic acid, suitable for large-scale applications. |
| Sample | Porosity (%) | Porosity (%) with PPy | Thickness (µm) | Porosity Through Thickness (%) | EIS Porosity (%) | Degree of Imidization (%) |
|---|---|---|---|---|---|---|
| Pure CNTs | 52.09 | - | - | - | - | - |
| PI/CNTs—90 °C | 50.59 | 38.57 | 34.8 | 37.08 | 12.2 | 38.3 |
| PI/CNTs—180 °C | 40.57 | 22.03 | 47.0 | 24.79 | 0.74 | 98.9 |
| PI/CNTs—250 °C | 36.99% | 10.19 | 50.3 | 16.06 | 0.42 | 100 |
| Deposition Time |
Time (s) |
Average Current (A) |
Charge (Q) |
|---|---|---|---|
| 60 s | 60 | 0.12 | 7.2 |
| 600 s | 600 | 0.055 | 33 |
| 700 s | 700 | 0.02 | 14 |
| Sample (Processing Conditions) | Rs (Ω) | Rct (Ω) | Cdl (µF) |
|---|---|---|---|
| 90 °C, 60 s (i) | 1.98 | 9.56 | 192.1 |
| 90 °C, 600 s (ii) | 2.12 | 15.84 | 138.6 |
| 90 °C, 700 s (iii) | 2.36 | 24.17 | 101.3 |
| Sample/Material System | Power Density (W/kg) | Energy Density (Wh/kg) | Reference |
|---|---|---|---|
| PI/CNTs—90 °C (This work) | 957.78 | 274.56 | This study |
| PI/CNTs—180 °C (This work) | 597.26 | 261.72 | This study |
| PI/CNTs—250 °C (This work) | 375.79 | 43.21 | This study |
| Ref. [29] | 250 | 15 | Ref. [29] |
| Ref. [30] | 141 | 148.3 | Ref. [30] |
| Ref. [31] | 20,600 | 141 | Ref. [31] |
| Ref. [32] | 142 | 119.722 | Ref. [32] |
| Deposition Time | Bulk Resistance (Ω) | Porosity (%) |
|---|---|---|
| 60 s | 17.7 | 12.2 |
| 600 s | 42.2 | 7.2 |
| 700 s | 61.9 | 4.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andezai, A.; Iroh, J.O. Sustainable Energy Storage Systems: Polypyrrole-Filled Polyimide-Modified Carbon Nanotube Sheets with Remarkable Energy Density. Energies 2025, 18, 2158. https://doi.org/10.3390/en18092158
Andezai A, Iroh JO. Sustainable Energy Storage Systems: Polypyrrole-Filled Polyimide-Modified Carbon Nanotube Sheets with Remarkable Energy Density. Energies. 2025; 18(9):2158. https://doi.org/10.3390/en18092158
Chicago/Turabian StyleAndezai, Andekuba, and Jude O. Iroh. 2025. "Sustainable Energy Storage Systems: Polypyrrole-Filled Polyimide-Modified Carbon Nanotube Sheets with Remarkable Energy Density" Energies 18, no. 9: 2158. https://doi.org/10.3390/en18092158
APA StyleAndezai, A., & Iroh, J. O. (2025). Sustainable Energy Storage Systems: Polypyrrole-Filled Polyimide-Modified Carbon Nanotube Sheets with Remarkable Energy Density. Energies, 18(9), 2158. https://doi.org/10.3390/en18092158






