Anaerobic Co-Digestion of Common Reed and Plant-Based Biowaste from Households
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrates and Inoculum
2.2. Experimental Setup
- G(t)—cumulative CH4 production at a specific time t (mL);
- G0—CH4 production potential (mL);
- Rmax—maximum daily CH4 production rate (mL day−1);
- λ—duration of lag phase (minimum time to produce CH4) (days);
- t—cumulative time for CH4 production (days);
- e—mathematical constant (2.71828).
2.3. Chemical Analyses
2.4. Calculations and Statistical Analyses
3. Results
3.1. Feedstock and Inoculum Characteristics
3.2. Methane Yield from Mono- and Co-Digestion of Reed Silage and Biowaste
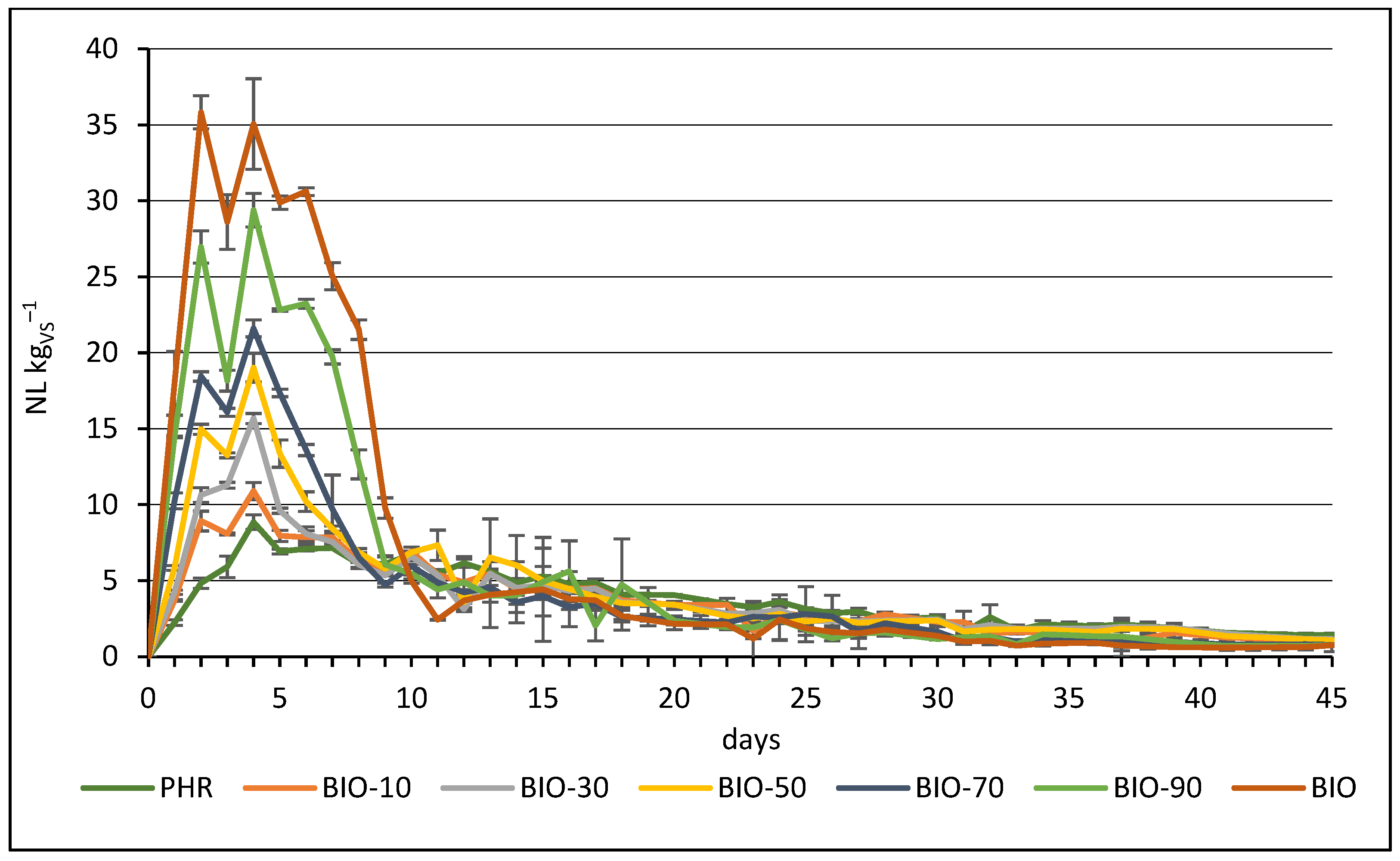
3.3. Daily Methane Production from Mono- and Co-Digestion of Reed Silage and Biowaste
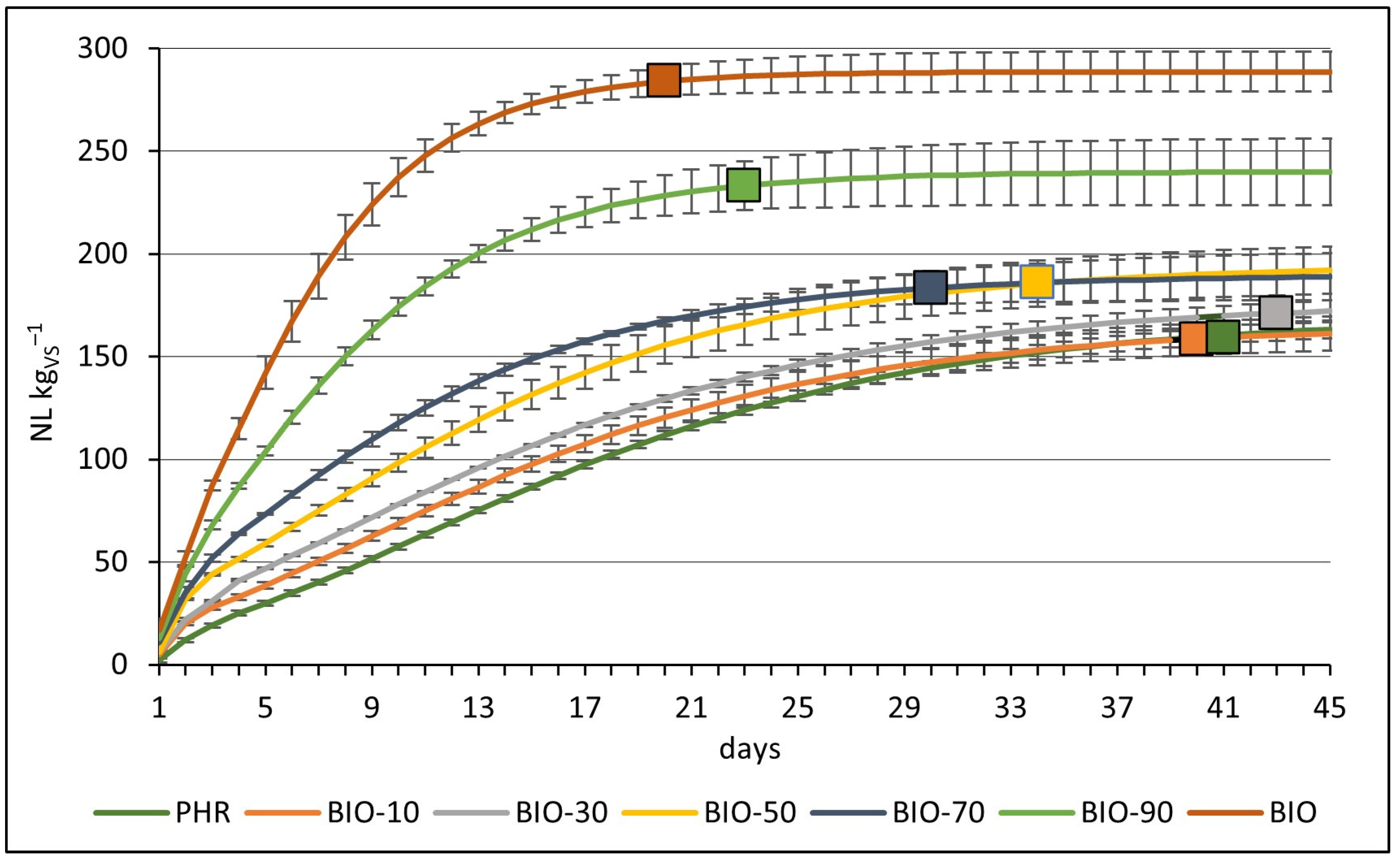
3.4. Cumulative Methane Production from Mono- and Co-Digestion of Reed Silage and Biowaste
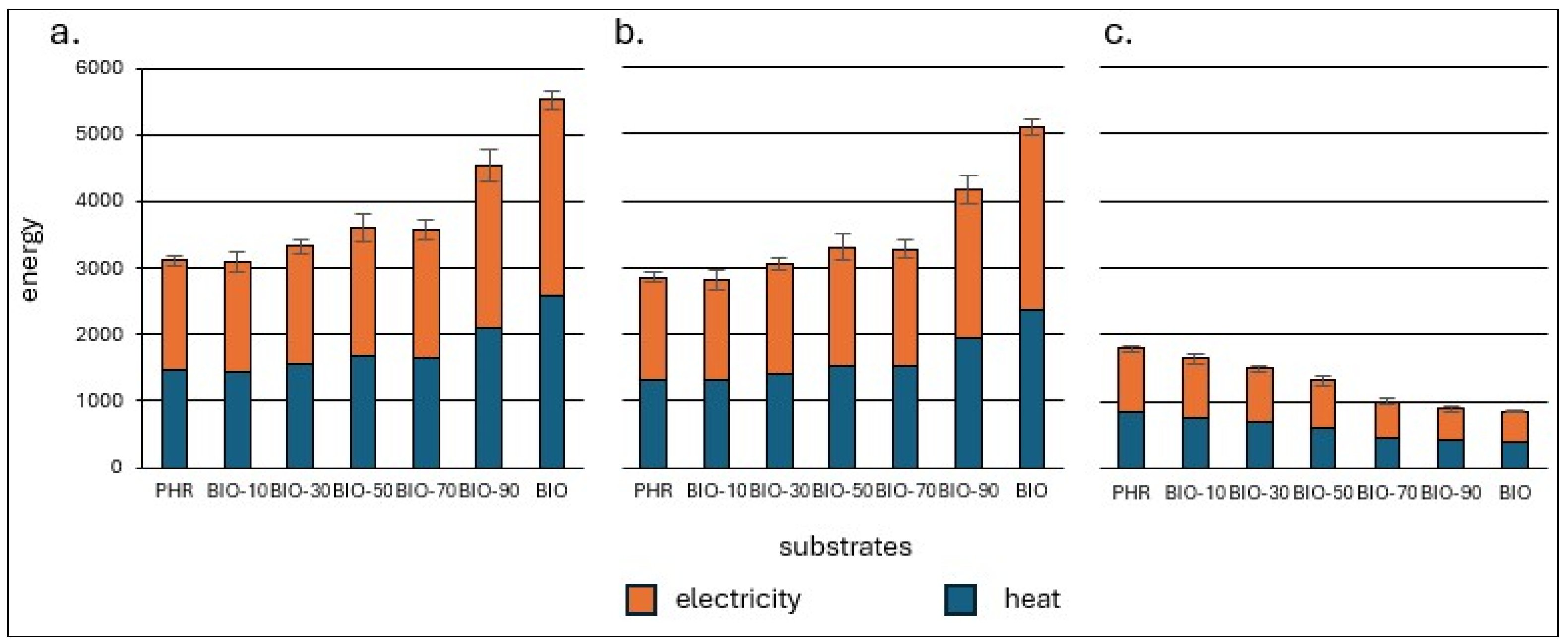
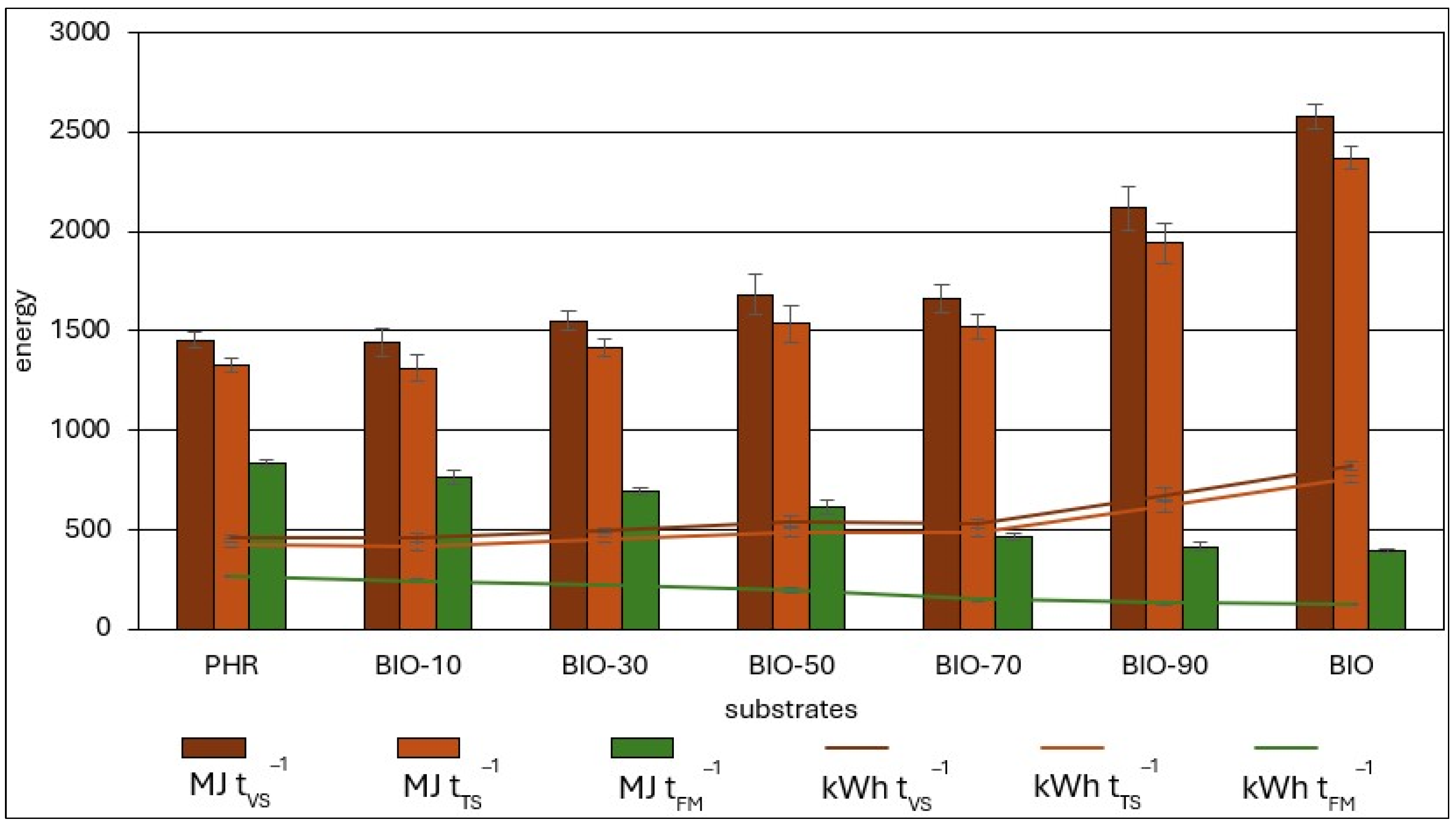
3.5. Energy Balance and GHG Emissions
4. Discussion
4.1. Feedstock Characteristics
4.2. Methane Production
4.3. Energy Balance and GHG Emissions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Eurostat. Municipal Waste by Waste Management Operations. Available online: https://ec.europa.eu/eurostat/databrowser/view/env_wasmun__custom_15352477/default/table?lang=en (accessed on 21 February 2025).
- Eurostat. Municipal Waste by Waste Management Operations. Available online: https://ec.europa.eu/eurostat/databrowser/product/page/ENV_WASMUN (accessed on 21 February 2025).
- Eurostat. Statistics Explained. Food Waste and Food Waste Prevention-Estimates. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Food_waste_and_food_waste_prevention_-_estimates (accessed on 21 February 2025).
- Statistics Poland. Statistical Analyses. In Environment 2024, 1st ed.; Statistics Poland: Warsaw, Poland, 2024. [Google Scholar]
- Eurostat. Food Waste and Food Waste Prevention by NACE Rev. 2 Activity-Tonnes of Fresh Mass. Available online: https://ec.europa.eu/eurostat/databrowser/view/env_wasfw/default/table?lang=en (accessed on 21 February 2025).
- Al Seadi, T.; Rutz, D.; Prassl, H.; Köttner, M.; Finsterwalder, T.; Volk, S.; Janssen, R. Biogas Handbook, 1st ed.; University of Southern Denmark Esbjerg: Esbjerg, Denmark, 2008. [Google Scholar]
- Linville, J.L.; Shen, Y.; Wu, M.M.; Urgun-Demirtas, M. Current State of Anaerobic Digestion of Organic Wastes in North America. Curr. Sustain. Renew. Energy Rep. 2015, 2, 136–144. [Google Scholar] [CrossRef]
- Meegoda, J.N.; Li, B.; Patel, K.; Wang, L.B. A Review of the Processes, Parameters, and Optimization of Anaerobic Digestion. Int. J. Environ. Res. Public Health 2018, 15, 2224. [Google Scholar] [CrossRef]
- Jameel, M.K.; Mustafa, M.A.; Ahmed, H.S.; Mohammed, A.J.; Ghazy, H.; Shakir, M.N.; Lawas, A.M.; khudhur Mohammed, S.; Idan, A.H.; Mahmoud, Z.H.; et al. Biogas: Production, Properties, Applications, Economic and Challenges: A Review. Results Chem. 2024, 7, 101549. [Google Scholar] [CrossRef]
- Costa, A.; Ely, C.; Pennington, M.; Rock, S.; Staniec, C.; Turgeon, J. Anaerobic Digestion and Its Applications, 1st ed.; U.S. Environmental Protection Agency: Washington, DC, USA, 2015.
- Lin, L.; Xu, F.; Ge, X.; Li, Y. Improving the Sustainability of Organic Waste Management Practices in the Food-Energy-Water Nexus: A Comparative Review of Anaerobic Digestion and Composting. Renew. Sustain. Energy Rev. 2018, 89, 151–167. [Google Scholar] [CrossRef]
- Angelidaki, I.; Sanders, W. Assessment of the Anaerobic Biodegradability of Macropollutants. Rev. Environ. Sci. Biotechnol. 2004, 3, 117–129. [Google Scholar] [CrossRef]
- Triolo, J.M.; Pedersen, L.; Qu, H.; Sommer, S.G. Biochemical Methane Potential and Anaerobic Biodegradability of Non-Herbaceous and Herbaceous Phytomass in Biogas Production. Bioresour. Technol. 2012, 125, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Melts, I.; Ivask, M.; Geetha, M.; Takeuchi, K.; Heinsoo, K. Combining Bioenergy and Nature Conservation: An Example in Wetlands. Renew. Sustain. Energy Rev. 2019, 111, 293–302. [Google Scholar] [CrossRef]
- Herrmann, C.; Prochnow, A.; Heiermann, M.; Idler, C. Biomass from Landscape Management of Grassland Used for Biogas Production: Effects of Harvest Date and Silage Additives on Feedstock Quality and Methane Yield. Grass Forage Sci. 2014, 69, 549–566. [Google Scholar] [CrossRef]
- Mosier, N. Features of Promising Technologies for Pretreatment of Lignocellulosic Biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef]
- Beer, F.; Wichtmann, W.; Villegas, L.; Agus, F. Paludiculture. In Recarbonizing Global Soils: A Technical Manual of Best Management Practices; FAO: Rome, Italy, 2021; Volume 5, ISBN 978-92-5-134900-7. [Google Scholar]
- Regulation (EU) 2024/1991 of the European Parliament and of the Council of 24 June 2024 on Nature Restoration and Amending Regulation (EU) 2022/869 (Text with EEA Relevance). 2024. Available online: https://climate-laws.org/document/regulation-eu-2024-1991-of-the-european-parliament-and-of-the-council-of-24-june-2024-on-nature-restoration-and-amending-regulation-eu-2022-869_93bc (accessed on 21 February 2025).
- Banaszuk, P.; Kamocki, A.K.; Wysocka-Czubaszek, A.; Czubaszek, R.; Roj-Rojewski, S. Closing the Loop-Recovery of Nutrients and Energy from Wetland Biomass. Ecol. Eng. 2020, 143, 105643. [Google Scholar] [CrossRef]
- Lizasoain, J.; Rincón, M.; Theuretzbacher, F.; Enguídanos, R.; Nielsen, P.J.; Potthast, A.; Zweckmair, T.; Gronauer, A.; Bauer, A. Biogas Production from Reed Biomass: Effect of Pretreatment Using Different Steam Explosion Conditions. Biomass Bioenerg. 2016, 95, 84–91. [Google Scholar] [CrossRef]
- Roj-Rojewski, S.; Wysocka-Czubaszek, A.; Czubaszek, R.; Kamocki, A.; Banaszuk, P. Anaerobic Digestion of Wetland Biomass from Conservation Management for Biogas Production. Biomass Bioenerg. 2019, 122, 126–132. [Google Scholar] [CrossRef]
- Ohlsson, L.-O.; Karlsson, S.; Rupar-Gadd, K.; Albers, E.; Welander, U. Evaluation of Laminaria Digitata and Phragmites Australis for Biogas Production and Nutrient Recycling. Biomass Bioenerg. 2020, 140, 105670. [Google Scholar] [CrossRef]
- Baute, K.; Van Eerd, L.; Robinson, D.; Sikkema, P.; Mushtaq, M.; Gilroyed, B. Comparing the Biomass Yield and Biogas Potential of Phragmites australis with Miscanthus x giganteus and Panicum virgatum Grown in Canada. Energies 2018, 11, 2198. [Google Scholar] [CrossRef]
- Dubrovskis, V.; Kazulis, V. Biogas Production Potential from Reeds. RE&PQJ 2012, 10, 886–889. [Google Scholar] [CrossRef]
- Pelegrin, J.; Holzem, R.M. Evaluating the Impacts of Phragmites Australis Pretreatment Methods on Biogas and Methane. MJUR 2017, 7, 244–259. [Google Scholar]
- Tran, G.V.; Unpaprom, Y.; Ramaraj, R. Effects of Co-Substrate Concentrations on the Anaerobic Co-Digestion of Common Reed and Cow Dung. AJARCDE 2019, 3, 28–32. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, H.; Chai, Y.; Wang, L.; Mi, X.; Zhang, L.; Ware, M.A. Biogas Properties and Enzymatic Analysis during Anaerobic Fermentation of Phragmites Australis Straw and Cow Dung: Influence of Nickel Chloride Supplement. Biodegradation 2017, 28, 15–25. [Google Scholar] [CrossRef]
- Hao, H.; Tian, Y.; Zhang, H.; Chai, Y. Copper Stressed Anaerobic Fermentation: Biogas Properties, Process Stability, Biodegradation and Enzyme Responses. Biodegradation 2017, 28, 369–381. [Google Scholar] [CrossRef]
- Zhang, H.; Han, X.; Tian, Y.; Li, Y.; Yang, K.; Hao, H.; Chai, Y.; Xu, X. Process Analysis of Anaerobic Fermentation of Phragmites Australis Straw and Cow Dung Exposing to Elevated Chromium (VI) Concentrations. J. Environ. Manag. 2018, 224, 414–424. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Xi, B.; Sun, W.; Xia, X.; Zhu, C.; He, X.; Li, M.; Yang, T.; Wang, P.; et al. Biogas Production Improvement and C/N Control by Natural Clinoptilolite Addition into Anaerobic Co-Digestion of Phragmites Australis, Feces and Kitchen Waste. Bioresour. Technol. 2015, 180, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Czubaszek, R.; Wysocka-Czubaszek, A.; Wichtmann, W.; Zając, G.; Banaszuk, P. Common Reed and Maize Silage Co-Digestion as a Pathway towards Sustainable Biogas Production. Energies 2023, 16, 695. [Google Scholar] [CrossRef]
- Caldeira, C.; De Laurentiis, V.; Corrado, S.; van Holsteijn, F.; Sala, S. Quantification of Food Waste per Product Group along the Food Supply Chain in the European Union: A Mass Flow Analysis. Resour. Conserv. Recycl. 2019, 149, 479–488. [Google Scholar] [CrossRef]
- Wang, K.; Yun, S.; Xing, T.; Li, B.; Abbas, Y.; Liu, X. Binary and Ternary Trace Elements to Enhance Anaerobic Digestion of Cattle Manure: Focusing on Kinetic Models for Biogas Production and Digestate Utilization. Bioresour. Technol. 2021, 323, 124571. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods: For the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA; American Water Works Association: Denver, CO, USA; Water Pollution Control Federation: Alexandria, VA, USA, 1998; ISBN 978-0-87553-235-6. [Google Scholar]
- Curkowski, A.; Mroczkowski, P.; Oniszk-Popławska, A.; Wśniewski, G. Agricultural Biogas-Production and Usage, 1st ed.; Mazowiecka Agencja Energetyczna Sp. z o.o.: Warsaw, Poland, 2009. (In Polish) [Google Scholar]
- The National Centre for Emissions Management. Emission Factors for CO2, SO2, NOx CO and Total Particulate Matter for Electrical Energy on the Basis of Information in National Database on Emissions of Greenhouse Gases and Other Substances for 2023; The National Centre for Emissions Management: Warsaw, Poland, 2024. (In Polish) [Google Scholar]
- The National Centre for Emissions Management. Calorific Values (CO) and CO2 Emission Factors (EC) in 2022 to Be Reported under the Emission Trading Scheme for 2025; The National Centre for Emissions Management: Warsaw, Poland, 2024. (In Polish) [Google Scholar]
- Cao, Y.; Natuhara, Y. Composition and Distribution of Common Reed (Phragmites australis) Along an Urbanized River: A Case from Central Japan. Appl. Ecol. Environ. Res. 2020, 18, 3935–3950. [Google Scholar] [CrossRef]
- Engloner, A.I.; Németh, K.; Gere, D.; Stefán, D.; Óvári, M. Effects of Water Depth and Water Level Fluctuation on the Total and Bio-Available Element Concentrations in Riverine Reed Stands. Ecol. Indic. 2020, 114, 106328. [Google Scholar] [CrossRef]
- Wenzel, M.; Kabengele, G.; Dahms, T.; Barz, M.; Wichtmann, W. Bioenergie aus Nassen Mooren. Thermische Verwertung von Halmgutartiger Biomasse aus Paludikultur, 1st ed.; Institut für Botanik und Landschaftsökologie, Universität Greifswald, Greifswald Moor Centrum: Greifswald, Germany, 2022. (In German) [Google Scholar]
- Wöhler-Geske, A.; Moschner, C.R.; Gellerich, A. Provenances and Properties of Thatching Reed (Phragmites australis). Appl. Agric. Forestry Res. 2016, 66, 1–10. [Google Scholar] [CrossRef]
- Czubaszek, R.; Wysocka-Czubaszek, A.; Wichtmann, W.; Banaszuk, P. Specific Methane Yield of Wetland Biomass in Dry and Wet Fermentation Technologies. Energies 2021, 14, 8373. [Google Scholar] [CrossRef]
- Baran, M.; Váradyová, Z.; Kráčmár, S.; Hedvábný, J. The Common Reed (Phragmites australis) as a Source of Roughage in Ruminant Nutrition. Acta Vet. Brno 2002, 71, 445–449. [Google Scholar] [CrossRef][Green Version]
- Asano, K.; Ishikawa, T.; Araie, A.; Ishida, M. Improving Quality of Common Reed (Phragmites communis Trin.) Silage with Additives. Asian-Australas. J. Anim. Sci. 2018, 31, 1747–1755. [Google Scholar] [CrossRef]
- Albrecht, K.; Neudecker, F.; Veigel, S.; Bodner, S.; Keckes, J.; Gindl-Altmutter, W. The Suitability of Common Reed (Phragmites australis) for Load-Bearing Structural Materials. J. Mater. Sci. 2023, 58, 15411–15420. [Google Scholar] [CrossRef]
- Temel, S.; Keskin, B.; Güner, Z.; Atalay, A.İ. Determination of Yield and Quality Characteristics of Common Reed (Phragmites australis (Cav.) Trin. Ex Steud) Harvested at Different Growth Stages. Turk. J. Field Crops 2023, 28, 70–78. [Google Scholar] [CrossRef]
- Borin, M.; Florio, G.; Barbera, A.; Cirelli, G.L.; Albergo, R.; Palazzo, S. Preliminary Evaluation of Macrophyte Wetland Biomasses to Obtain Second Generation Ethanol. In Proceedings of the 19th European Biomass Conference and Exhibition, Berlin, Germany, 6–10 June 2011. [Google Scholar]
- López-González, D.; Avalos-Ramirez, A.; Giroir-Fendler, A.; Godbout, S.; Fernandez-Lopez, M.; Sanchez-Silva, L.; Valverde, J.L. Combustion Kinetic Study of Woody and Herbaceous Crops by Thermal Analysis Coupled to Mass Spectrometry. Energy 2015, 90, 1626–1635. [Google Scholar] [CrossRef]
- Obolewski, K.; Strzelczak, A.; Kiepas-Kokot, A. Chemical Composition of Reed Phragmites australis (Cav.) Trin. Ex Steud. Versus Density and Structure of Periphyton In Various Aquatic Ecosystems. J. Elementol. 2007, 12, 63–79. [Google Scholar]
- Jiménez-Rosado, M.; Perez-Puyana, V.; Romero, A. The Use of Biowaste for the Production of Biodegradable Superabsorbent Materials. Curr. Opin. Food Sci. 2023, 49, 100975. [Google Scholar] [CrossRef]
- Sharma, A.; Kuthiala, T.; Thakur, K.; Thatai, K.S.; Singh, G.; Kumar, P.; Arya, S.K. Kitchen Waste: Sustainable Bioconversion to Value-Added Product and Economic Challenges. Biomass Conv. Bioref. 2025, 15, 1749–1770. [Google Scholar] [CrossRef]
- Rex, P.; Meenakshisundaram, N.; Barmavatu, P. Sustainable Valorisation of Kitchen Waste through Greenhouse Solar Drying and Microwave Pyrolysis—Technology Readiness Level for the Production of Biochar. J. Environ. Health Sci. Eng. 2024, 22, 381–395. [Google Scholar] [CrossRef]
- de Abreu, Í.B.S.; de Sousa, M.H.; da Silva, A.P.; de Araújo Padilha, C.E.; Sales, A.T.; da Silva, A.S.A.; Dutra, E.D.; Menezes, R.S.C. Global Variability of Food Waste Chemical Composition and Its Consequences on the Production of Biofuels and Chemical Compounds. J. Mater. Cycles Waste Manag. 2023, 25, 1309–1324. [Google Scholar] [CrossRef]
- Hanc, A.; Novak, P.; Dvorak, M.; Habart, J.; Svehla, P. Composition and Parameters of Household Bio-Waste in Four Seasons. Waste Manag. 2011, 31, 1450–1460. [Google Scholar] [CrossRef]
- Škorjanc, A.; Goričanec, D.; Urbancl, D. Assessing Energy Potential and Chemical Composition of Food Waste Thermodynamic Conversion Products: A Literature Review. Energies 2024, 17, 1897. [Google Scholar] [CrossRef]
- Meng, Q.; Liu, H.; Zhang, H.; Xu, S.; Lichtfouse, E.; Yun, Y. Anaerobic Digestion and Recycling of Kitchen Waste: A Review. Environ. Chem. Lett. 2022, 20, 1745–1762. [Google Scholar] [CrossRef]
- Montoneri, E.; Boffa, V.; Savarino, P.; Perrone, D.; Ghezzo, M.; Montoneri, C.; Mendichi, R. Acid Soluble Bio-Organic Substances Isolated from Urban Bio-Waste. Chemical Composition and Properties of Products. Waste Manag. 2011, 31, 10–17. [Google Scholar] [CrossRef]
- Wo, D.; Bi, G.; Li, L.; Kong, X.; Jiang, E.; Xie, J. Iron-Fortified Anaerobic Co-Digestion Performance of Kitchen Waste and Pennisetum Hybrid. Bioenerg. Res. 2023, 16, 651–659. [Google Scholar] [CrossRef]
- Hu, N.; Tong, Z.; Li, F.; Zhang, X.; Gao, H.; Zhou, J.A. Two-Stage Strategy Combining Vermicomposting and Membrane-Covered Aerobic Composting to Achieve Value-Added Recycling of Kitchen Waste Solid Residues. Waste Dispos. Sustain. Energy 2024, 6, 501–510. [Google Scholar] [CrossRef]
- Hu, F.; Fu, N.; Wei, Q.; Liu, S.; Hu, Y.; Zhang, S.; Wang, X.; Peng, X.; Dai, H.; Wei, Y. Effect of Alkali Pretreatment Time on Kitchen Waste Anaerobic Digestion Performance Enhanced by Alkali Pretreatment Combined with Bentonite: Performance Enhancement, Microbial Community Structure, and Functional Gene Analysis. Environ. Sci. Pollut. Res. 2024, 31, 7167–7178. [Google Scholar] [CrossRef] [PubMed]
- Sathya, T.A.; Alarjani, K.M.; Elshikh, M.S.; Flanetraj, S.R.; Ponnuswamy, V. Co-Composting of Green Leaves and Kitchen Waste: Characterization of Organic Amendments, Microbial Activity and Analysis of Defence Enzymes in Plants. Biomass Conv. Bioref. 2024, 1–12. [Google Scholar] [CrossRef]
- Gautam, B.; Tiwari, S.; Pokhrel, M.R.; Tomberlin, J.K.; Khanal, P. Expanding Black Soldier Fly (BSF; Hermetia illucens; Diptera: Stratiomyidae) in the Developing World: Use of BSF Larvae as a Biological Tool to Recycle Various Organic Biowastes for Alternative Protein Production in Nepal. Biotechnol. Rep. 2025, 45, e00879. [Google Scholar] [CrossRef]
- Slopiecka, K.; Liberti, F.; Massoli, S.; Bartocci, P.; Fantozzi, F. Chemical and Physical Characterization of Food Waste to Improve Its Use in Anaerobic Digestion Plants. Energy Nexus 2022, 5, 100049. [Google Scholar] [CrossRef]
- Kaparaju, P.; Rintala, J. Anaerobic Co-Digestion of Potato Tuber and Its Industrial by-Products with Pig Manure. Resour. Conserv. Recycl. 2005, 43, 175–188. [Google Scholar] [CrossRef]
- Dias, R.D.C.; Melo, C.A.D.; Faria, A.T.; Silva, D.V.; Ferreira, M.K.; dos Reis, M.R. Effects of Tembotrione on Potato Crop in a Tropical Soil. Commun. Soil Sci. Plant Anal. 2019, 50, 1652–1661. [Google Scholar] [CrossRef]
- Czubaszek, R.; Wysocka-Czubaszek, A.; Tyborowski, R. Methane Production Potential from Apple Pomace, Cabbage Leaves, Pumpkin Residue and Walnut Husks. Appl. Sci. 2022, 12, 6128. [Google Scholar] [CrossRef]
- Nagy, G. The Application and Treatment of Freshwater Macrophytes as Potential Biogas Base Materials: A Review. Renew. Sustain. Energy Rev. 2024, 199, 114513. [Google Scholar] [CrossRef]
- Granéli, W. Reed Phragmites Australis (Cav.) Trin. Ex Steudel as an Energy Source in Sweden. Biomass 1984, 4, 183–208. [Google Scholar] [CrossRef]
- Brix, H.; Sorrell, B.K.; Lorenzen, B. Are Phragmites-Dominated Wetlands a Net Source or Net Sink of Greenhouse Gases? Aquat. Bot. 2001, 69, 313–324. [Google Scholar] [CrossRef]
- Köbbing, J.F.; Thevs, N.; Zerbe, S. The Utilisation of Reed (Phragmites australis): A Review. Mires Peat 2013, 13, 1–14. [Google Scholar]
- Hartung, C.; Andrade, D.; Dandikas, V.; Eickenscheidt, T.; Drösler, M.; Zollfrank, C.; Heuwinkel, H. Suitability of Paludiculture Biomass as Biogas Substrate—Biogas Yield and Long-Term Effects on Anaerobic Digestion. Renew. Energy 2020, 159, 64–71. [Google Scholar] [CrossRef]
- Eller, F.; Ehde, P.M.; Oehmke, C.; Ren, L.; Brix, H.; Sorrell, B.K.; Weisner, S.E.B. Biomethane Yield from Different European Phragmites australis Genotypes, Compared with Other Herbaceous Wetland Species Grown at Different Fertilization Regimes. Resources 2020, 9, 57. [Google Scholar] [CrossRef]
- Herrmann, A.; Rath, J. Biogas Production from Maize: Current State, Challenges, and Prospects. 1. Methane Yield Potential. Bioenergy Res. 2012, 5, 1027–1042. [Google Scholar] [CrossRef]
- Kreuger, E.; Nges, I.; Björnsson, L. Ensiling of Crops for Biogas Production: Effects on Methane Yield and Total Solids Determination. Biotechnol. Biofuels. 2011, 4, 44. [Google Scholar] [CrossRef]
- Brown, A.E.; Ford, J.S.; Bale, C.S.E.; Camargo-Valero, M.A.; Cheffins, N.J.; Mason, P.E.; Price-Allison, A.M.; Ross, A.B.; Taylor, P.G. An Assessment of Road-Verge Grass as a Feedstock for Farm-Fed Anaerobic Digestion Plants. Biomass Bioenerg. 2020, 138, 105570. [Google Scholar] [CrossRef]
- Czubaszek, R.; Wysocka-Czubaszek, A.; Banaszuk, P.; Zając, G.; Wassen, M.J. Grass from Road Verges as a Substrate for Biogas Production. Energies 2023, 16, 4488. [Google Scholar] [CrossRef]
- Al-Iraqi, A.R.; Gandhi, B.P.; Folkard, A.M.; Barker, P.A.; Semple, K.T. Determine the Optimal Parameters for Biogas Production from Common Reed (Phragmites australis). Bioenerg. Res. 2024, 17, 1302–1314. [Google Scholar] [CrossRef]
- Willfors, A. Exploring Biogas from Common Reed. Available online: https://research.fi/en/results/publication/0694257524 (accessed on 29 March 2025).
- Al-Iraqi, A.R.; Gandhi, B.P.; Folkard, A.M.; Barker, P.A.; Semple, K.T. Influence of Inoculum to Substrate Ratio and Substrates Mixing Ratio on Biogas Production from the Anaerobic Co-Digestion of Phragmites australis and Food Waste. Bioenerg. Res. 2024, 17, 1277–1287. [Google Scholar] [CrossRef]
- Xu, F.; Li, Y. Solid-State Co-Digestion of Expired Dog Food and Corn Stover for Methane Production. Bioresour. Technol. 2012, 118, 219–226. [Google Scholar] [CrossRef]
- Brown, D.; Li, Y. Solid State Anaerobic Co-Digestion of Yard Waste and Food Waste for Biogas Production. Bioresour. Technol. 2013, 127, 275–280. [Google Scholar] [CrossRef]
- Li, W.; Khalid, H.; Zhu, Z.; Zhang, R.; Liu, G.; Chen, C.; Thorin, E. Methane Production through Anaerobic Digestion: Participation and Digestion Characteristics of Cellulose, Hemicellulose and Lignin. Appl. Energy 2018, 226, 1219–1228. [Google Scholar] [CrossRef]
- Jung, S.-J.; Kim, S.-H.; Chung, I.-M. Comparison of Lignin, Cellulose, and Hemicellulose Contents for Biofuels Utilization among 4 Types of Lignocellulosic Crops. Biomass Bioenerg. 2015, 83, 322–327. [Google Scholar] [CrossRef]
- Cotana, F.; Cavalaglio, G.; Pisello, A.; Gelosia, M.; Ingles, D.; Pompili, E. Sustainable Ethanol Production from Common Reed (Phragmites australis) through Simultaneuos Saccharification and Fermentation. Sustainability 2015, 7, 12149–12163. [Google Scholar] [CrossRef]
- Elhaak, M.A.; Mohsen, A.A.; Hamada, E.-S.A.M.; El-Gebaly, F.E. Biofuel Production from Phragmites australis (Cav.) and Typha domingensis (Pers.) Plants of Burullus Lake. Egypt. J. Exp. Biol. (Bot.) 2015, 11, 237. [Google Scholar]
- Murawski, M.; Grzelak, M.; Waliszewska, M.; Knioła, A.; Czekała, W. Energy Value and Yielding from Extensively Used Meadows. Fragm. Agron. 2015, 32, 71–78. (In Polish) [Google Scholar]
- Honoré, M.; Pimbert, S.; Lecompte, T. Characterisation of Plant Flours for Biocomposite Applications Focussing on Phragmites australis Properties. Biosyst. Eng. 2020, 197, 367–377. [Google Scholar] [CrossRef]
- Van Tran, G.; Unpaprom, Y.; Ramaraj, R. Methane Productivity Evaluation of an Invasive Wetland Plant, Common Reed. Biomass Conv. Bioref. 2020, 10, 689–695. [Google Scholar] [CrossRef]
- Waliszewska, B.; Grzelak, M.; Gaweł, E.; Spek-Dźwigała, A.; Sieradzka, A.; Czekała, W. Chemical Characteristics of Selected Grass Species from Polish Meadows and Their Potential Utilization for Energy Generation Purposes. Energies 2021, 14, 1669. [Google Scholar] [CrossRef]
- Holloway, W.D.; Tasman-Jones, C.; Maher, K. Towards an Accurate Measurement of Dietary Fibre. N. Z. Med. J. 1977, 85, 420–423. [Google Scholar] [PubMed]
- Hartley, R.D. The Lignin Fraction of Plant Cell Walls. Am. J. Clin. Nutr. 1978, 31, S90–S93. [Google Scholar] [CrossRef]
- Szymańska-Chargot, M.; Chylińska, M.; Gdula, K.; Kozioł, A.; Zdunek, A. Isolation and Characterization of Cellulose from Different Fruit and Vegetable Pomaces. Polymers 2017, 9, 495. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Kong, C.-X.; Mei, Z.-L.; Li, J. A Review of the Anaerobic Digestion of Fruit and Vegetable Waste. Appl. Biochem. Biotechnol. 2017, 183, 906–922. [Google Scholar] [CrossRef]
- Dhanalakshmi Sridevi, V.; Rema, T.; Srinivasan, S.V. Studies on Biogas Production from Vegetable Market Wastes in a Two-Phase Anaerobic Reactor. Clean Technol. Environ. Policy 2015, 17, 1689–1697. [Google Scholar] [CrossRef]
- Ganesh, R.; Torrijos, M.; Sousbie, P.; Lugardon, A.; Steyer, J.P.; Delgenes, J.P. Single-Phase and Two-Phase Anaerobic Digestion of Fruit and Vegetable Waste: Comparison of Start-up, Reactor Stability and Process Performance. Waste Manag. 2014, 34, 875–885. [Google Scholar] [CrossRef]
- Masebinu, S.O.; Akinlabi, E.T.; Muzenda, E.; Aboyade, A.O.; Mbohwa, C. Experimental and Feasibility Assessment of Biogas Production by Anaerobic Digestion of Fruit and Vegetable Waste from Joburg Market. Waste Manag. 2018, 75, 236–250. [Google Scholar] [CrossRef]
- Borowski, S.; Cieciura-Włoch, W.; Liczbiński, P. Enhancement of Biogas Production from Vegetable Waste by Application of Mineral Fertilizers. Bioenergy Res. 2024, 17, 972–982. [Google Scholar] [CrossRef]
- Pavi, S.; Kramer, L.E.; Gomes, L.P.; Miranda, L.A.S. Biogas Production from Co-Digestion of Organic Fraction of Municipal Solid Waste and Fruit and Vegetable Waste. Bioresour. Technol. 2017, 228, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Mata-Alvarez, J.; Macé, S.; Llabrés, P. Anaerobic Digestion of Organic Solid Wastes. An Overview of Research Achievements and Perspectives. Bioresour. Technol. 2000, 74, 3–16. [Google Scholar] [CrossRef]
- Balcioglu, G.; Jeswani, H.K.; Azapagic, A. Evaluating the Environmental and Economic Sustainability of Energy from Anaerobic Digestion of Different Feedstocks in Turkey. Sustain. Prod. Consum. 2022, 32, 924–941. [Google Scholar] [CrossRef]
- Tonini, D.; Hamelin, L.; Alvarado-Morales, M.; Astrup, T.F. GHG Emission Factors for Bioelectricity, Biomethane, and Bioethanol Quantified for 24 Biomass Substrates with Consequential Life-Cycle Assessment. Bioresour. Technol. 2016, 208, 123–133. [Google Scholar] [CrossRef]
- Tanneberger, F.; Birr, F.; Couwenberg, J.; Kaiser, M.; Luthardt, V.; Nerger, M.; Pfister, S.; Oppermann, R.; Zeitz, J.; Beyer, C.; et al. Saving Soil Carbon, Greenhouse Gas Emissions, Biodiversity and the Economy: Paludiculture as Sustainable Land Use Option in German Fen Peatlands. Reg. Environ. Change 2022, 22, 69. [Google Scholar] [CrossRef]
| Parameter | Biowaste | Reed Silage | Inoculum |
|---|---|---|---|
| Total solids (TSs), % | 16.71 ± 0.05 a * | 62.85 ± 0.99 b | 6.03 ± 0.19 c |
| Volatile solids (VSs), %TSs | 91.95 ± 0.41 a | 91.16 ± 0.27 a | 76.54 ± 0.19 b |
| Total Kjeldahl nitrogen (TKN), g kgDM−1 | 14.23 ± 0.20 a | 14.60 ± 0.53 a | 82.16 ± 2.07 b |
| Total organic carbon (TOC), g kgDM−1 | 405.80 ± 7.11 a | 379.11 ± 7.81 b | 412.80 ± 2.19 a |
| C:N | 29:1 | 26:1 | 5:1 |
| Feedstock | Specific Methane Production | Maximum Daily Methane Production | Methane Content |
|---|---|---|---|
| NL kgVS−1 | NL kgVS−1 | % | |
| PHR | 160.40 ± 4.09 ab * | 5.94 ± 0.09 a | 57.0 ± 1.7 a |
| BIO-10 | 158.57 ± 7.88 a | 6.18 ± 0.23 a | 58.0 ± 1.0 ab |
| BIO-30 | 171.06 ± 5.09 abc | 6.31 ± 0.09 a | 56.3 ± 1.2 a |
| BIO-50 | 185.58 ± 11.17 c | 8.30 ± 0.34 a | 58.0 ± 1.7 ab |
| BIO-70 | 183.41 ± 7.77 bc | 9.69 ± 1.12 a | 61.3 ± 0.6 b |
| BIO-90 | 233.28 ± 11.91 d | 17.18 ± 1.66 b | 67.7 ± 1.2 c |
| BIO | 284.03 ± 7.03 e | 27.81 ± 3.58 c | 74.0 ± 1.0 d |
| PHR | BIO-10 | BIO-30 | BIO-50 | BIO-70 | BIO-90 | BIO |
|---|---|---|---|---|---|---|
| Increase in the amount of methane in relation to reed [%] | ||||||
 | ||||||
| −1.14 | 6.65 | 15.70 | 14.35 | 45.44 | 77.08 | |
| 43.53 | 44.17 | 39.77 | 34.66 | 35.43 | 17.87 | |
 | ||||||
| Decrease in the amount of methane in relation to biowaste [%] | ||||||
| PHR | BIO-10 | BIO-30 | BIO-50 | BIO-70 | BIO-90 | BIO |
| Feedstock | CO2 Emissions Avoided | ||
|---|---|---|---|
| kgCO2 tTS−1 | |||
| Electricity | Heat | Total | |
| PHR | 252.79 | 125.84 | 378.62 |
| BIO-10 | 249.97 | 124.44 | 374.41 |
| BIO-30 | 269.84 | 134.33 | 404.17 |
| BIO-50 | 293.02 | 145.87 | 438.89 |
| BIO-70 | 290.02 | 144.37 | 434.39 |
| BIO-90 | 369.93 | 184.15 | 554.08 |
| BIO | 451.57 | 224.79 | 676.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czubaszek, R.; Wysocka-Czubaszek, A. Anaerobic Co-Digestion of Common Reed and Plant-Based Biowaste from Households. Energies 2025, 18, 2178. https://doi.org/10.3390/en18092178
Czubaszek R, Wysocka-Czubaszek A. Anaerobic Co-Digestion of Common Reed and Plant-Based Biowaste from Households. Energies. 2025; 18(9):2178. https://doi.org/10.3390/en18092178
Chicago/Turabian StyleCzubaszek, Robert, and Agnieszka Wysocka-Czubaszek. 2025. "Anaerobic Co-Digestion of Common Reed and Plant-Based Biowaste from Households" Energies 18, no. 9: 2178. https://doi.org/10.3390/en18092178
APA StyleCzubaszek, R., & Wysocka-Czubaszek, A. (2025). Anaerobic Co-Digestion of Common Reed and Plant-Based Biowaste from Households. Energies, 18(9), 2178. https://doi.org/10.3390/en18092178







