Recent Progress in Metal Borohydrides for Hydrogen Storage
Abstract
:1. Introduction

| M(BH4)n | CAS No. | Density (g/mol) | Density (g/cm3) | Hydrogen Density (mass%) | Hydrogen Density (kg/m3) |
|---|---|---|---|---|---|
| LiBH4 | 16949-15-8 | 21.78 | 0.66 | 18.5 | 122.1 |
| NaBH4 | 16940-66-2 | 37.83 | 1.07 | 10.7 | 114.5 |
| KBH4 | 13762-51-1 | 53.94 | 1.17 | 7.5 | 87.8 |
| RbBH4 | 20346-99-0 | 100.31 | 1.92 | 4.0 | 76.8 |
| CsBH4 | - | 147.75 | 2.42 | 2.7 | 65.3 |
| Be(BH4)2 | 17440-85-6 | 38.70 | 0.702 | 20.8 | 146.0 |
| Mg(BH4)2 | 16903-37-0 | 53.99 | 0.989 | 14.9 | 147.4 |
| Ca(BH4)2 | 17068-95-0 | 69.76 | (1.07) | 11.6 | (124.1) |
| Mn(BH4)2 | - | 84.62 | (1.24) | 9.5 | (117.8) |
| Al(BH4)3 | 16962-07-5 | 71.51 | 0.79 | 16.9 | 133.5 |
| Zr(BH4)4 | 12370-59-1 | 150.6 | 1.179 | 10.7 | 126.2 |
| Hf(BH4)4 | 53608-70-1 | 237.6 | 1.65 | 6.8 | 112.2 |
2. Fundamentals of Hydrogen Storage Properties

3. Improvement of Hydrogen Storage Properties
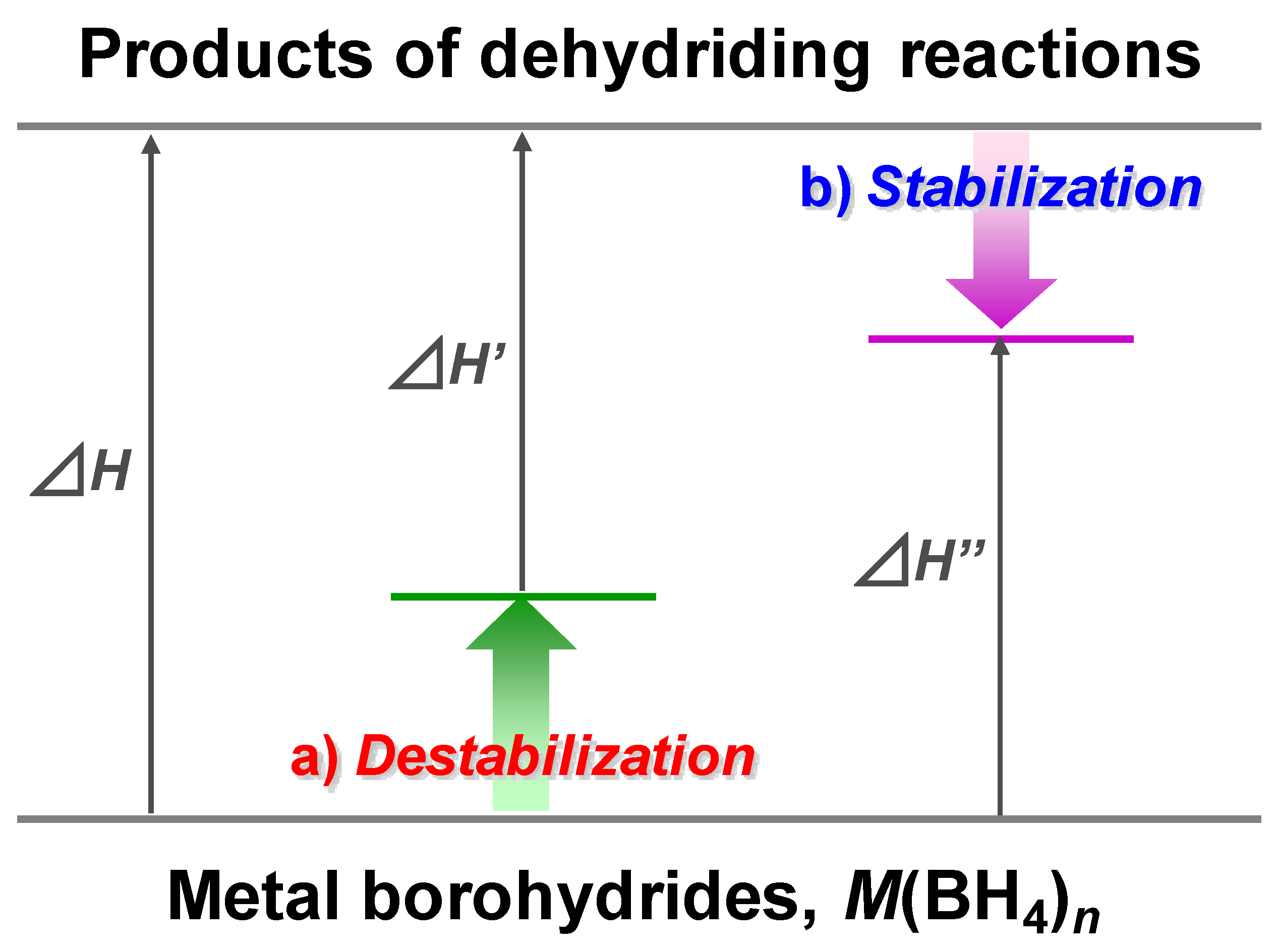
3.1. Tailoring Thermodynamics
| Borohydrides | Hydrogen (mass%) | Conditions: temp (K) [pressure (MPa)] | Reference | |||
|---|---|---|---|---|---|---|
| Ideal | Obs (First Dehyd) | Obs (Rehyd) | First Dehyd | Rehyd | ||
| ZrLi(BH4)5 | 11.7 | 595–873 | [107] | |||
| ZrLi2(BH4)6 | 12.5 | 650–873 | [107] | |||
| LiK(BH4)2 | 10.6 | 653– | [108] | |||
| LiSc(BH4)4 | 14.5 | 4.38–6.4 m | 415–673 | 673 [7] | [109,111] | |
| LiZn2(BH4)5 * | 9.5 | 238–473 | [112] | |||
| NaZn2(BH4)5 * | 8.8 | 241– | [112] | |||
| NaSc(BH4)4 | 12.7 | 0.97 m | 440–750 | [113] | ||
| Na2Mn(BH4)4 | 10.1 | 2.9 | 393 | [114] | ||
| KSc(BH4)4 | 11.2 | 4.4 p | 470–580 | [117] | ||
| LiBH4/Mg(BH4)2 | 16.0 | 12.5 | 2.5 | 513–773 | 673 [10] | [115] |
| xLiBH4 + (1-x)Ca(BH4)2 (0 < x < 1) | 9.6–18.5 | 10 (x = 0.4, 0.6) | 4 (x = 0.4) | 473–773 | 673 [9] | [116] |

| Reaction | Hydrogen (mass%) | Conditions: temp (K) [pressure (MPa)] | Theor/Exp ∆Hdehyd (kJ mol−1 H2) | Reference | |||
|---|---|---|---|---|---|---|---|
| Ideal | Obs (First Dehyd) | Obs (Rehyd) | First Dehyd | Rehyd | |||
| LiBH4 + 1/2 MgH2 = LiH + 1/2 MgB2 + 2 H2 | 11.5 | 8.0–10.6 | 8.0–10 | 543–723 | 503–723 [10] | T 50.4–66.8/E 40.5 | [29,120,124,134,135,136,142,145,156,168,172,186] |
| LiBH4 + 2 LiNH2 = Li3BN2 + 4 H2 | 11.9 | 7.8–12 | 0.1 | 622–673 | 573 [5] | E 23 | [121,123,124,127,128,129,130,132,149,160,166] |
| LiBH4 + 1/2 Al = LiH + 1/2 AlB2 + 3/2 H2 | 8.6 | 6.8–7.2 | 5–7.6 | 553–823 | 573–773 [10–15.5] | T 18.8–57.9 | [134,135,137,163,164,165] |
| LiBH4 + 1/2 LiAlH4 = 3/2 LiH + 1/2 AlB2 + 9/4 H2 | 11.1 | 6–10 | 4.8–5.1 | 327–773 | 623–873 [4–7] | [157] | |
| LiBH4 + 1/2 Mg = LiH + 1/2 MgB2 + 3/2 H2 | 8.9 | 5.6 | 648–773 | T 46.4 | [134,135] | ||
| LiBH4 + 1/6 CaH2 = LiH + 1/6 CaB6 + 10/6 H2 | 11.7 | 5.1–11.1 | 9–11.1 | 423–773 | 673 [10] | T 45.4–66.5 | [19,135,137,142,144,168] |
| LiBH4 + 1/2 ScH2 = LiH + 1/2 ScB2 + 2 H2 | 8.9 | 4.5 | 553–723 | T 34.1 | [134,135,142,143] | ||
| LiBH4 + 1/6 CeH2 = LiH + 1/6 CeB6 + 10/6 H2 | 7.4 | 6.1–6.2 | 6.0 | 443–723 | 623 [10] | T 44.1 | [144,168,177] |
| LiBH4 + 1/4 YH3 = LiH + ¼ YB4 + 15/8 H2 | 8.4 | 7.2 | 623 | [168] | |||
| LiBH4 + 1/4 MgH2 + 1/4 Al = LiH + 1/4 MgAlB4 + 7/4 H2 | 10.0 | 9.4 | 6 | 533–673 | 673 [4] | E 57 | [159] |
| Ca(BH4)2 + MgH2 = CaH2 + MgB2 + 4 H2 | 8.4 | 7.1 | 623–723 | T 47 | [135] | ||
| Ca(BH4)2 + MgH2 = 2/3 CaH2 + 1/3 CaB6 + Mg + 13/3 H2 | 9.1 | 8.1 | 5.5 | 593–773 | 623 [9] | T 45 | [187] |
| Mg(BH4)2 + LiNH2 = Li-Mg + BN-related + 5 H2 | 13.1 | 11.4 | 433–873 | [174] | |||
| NaBH4 + 1/2 MgH2 = Na + 1/2 MgB2 + 5/2 H2 | 9.9 | 9 | 6 | 330–723 | 723 [5] | T 62 | [183,186] |
| NaBH4 + 2 NaNH2 = Na3BN2 + 4 H2 * | 7.0 | 500–773 | [173] | ||||
| x LiBH4 + y (LiNH2)2 + z (MgH2) = Li3BN2 + Mg3N2 + LiH + H2 | 453 [15] | [140,141,155] | |||||
| x:y:z = 2:1:1 | 13.0 | 8.5 | 2.9 | 413–743 | |||
| 2:0.5:1 | 13.6 | 8.6 | 3.7 | 413–743 | |||
| 2:1:2 | 11.8 | 6.6 | 3.1 | 428–743 | |||
| 1:1:1 | 11.3 | 5.6 | 2.7 | 428–743 | |||
| 3:1:1.5 | 13.4 | 9.1 | 413–743 | ||||
| Reaction | Hydrogen (mass%) | Conditions: temp (K) [pressure (MPa)] | Reference | |||
|---|---|---|---|---|---|---|
| Ideal | Obs (First Hyd) | Obs (Dehyd) | First Hyd | Dehyd | ||
| LiH + 1/2 MgB2 + 2 H2 = LiBH4 + 1/2 MgH2 | 11.5 | 3.2–11 | 3.0–8.0 | 538–673 [9–35] | 538–723 [0–0.6] | [120,145,182,186,187] |
| LiF + 1/2 MgB2 + H2 = LiBH4−yFy + 1/2 MgF2 + LiH1−xFx | 6.6 | 6.4 | 663 [6] | 693 [0.5] | [189] | |
| Li7Sn2 + 7/2 MgB2 + 14 H2 = 7 LiBH4 + 7/4 Mg2Sn + 1/4 Sn | 5.9 | 2.5–3 | 573–673 [20–30] | [186] | ||
| CaH2 + MgB2 + 4H2 = Ca(BH4)2 + MgH2 | 8.4 | 4.7–7 | 573–673 [20–35] | [186,187] | ||
| NaH + 1/2 MgB2 + 2 H2 = NaBH4 + 1/2 MgH2 | 7.9 | 6.2–6.7 | 573–673 [20–35] | [186] | ||
| 1/3 CaB6 + 2/3 CaH2 + 10/3 H2 = Ca(BH4)2 | 9.6 | 4.8 | 623 [10] | 543–573 | [186] | |
| 2/3 CaH2 + 1/3 CaB6 + 1/2 Mg + 23/6 H2 = Ca(BH4)2 + 1/2 MgH2 | 9.3 | 4.9–5.9 | 623–673 [9] | [192] | ||
| MgNi2.5B2 + 2 LiH + 4 MgH2 + 4H2 = 2 LiBH4 + 5/2 Mg2NiH4 | 2.5 | 1.0 | 1.0 | 623 [16] | 613 [0.4] | [167] |
| MgB2 + 4 H2 = Mg(BH4)2 | 14.9 | 11.0 | 673 [95] | [73,74] | ||
3.2. Promoting Kinetics
| M(BH4)n | Additives | Hydrogen (mass%) | Conditions: temp (K) [pressure (MPa)] | Toxic Byproduct | Reference | |||
|---|---|---|---|---|---|---|---|---|
| Type | Amount | Obs (First Dehyd) | Obs (Rehyd) | First Dehyd | Rehyd | |||
| LiBH4 | SiO2 | 10–25 mass% | 9–10 m | 423–873 | [19,20,204] | |||
| TiO2 | 25–80 mass% | 4–9 m | 3.5–8.3 m | 373–873 | 873 [7–10] | [197,209] | ||
| ZrO2 | 25 mass% | 8–9 m | 448–873 | [197] | ||||
| V2O3 | 25 mass% | 8–9 m | 8 m | 448–873 | 873 [10] | [197] | ||
| SnO2 | 25 mass% | 8–9 m | 448–873 | [197] | ||||
| Nb2O5 | 50–80 mass% | 4–6 m | 373–873 | [209] | ||||
| Fe2O3 | 50–66.7 mass% | 5.7–9 m | 373–873 | [209] | ||||
| V2O5 | 50–66.7 mass% | 5.7–9 m | 373–873 | [209] | ||||
| TiCl3 | 10–88 mass% | 2.8–9.2 m | 3.4 m | 373–873 | 773 [7] | B2H6 | [197,208] | |
| CoCl2 | 5–100 mol% | 10.5–18.3 p | 503–873 | B2H6 | [213] | |||
| TiH2 | 10–50 mol% | 6–15 m | 2.5–4.5 m | 573–873 | 773 [7] | [208] | ||
| TiF3 | 10–50 mol% | 6.4–14 m | 0.2–4.0 m | 373–773 | 623–773[7–10] | B2H6 | [208,211] | |
| ZnF2 | 10–50 mol% | 3.7–7 m | 1–4 m | 393–773 | 773 [7] | B2H6 | [208] | |
| mixture of MgCl2/TiCl3 | 30 mol% | 5 m | 4.5 m | 333–873 | 873 [7] | [198] | ||
| Mg | 10–20 mol% | 9 m | 333–873 | [198] | ||||
| Al | 20 mol% | 7.8 m | 3.5 m | 353–873 | 873 [10] | [198] | ||
| Sc | 33 mol% | 2.9 m | 673–773 | [134] | ||||
| Ti | 33 mol% | 2.5 m | 673–773 | [134] | ||||
| V | 33 mol% | 4.4 m | 673–773 | [134] | ||||
| Cr | 33 mol% | 4.4 m | 673–773 | [134] | ||||
| LiBH4 | MgH2 | 80 mass% | 8.8–9.2 m | 8.5 m | 627–853 | 673 [10] | [126,172] | |
| graphite | 30 mass% | 9.9 p | 2.6 p | 663–773 | 673 [10] | [212] | ||
| activated carbon | 30 mass% | 11.2 p | 4.6 p | 623–773 | 673 [10] | [212] | ||
| single-walled carbon nanotubes | 30 mass% | 11.4 -12.3 p | 3.7 p | 553–773 | 673 [10] | [205,212] | ||
| single-walled carbon nanotubes | 9.1–50 mass% | 5–11.8 p | 3.7–6.1 p | 723 (iso) | 673 [10] | [207] | ||
| mesoporous carbon | 50 mass% | 7 m | 6 m | 605–873 | 623 [3] | [200] | ||
| mixture of TiF3/SiO2 | 50 mass% | 8.3 m | 4 m | 343–823 | 773 [4.5] | [204] | ||
| Pt/C | 10–50 mass% | 9.2–15.7 m | 6.1 m | 553–973 | 873 [3] | [206] | ||
| LiBH4 + 1/2MgH2 | Ti-iso | 5–10 mol% | 6.5–8.4 m | 6.0 m | 673 (iso) | 623 [5] | [170,136] | |
| Zr-iso | 10 mol% | 5.5 m | 673 (iso) | [150] | ||||
| ZrCl4 | 10 mol% | 7.5 m | 673 (iso) | [150] | ||||
| SiO2 | 5 mol% | 9.3 m | 673 (iso) | [136] | ||||
| VCl3 | 5 mol% | 9.1 m | 673 (iso) | [136] | ||||
| graphite | 10 mass% | 9.5 p | 723 (iso) | [168] | ||||
| carbon nanofibers | 10 mass% | 10.0 p | 723 (iso) | [168] | ||||
| activated carbon | 10 mass% | 10.0 p | 723 (iso) | [168] | ||||
| single-walled carbon nanotubes | 10 mass% | 10.0 p | 6.7 p | ~573–773 | 673 [7.5] | [168] | ||
| muti-walled carbon nanotubes | 10 mass% | 10.0 p | 723 (iso) | [168] | ||||
| TiF3 | 5 mol% | 9.7 p | 573 (iso) | [72] | ||||
| Li3BN2H8 | Pt/Vulcan carbon | 1–10 mass% | 9–13 m | 1.4 m | 388–673 | 423 [8.4] | NH3 | [130] |
| Pd | 5–10 mass% | 11.8–13 m | 473–673 | [130] | ||||
| PdCl2 | 8.3 mass% | 10.4 m | 473–673 | [130] | ||||
| CoCl2 | 5 mass% | 8–10 m | 388–493 | [149] | ||||
| Li4BN3H10 | NiCl2 | 11 mass% | 7.6 p | 433–673 | NH3 | [161] | ||
| Ca(BH4)2 + MgH2 | Ti-iso | 1 mass% | 7.1 m | 523–723 | [190] | |||
| Ca(BH4)2 | NbF5 | 2 mol% | 8.3 m | 4.6–5.0 m | ~593–823 | 623 [9] | [87] | |
| NbCl5 | 2 mol% | 4.1–5.0 m | 3.1–4.5 m | ~593–823 | 623 [9] | [87] | ||
| TiF3 | 2 mol% | 4.1–5.0 m | 2.5–4.2 m | ~593–823 | 623 [9] | [87] | ||
| TiCl3 | 2 mol% | 4.1–5.0 m | 3.5–4.4 m | ~593–823 | 623 [9] | [87] | ||
| Mg(BH4)2 | TiCl3 | 25 mass% | 13.7 p | 361–800 | [58] | |||
| TiO2 | 25 mass% | 13.7 p | 483–800 | [58] | ||||
| Reaction | Type of Nano Scaffold (size (nm)) | Loading Ratio (mass%) | Hydrogen (mass%) | Conditions: temp (K) [pressure (MPa)] | Reference | ||
|---|---|---|---|---|---|---|---|
| Obs (First Dehyd) | Obs (Rehyd) | First Dehyd | Rehyd | ||||
| LiBH4 = LiH + B + 3/2 H2 | Nanoporous Carbon (13–25) | 25–50 | 4.6–6.4 m | 503–873 | 673 [10] | [212] | |
| Activated carbon (1.75–3.2) | 28.4 | 11.2 p | 6.6 p | 493–773 | 573 [5] | [213] | |
| Mesoporous carbon (4) | 33 | 3.4 m | 473–773 | [214] | |||
| Nanoporous Carbon (2) | 8.8 p | 493–673 | [219] | ||||
| Li3BN2H8 = Li3BN2 + 4 H2 * | Nanoporous carbon scaffolds (16 ± 3) | 33 | 11.1 p | 3.8 p | 523–673 | 573 [5] | [160] |
| activated carbon AX-21 (2) | 33 | 10.7 p | 4.0 p | 438–673 | 573 [5] | [160] | |
| Mg(BH4)2 = MgB2 + 4 H2 | Activated carbon (<2) | 44 ± 3 | 6.0 m | 443–773 | [216] | ||
| LiBH4 + 3.75 mass%Ni | Nanoporous carbon (2–3) | 25 | 14 p | 10 p | 473–673 | 593 [4] | [220] |
| LiBH4 + 1/2 MgH2 = LiH + 1/2 MgB2 + 2 H2 | Nanoporous carbon aerogel (~21) | 4.7 m | 4 m | 533–743 | 643–663 [5–10] | [221] | |
4. Conclusions
Acknowledgments
References
- Schlapbach, L.; Züttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Orimo, S.; Nakamori, Y.; Eliseo, J.; Züttel, A.; Jensen, C.M. Complex Hydrides for Hydrogen Storage. Chem. Rev. 2007, 107, 4111–4132. [Google Scholar] [CrossRef] [PubMed]
- Nakamori, Y.; Orimo, S. Borohydrides as hydrogen storage materials. In Solid-State Hydrogen Storage; Walker, G., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2008; pp. 420–449. [Google Scholar]
- Miwa, K.; Ohba, N.; Towata, S.; Nakamori, Y.; Orimo, S. First-principles Study on Lithium Borohydride LiBH4. Phys. Rev. B 2004, 69, 245120. [Google Scholar] [CrossRef]
- Ge, Q. Structure and Energetics of LiBH4 and Its surfaces: A First-Principles Study. J. Phys. Chem. A 2004, 108, 8682–8690. [Google Scholar] [CrossRef]
- Vajeeston, P.; Ravindran, P.; Kjekshus, A.; Fjellvåg. Structrual Stability of Alkali Boron Tetrahydrides ABH4 (A = Li, Na, K, Rb, Cs) From First Principle Calculation. J. Alloys Compd. 2005, 387, 97–104. [Google Scholar] [CrossRef]
- Łodziana, Z.; Vegge, T. Structural Stability of Complex Hydrides: LiBH4 Revisited. Phys. Rev. Lett. 2004, 93, 14550. [Google Scholar] [CrossRef]
- Orgaz, E.; Membrillo, A.; Castañeda, R.; Aburto, A. Electronic Structure of Ternary Hydrides Based on Light Elements. J. Alloys Compd. 2005, 404–406, 176–180. [Google Scholar] [CrossRef]
- Schlesinger, H.I.; Brown, H.C.; Finholt, A.E.; Gilbreath, J.R.; Hoekstra, H.R.; Hyde, E.K. Sodium Borohydride, Its Hydrolysis and its Use as a Reducing Agent and in the Generation of Hydrogen. J. Am. Chem. Soc. 1953, 75, 215–219. [Google Scholar] [CrossRef]
- Liu, B.H.; Li, Z.P.; Suda, S. Nickel- and cobalt-based catalysts for hydrogen generation by hydrolysis of borohydride. J. Alloys Compd. 2006, 415, 288–293. [Google Scholar] [CrossRef]
- Wang, P.; Kang, X.D. Hydrogen-rich boron-containing materials for hydrogen storage. Dalton Trans. 2008, 40, 5400–5413. [Google Scholar] [CrossRef] [PubMed]
- Grochala, W.; Edwards, P.P. Thermal Decomposition of the Non-Interstitial Hydrides for the Storage and Production of Hydrogen. Chem. Rev. 2004, 104, 1283–1315. [Google Scholar] [CrossRef] [PubMed]
- Sakintuna, B.; Lamari-Darkrimb, F.; Hirscherc, M. Metal Hydride Materials for Solid Hydrogen Storage: A review. Int. J. Hydrogen Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Chen, P.; Zhu, M. Recent progress in hydrogen storage. Mater. Today. 2008, 11, 36–43. [Google Scholar] [CrossRef]
- Rönnebro, E. Development of group II borohydrides as hydrogen storage materials. Curr. Opin. Solid State Mater. Sci. 2010. [Google Scholar] [CrossRef]
- George, L.; Saxena, S.K. Structural Stability of Metal Hydrides, Alanates and Borohydrides of Alkali and Alkali-Earth Elements: A Review. Int. J. Hydrogen Energy 2010, 35, 5454–5470. [Google Scholar] [CrossRef]
- Jain, I.P.; Jain, P.; Jain, A. Novel hydrogen storage materials: A review of lightweight complex hydrides. J. Alloys Compd. 2010, 503, 303–339. [Google Scholar] [CrossRef]
- Hagemann, H.; Černý, R. Synthetic approaches to inorganic borohydrides. Dalton Trans. 2010, 39, 6006–6012. [Google Scholar] [CrossRef] [PubMed]
- Züttel, A.; Wenger, P.; Rentsch, S.; Sudan, P. LiBH4 A New Hydrogen Storage Material. J. Power Sources 2003, 118, 1–7. [Google Scholar] [CrossRef]
- Züttel, A.; Rentsch, S.; Fischer, P.; Wenger, P.; Sudan, P.; Mauron, Ph.; Emmenegger, Ch. Hydrogen Storage Properties of LiBH4. J. Alloys Compd. 2003, 356–357, 515–520. [Google Scholar] [CrossRef]
- Nakamori, Y.; Orimo, S. Destabilization of Li-based Complex Hydrides. J. Alloys Compd. 2004, 370, 271–275. [Google Scholar] [CrossRef]
- Orimo, S.; Nakamor, Y.; Züttel, A. Material Properties of MBH4 (M = Li, Na, and K). Mater. Sci. Eng. B 2004, 108, 51–53. [Google Scholar] [CrossRef]
- Orimo, S.; Nakamori, Y.; Kitahara, G.; Miwa, K.; Ohba, N.; Towata, S.; Züttel, A. Dehydriding and Rehydriding Reactions of LiBH4. J. Alloys Compd. 2005, 404–406, 427–430. [Google Scholar] [CrossRef]
- Nakamori, Y.; Orimo, S.; Tsutaoka, T. Dehydriding Reaction of Metal Hydrides and Alkali Borohydrides Enhanced by Microwave Irradiation. Appl. Phys. Lett. 2006, 88, 112104. [Google Scholar] [CrossRef]
- Züttel, A.; Borgschulte, A.; Orimo, S. Tetrahydroborates as New Hydrogen Storage Materials. Scripta Mater. 2007, 56, 823–828. [Google Scholar] [CrossRef]
- Orimo, S.; Nakamori, Y.; Ohba, N.; Miwa, K.; Aoki, M.; Miwa, S.; Züttel, A. Experimental Studies on Intermediate Compound of LiBH4. Appl. Phys. Lett. 2006, 89, 021920. [Google Scholar] [CrossRef]
- Ohba, N.; Miwa, K.; Aoki, M.; Noritake, T.; Towata, S.; Nakamori, Y.; Orimo, S.; Züttel, A. First-Principles Study on the Stability of Intermediate Compounds of LiBH4. Phys. Rev. B 2006, 74, 075110. [Google Scholar] [CrossRef]
- Hwang, S.-J.; Bowman, R.C., Jr.; Reiter, J.W.; Rijssenbeek, J.; Soloverchik, G.L.; Zhao, J.-C.; Kabbour, H.; Ahn, C.C. NMR Confirmation for Formation of [B12H12]2− Complexes during Hydrogen Desorption from Metal Borohydrides. J. Phys. Chem. C 2008, 112, 3164–3169. [Google Scholar] [CrossRef]
- Ozolin, V.; Majzoub, E.H.; Wolverton, C. First-Principles Predition of Thermodynamically Reversible Hydrogen Storage Reactions in the Li-Mg-Ca-B-H. System. J. Am. Chem. Soc. 2009, 131, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Her, J.-H.; Yousufuddin, M.; Zhou, W.; Jalisatgi, S.S.; Kulleck, J.G.; Zan, J.A.; Hwang, S.-J.; Bowman, R.C., Jr.; Udovic, T.J. Crystal Structure of Li2B12H12: A Possible Intermediate Phase in the Decomposition of LiBH4. Inorg. Chem. 2008, 47, 9757–9759. [Google Scholar] [CrossRef] [PubMed]
- Friedrichs, O.; Remhof, A.; Hwang, S.-J.; Züttel, A. Role of Li2B12H12 for the Formation and Decomposition of LiBH4. Chem. Mater. 2009, 22, 3265–3268. [Google Scholar] [CrossRef]
- Soulié, J-Ph.; Renaudin, G.; Černy, R.; Yvon, K. Lithium Boro-hydride LiBH4. I. Crystal Structure. J. Alloys Compd. 2002, 346, 200–205. [Google Scholar] [CrossRef]
- Renaudin, G.; Gomes, S.; Hagemann, H.; Keller, L.; Yvon, K. Structural and Spectroscopic Studies on the Alkali Borohydrides MBH4 (M = Na, K, Rb, Cs). J. Alloys Compd. 2004, 375, 98–106. [Google Scholar] [CrossRef]
- Frankcombe, T.J.; Kroes, G.-J. Quasiharmonic Approximation Applied to LiBH4 and Its Decomposition Products. Phys. Rev. B 2006, 73, 174302. [Google Scholar] [CrossRef]
- Hartman, M.R.; Rush, J.J.; Udovic, T.J.; Bowman, R.C., Jr.; Hwang, S.-J. Structure and Vibrational Dynamics of Isotopically Labeled Lithium Borohydride Using Neutron Diffraction and Spectroscopy. J. Solid State Chem. 2007, 180, 1298–1305. [Google Scholar] [CrossRef]
- Łodziana, Z.; Züttel, A.; Zielinski, P. Titanium and Native Defects in LiBH4 and NaAlH4. J. Phys: Condens. Matter. 2008, 20, 465210. [Google Scholar] [CrossRef]
- Borgschulte, A.; Züttel, A.; Hug, P.; Racu, A.-M.; Schoenes, J. Hydrogen-Deuterium Exchange in Bulk LiBH4. J. Phys. Chem. A 2008, 112, 4749–4753. [Google Scholar] [CrossRef] [PubMed]
- Buchter, F.; Łodziana, Z.; Mauron, Ph.; Remhof, A.; Friedrichs, Q.; Borgschulte, A.; Züttel, A.; Sheptyakov, D.; Strässle, Th.; Ramirez-Cuesta, A.J. Dynamical Properties and Temperature Induced Molecular Disordering of LiBH4 and LiBD4. Phys. Rev. B 2008, 78, 094302. [Google Scholar] [CrossRef]
- Skripov, A.V.; Soloninin, A.V.; Filinchuk, Y.; Chernyshov, D. Nuclear Magnetic Resonance Study of Rotational Motion and The Phase transition in LiBH4. J. Phys. Chem. C 2008, 112, 18701–18705. [Google Scholar] [CrossRef]
- Corey, R.L.; Shane, D.T.; Bowman, R.C., Jr.; Conradi, M.S. Atomic Motions in LiBH4 by NMR. J. Phys. Chem. C 2008, 112, 18706–18710. [Google Scholar] [CrossRef]
- Gremaud, R.; Łodziana, Z.; Hug, P.; Willenberg, B.; Racu, A.-M.; Schoenes, J.; Ramirez-Cuesta, A.J.; Clark, S.J.; Refson, K.; Züttel, A.; Borgschulte, A. Evidence for Hydrogen Transport in Deuterated LiBH4 from Low-Temperature Raman-Scattering Measurement and First-Principles Calculations. Phys. Rev. B 2008, 80, 100301. [Google Scholar] [CrossRef]
- Filinchuk, Y.; Chernyshov, D.; Nevidomskyy, A.; Dmitriev, V. High-Pressure Polymorphism as a Step towards Destabilization of LiBH4. Angew. Chem. Int. Ed. 2008, 47, 529–532. [Google Scholar] [CrossRef]
- Shane, D.T.; Bowman, R.C., Jr.; Conradi, M.S. Exchange of Hydrogen Atoms between BH4 in LiBH4. J. Phys. Chem. C 2009, 113, 5039–5042. [Google Scholar] [CrossRef]
- Ramzan, M.; Ahuja, R. Ab initio Molecular Dynamics Study of the Hydrogen-Deuterium Exchange in Bulk Lithiumborohydride (LiBH4). Appl. Phys. Lett. 2009, 94, 141903. [Google Scholar] [CrossRef]
- Hagemann, H.; Filinchuk, Y.; Chernyshov, D.; van Beek, W. Lattice Anharmonicity and Structural Evolution of LiBH4: an Insight from Raman and X-ray Diffraction Experiments. Phase Transitions 2009, 82, 344–355. [Google Scholar] [CrossRef]
- Andresen, E.R.; Gremaud, R.; Borgschulte, A.; Ramirez-Cuesta, A.J.; Züttel, A.; Hamm, P. Vibrational Dynamics of LiBH4 by Infrared Pump-Probe and 2D Spectroscopy. J. Phys. Chem. A 2009, 113, 12838–12846. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Sholl, D.S. The Role of Interstitial H2 in Hydrogen Diffusion in Light Metal Borohydrides. Phys. Chem. Chem. Phys. 2009, 11, 11106–11109. [Google Scholar] [CrossRef] [PubMed]
- Galvez-Ruiz, J.C.; Sanchez, M. Structural Analysis of Alkali Metal Tetrahydroborates: The Role of Metal and Coordination from in the [BH4]− Anion Structure. J. Mol. Struc.: Theochem. 2009, 908, 114–116. [Google Scholar] [CrossRef]
- Remhof, A.; Gremaud, R.; Buchter, F.; Lodziana, Z.; Embs, J.P.; Ramirez-Cuesta, A.J.; Borgschulte, A.; Züttel, A. Hydrogen Dynamics in Lightweight Tetrahydroborates. Z. Phys. Chem. 2010, 224, 263–278. [Google Scholar] [CrossRef]
- Remhof, A.; Lodziana, Z.; Martelli, P.; Friedrichs, O.; Züttel, A.; Skripov, A.V.; Embs, J.P.; Strässle, T. Rotational Motion of BH4 Units in MBH4 (M = Li, Na, K) from Quasielastic Neutron Scattering and Density Functional Calculations. Phys. Rev. B 2010, 81, 214304. [Google Scholar] [CrossRef]
- Mauron, P.; Buchter, F.; Friedrichs, O.; Remhof, A.; Bielmann, M.; Zwichy, C.N.; Züttel, A. Stability and Reversibility of LiBH4. J. Phys. Chem. B 2008, 112, 906–910. [Google Scholar] [CrossRef] [PubMed]
- Friedrichs, O.; Buchter, F.; Borgschulte, A.; Remhof, A.; Zwicky, C.N.; Mauron, P.H.; Bielmann, M.; Züttel, A. Direct Synthesis of Li[BH4] and Li[BD4] from the Elements. Acta Mater. 2007, 56, 949–954. [Google Scholar] [CrossRef]
- Remhof, A.; Friedrichs, O.; Buchter, F.; Mauron, P.H.; Züttel, A.; Wallacher, D. Solid-State Synthesis of LiBD4 Observed by In Situ Neutron Diffraction. Phys. Chem. Chem. Phys. 2008, 10, 5859–5862. [Google Scholar] [CrossRef] [PubMed]
- Friedrichs, O.; Borgschulte, A.; Kato, S.; Buchter, F.; Gremaud, R.; Remhof, A.; Züttel, A. Low-Temperature Synthesis of LiBH4 by Gas-Solid Reaction. Chem. Eur. J. 2009, 15, 5531–5534. [Google Scholar] [CrossRef] [PubMed]
- Friedrichs, O.; Remhof, A.; Borgschulte, A.; Buchter, F.; Orimo, S.; Züttel, A. Breaking the passivation—the road to a solvent fee borohydride synthesis. Phys. Chem. Chem. Phys. 2010, 12, 10919–10922. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, T.; Buchter, F.; Mauron, P.; Bielman, M.; Nakamori, Y.; Orimo, S.; Ohba, N.; Miwa, K.; Towata, S.; Züttel, A. Hydrogen Storage Properties of Mg(BH4)2. J. Alloys Compd. 2008, 459, 583–588. [Google Scholar] [CrossRef]
- Chłopek, K.; Frommen, C.; Léon, A.; Zabara, O.; Fichtner, M. Synthesis and Properties of Magnesium Tetrahydroborate, Mg(BH4)2. J. Mater. Chem. 2007, 17, 3496–3503. [Google Scholar] [CrossRef]
- Li, H.-W.; Kikuchi, K.; Nakamori, Y.; Miwa, K.; Towata, S.; Orimo, S. Effects of Ball Milling and Additives on Dehydriding Behaviors of Well-crystallized Mg(BH4)2. Scripta Mater. 2007, 57, 679–682. [Google Scholar] [CrossRef]
- Riktor, M.D.; Sørby, M.H.; Chłopek, K.; Fichtner, M.; Buchter, F.; Züttel, A.; Hauback, B.C. In Situ Synchrotron Diffraction Studies of Phase Transitions and Thermal Decomposition of Mg(BH4)2 and Ca(BH4)2. J. Mater. Chem. 2007, 17, 4939–4942. [Google Scholar] [CrossRef]
- Matsunaga, T.; Buchter, F.; Miwa, K.; Towata, S.; Orimo, S.; Züttel, A. Magnesium borohydride: A New Hydrogen Storage Material. Renew. Energy 2008, 33, 193–196. [Google Scholar] [CrossRef]
- Varin, R.A.; Chiu, Ch.; Wronski, Z.S. Mechano-Chemical Activation Synthesis (MCAS) of Disordered Mg(BH4)2 Using NaBH4. J. Alloys Compd. 2008, 462, 201–208. [Google Scholar] [CrossRef]
- Li, H.-W.; Kikuchi, K.; Nakamori, Y.; Ohba, N.; Miwa, K.; Towata, S.; Orimo, S. Dehydriding and Rehydriding Processes of Well-Crystallized Mg(BH4)2 Accompanying with Formation of Intermediate Compounds. Acta Mater. 2008, 56, 1342–1347. [Google Scholar] [CrossRef]
- Hanada, N.; Ch1opek, K.; Frommen, C.; Lohstroh, W.; Fichtner, M. Thermal Decomposition of Mg(BH4)2 Under He flow and H2 Pressure. J. Mater. Chem. 2008, 18, 2611–2614. [Google Scholar] [CrossRef]
- Yan, Y.; Li, H.-W.; Nakamori, Y.; Ohba, N.; Miwa, K.; Towata, S.; Orimo, S. Differential Scanning Calorimetry Measurements of Magnesium Borohydride Mg(BH4)2. Mater. Trans. 2008, 49, 2751–2752. [Google Scholar] [CrossRef]
- Soloveichika, G.L.; Gao, Y.; Rijssenbeek, J.; Andrus, M.; Kniajanski, S.; Bowman, R.C., Jr.; Hwangc, S.-J.; Zhao, J.-C. Magnesium Borohydride as a Hydrogen Storage Material: Properties and Dehydrogenation Pathway of Unsolvated Mg(BH4)2. Int. J. Hydrogen Energy 2009, 34, 916–928. [Google Scholar] [CrossRef]
- Li, H.-W.; Miwa, K.; Ohba, N.; Fujita, T.; Sato, T.; Yan, Y.; Towata, S.; Chen, M.W.; Orimo, S. Formation of Intermediate Compound with B12H12 Cluster: Experimental and Theoretical Studies on Magnesium Borohydride Mg(BH4)2. Nanotechnology 2009, 20, 204013. [Google Scholar] [CrossRef] [PubMed]
- Ozolins, V.; Majzoub, E.H.; Wolverton, C. First-Principles Prediction of a Ground State Crystal Structure of Magnesium Borohydride. Phys. Rev. Lett. 2008, 100, 135501. [Google Scholar] [CrossRef]
- van Setten, M.J.; Lohstroh, W.; Fichtner, M. A New Phase in the Decomposition of Mg(BH4)2: First-Principles Simulated Annealing. J. Mater. Chem. 2009, 19, 7081–7087. [Google Scholar] [CrossRef]
- Kulkarni, A.D.; Wang, L.L.; Johnson, D.D.; Sholl, D.S.; Johnson, J.K. First-principles Characterization of Amorphous Phases of MB12H12, M = Mg, Ca. J. Phys. Chem. C 2010, 114, 14601–14605. [Google Scholar]
- Li, S.; Willis, M.; Jena, P. Reaction Intermediates during the Dehydrogenation of Metal Borohydrides: A Cluster Perspective. J. Phys. Chem. C 2010, 114, 16849–16854. [Google Scholar] [CrossRef]
- Chen, X.; Lingam, H.K.; Huang, Z.; Yisgedu, T.; Zhao, J.-C.; Shore, S.G. Thermal Decomposition Behavior of Hydrated Magnesium Dodecahydrododecaborates. J. Phys. Chem. Lett. 2010, 1, 201–204. [Google Scholar] [CrossRef]
- Newhouse, R.J.; Stavila, V.; Hwang, S.-J.; Klebanoff, L.E.; Zhang, J.Z. Reversibility and Improved Hydrogen Release of Magnesium Borohydride. J. Phys. Chem. C 2010, 114, 5224–5232. [Google Scholar] [CrossRef]
- Severa, G.; Rönnebro, E.; Jensen, C.M. Direct Hydrogenation of Magnesium Boride to Magnesium Borohydride: Demonstration of >11 Weight Percent Reversible Hydrogen Storage. Chem. Commun. 2010, 46, 421–423. [Google Scholar] [CrossRef]
- Li, H.-W.; Matsunaga, T.; Yan, Y.; Maekawa, H.; Ishikiriyama, M.; Orimo, S. Nanostructure-induced hydrogenation of layered compound MgB2. J. Alloys Compd. 2010, 505, 654–656. [Google Scholar] [CrossRef]
- Pistidda, C.; Garroni, S.; Dolci, F.; Bardají, E.G.; Khandelwal, A.; Nolis, P.; Dornheim, M.; Gosalawit, R.; Jensen, T.; Cerenius, Y.; Suriñach, S.; Baró, M.D.; Lohstroh, W.; Fichtner, M. Synthesis of amorphous Mg(BH4)2 from MgB2 and H2 at room temperature. J. Alloys Compd. 2010, 508, 212–215. [Google Scholar] [CrossRef]
- Chong, M.; Karkamkar, A.; Autrey, T.; Orimo, S.; Jalisatgi, S.; Jensen, C.M. Reversible dehydrogenation of magnesium borohydride to magnesium triborane in the solid state under moderate conditions. Chem. Commun. 2011, 47, 1330–1332. [Google Scholar] [CrossRef]
- Miwa, K.; Aoki, M.; Noritake, T.; Ohba, N.; Nakamori, Y.; Towata, S.; Züttel, A.; Orimo, S. Thermodynamical Stability of Calcium Borohydride Ca(BH4)2. Phys. Rev. B 2006, 74, 155122. [Google Scholar] [CrossRef]
- Kim, J.-H.; Jin, S.-A.; Shim, J.-H.; Cho, Y.W. Thermal Decomposition Behavior of Calcium Borohydride Ca(BH4)2. J. Alloys Compd. 2008, 461, L20–L22. [Google Scholar] [CrossRef]
- Aoki, M.; Miwa, K.; Noritake, T.; Ohba, N.; Matsumoto, M.; Li, H.-W.; Nakamori, Y.; Towata, S.; Orimo, S. Structural and Dehydriding Properties of Ca(BH4)2. Appl. Phys. A 2008, 92, 601–605. [Google Scholar] [CrossRef]
- Mao, J.; Guo, Z.; Poh, C.K.; Ranjbar, A.; Guo, Y.; Yu, X.; Liu, H. Study on the dehydrogenation kinetics and thermodynamics of Ca(BH4)2. J. Alloys Compd. 2010, 500, 200–205. [Google Scholar] [CrossRef]
- Riktor, M.D.; Sørby, M.H.; Chopek, K.; Fichtner, M.; Haubac, B.C. The Identification of a Hitherto Unknown Intermediate Phase CaB2HX from Decomposition of Ca(BH4)2. J. Mater. Chem. 2009, 19, 2754–2759. [Google Scholar] [CrossRef]
- Frankcombe, T.J. Calcium Borohydride for Hydrogen Storage: A Computational Study of Ca(BH4)2 Crystal Structures and the CaB2Hx Intermediate. J. Phys. Chem. C 2010, 114, 9503–9509. [Google Scholar] [CrossRef]
- Wang, L.-L.; Graham, D.D.; Robertson, I.M.; Johnson, D.D. On the Reversibility of Hydrogen-Storage Reactions in Ca(BH4)2: Characterization via Experiment and Theory. J. Phys. Chem. C 2009, 113, 20088–20096. [Google Scholar] [CrossRef]
- Stavila, V.; Her, J.-H.; Zhou, W.; Hwang, S.-J.; Kim, C.; Ottley, L.A.M.; Udovic, T.J. Probing the structure, stability and hydrogen storage properties of calcium dodecahydro-closo-dodecaborate. J. Solid State Chem. 2010, 183, 1133–1140. [Google Scholar] [CrossRef]
- Rönnebro, E.; Majzoub, E.H. Calcium Borohydride for Hydrogen Storage: Catalysis and Reversibility. J. Phys. Chem. B 2007, 111, 12045–12047. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Jin, S.-A.; Shim, J.-H.; Cho, Y.W. Reversible Hydrogen Storage in Calcium Borohydride Ca(BH4)2. Scripta Mater. 2008, 58, 481–483. [Google Scholar] [CrossRef]
- Kim, J.-H.; Shim, J.-H.; Cho, Y.W. On the Reversibility of Hydrogen Storage in Ti- and Nb-catalyzed Ca(BH4)2. J. Power Sources. 2008, 181, 140–143. [Google Scholar] [CrossRef]
- Sato, T.; Miwa, K.; Nakamori, Y.; Ohoyama, K.; Li, H.-W.; Noritake, T.; Aoki, M.; Towata, S.; Orimo, S. Experimental and computational studies on solvent-free rare-earth metal borohydrides R(BH4)3 (R = Y, Dy, and Gd). Phys. Rev. B 2008, 77, 104114. [Google Scholar] [CrossRef]
- Yan, Y.; Li, H.-W.; Sato, T.; Umeda, N.; Miwa, K.; Towata, S.; Orimo, S. Dehydriding and rehydriding properties of yttrium borohydride Y(BH4)3 prepared by liquid-phase synthesis. Int. J. Hydrogen Energy 2009, 34, 5732–5736. [Google Scholar] [CrossRef]
- Frommen, C.; Aliouane, N.; Deledda, S.; Fonnelop, J.E.; Grove, H.; Lieutenant, K.; Llamas-Jansa, I.; Sartori, S.; Sorby, M.H.; Hauback, B.C. Crystal structure, polymorphism, and thermal properties of yttrium borohydride Y(BH4)3. J. Alloys Compd. 2010, 496, 710–716. [Google Scholar] [CrossRef]
- Ravnsbæk, D.B.; Filinchuk, Y.; Cerny, Radovan.; Ley, M.B.; Haase, D.; Jakobsen, H.J.; Skibsted, J.; Jensen, T.R. Thermal Polymorphism and Decomposition of Y(BH4)3. Inorg. Chem. 2010, 49, 3801–3809. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-S.; Shim, J.-H.; Cho, Y.W. Polymorphism and Thermodynamics of Y(BH4)3 from First Principles. J. Phys. Chem. C 2010, 114, 12833–12837. [Google Scholar] [CrossRef]
- Jaroń, T.; Grochala, W. Y(BH4)3—an Old–New Ternary Hydrogen Store aka Learning from a Multitude of Failures. Dalton Trans. 2010, 39, 160–166. [Google Scholar] [CrossRef]
- Gennari, F.C.; Esquivel, M.R. Synthesis and Dehydriding Process of Crystalline Ce(BH4)3. J. Alloys Compd. 2009, 485, L47–L51. [Google Scholar] [CrossRef]
- Jeon, E.; Cho, Y.W. Mechanochmical Synthesis and Thermal Decomposition of Zinc Borohydride. J. Alloys Compd. 2006, 422, 273–275. [Google Scholar] [CrossRef]
- Srinivasan, S.; Escobar, D.; Jurczyk, M.; Goswami, Y.; Stefanakos, E. Nanocatalyst Doping of Zn(BH4)2. J. Alloys. Compd. 2008, 462, 294–302. [Google Scholar] [CrossRef]
- Titov, L.V.; Eremin, E.R. Complex of Aluminum Borohydride with Calcium Borohydride. Russ. Chem. B. 1975, 24, 1095–1096. [Google Scholar] [CrossRef]
- Fang, Z.Z.; Ma, L.P.; Kang, X.D.; Wang, P.J.; Wang, P.; Cheng, H.M. In situ Formation and Rapid Decomposition of Ti(BH4)3 by Mechanical Milling LiBH4 with TiF3. Appl. Phys. Lett. 2009, 94, 044104. [Google Scholar] [CrossRef]
- Gennari, F.C.; Albanesi, L.F.; Rios, I.J. Synthesis and Thermal Stability of Zr(BH4)4 and Zr(BD4)4 Produced by Mechanochemical Processing. Inorg. Chim. Acta. 2009, 362, 3731–3737. [Google Scholar] [CrossRef]
- Łodziana, Z. Multivalent metal tetrahydroborides of Al, Sc, Y, Ti, and Zr. Phy. Rev. B 2010, 81, 144108. [Google Scholar] [CrossRef]
- Choudhury, P.; Srinivasan, S.S; Bhethanabotla, V.R.; Goswami, Y.; McGrath, K.; Stefanakos, E.K. Nano-Ni Doped Li-Mn-B-H System as a New Hydrogen Storage Candidate. Int. J. Hydrogen Energy. 2009, 34, 6325–6334. [Google Scholar]
- Varin, R.A.; Zbroniec, L. The Effects of Ball Milling Nanometric Nickel on the Hydrogen Desorption from Lithium Borohydride and Manganese Chloride (3 LiBH4 + MnCl2) Mixture. Int. J. Hydrogen Energy. 2010, 35, 3588–3597. [Google Scholar] [CrossRef]
- Černý, R.; Penin, N.; Hagemann, H.; Filinchuk, Y. The First Crystallographic and Spectroscopic Characterization of a 3d-Metal Borohydride: Mn(BH4)2. J. Phys. Chem. C 2009, 113, 9003–9007. [Google Scholar]
- Nakamori, Y.; Miwa, K.; Ninoyiya, A.; Li, H.; Ohba, N.; Towata, S.; Züttel, A.; Orimo, S. Correlation between Thermodynamical Stabilities of Metal Borohydrides and Cation Electronegativities: First-Principles Calculations and Experiments. Phys. Rev. B 2006, 74, 045126. [Google Scholar] [CrossRef]
- Nakamori, Y.; Li, H.-W.; Mastuo, M.; Miwa, K.; Towata, S.; Orimo, S. Development of Metal Borohydrides for Hydrogen Storage. J. Phys. Chem. Sol. 2008, 69, 2292–2296. [Google Scholar] [CrossRef]
- Sugiyama, J.; Ikedo, Y.; Noritake, T.; Ofer, O.; Goko, T.; Månsson, M.; Miwa, K.; Ansaldo, E.J.; Brewer, J.H.; Chow, K.H.; Towata, S. Microscopic Indicator for Thermodynamic Stability of Hydrogen Storage Materials Provided by Positive Muon-Spin Rotation. Phys. Rev. B 2010, 81, 092103. [Google Scholar] [CrossRef]
- Li, H.-W.; Orimo, S.; Nakamori, Y.; Miwa, K.; Ohba, N.; Towata, S.; Züttel, A. Materials Designing of Metal Borohydrides: Viewpoints from Thermodynamically Stabilities. J. Alloys Compd. 2007, 446–447, 315–318. [Google Scholar] [CrossRef]
- Nickels, E.A.; Jones, M.O.; David, W.I.F.; Johnson, S.R.; Lowton, R.L.; Sommariva, M.; Edwards, P.P. Tuning the Decomposition Temperature in Complex Hydrides: Synthesis of a Mixed Alkali Metal Borohydride. Angew. Chem. Int. Ed. 2008, 47, 2817–2819. [Google Scholar] [CrossRef]
- Hagemann, H.; Longhini, M.; Kaminski, J.W.; Wesolowski, T.A.; Černy, R.; Penin, N.; Sørby, M.H.; Hauback, B.C.; Severa, G.; Jensen, C.M. LiSc(BH4)4: A Novel Salt of Li+ and Discrete Sc(BH4)4− Complex Anions. J. Phys. Chem. A 2008, 112, 7551–7555. [Google Scholar] [CrossRef] [PubMed]
- Seballos, J.; Zhang, J.Z.; Rönnebro, E.; Herberg, J.L.; Majzoub, E.H. Metastabilility and Crystal Structure of the Bialkali Complex Metal Borohydride NaK(BH4)2. J. Alloys Compd. 2009, 476, 446–450. [Google Scholar] [CrossRef]
- Kim, C.; Hwang, S.-J.; Bowman, R.C., Jr.; Reiter, J.W.; Zan, J.A.; Kulleck, J.G.; Kabbour, H.; Majzoub, E.H.; Ozolins, V. LiSc(BH4)4 as a Hydrogen Storage Material: Multinuclear High-Resolution Solid-State NMR and First-Principles Density Functional Theory Studies. J. Phys. Chem. C 2009, 113, 9956–9968. [Google Scholar] [CrossRef]
- Ravnsbæk, D.; Filinchuk, Y.; Cerenius, Y.; Jakobsen, H.J.; Besenbacher, F.; Skibsted, J.; Jensen, T.R. A Series of Mixed-Metal Borohydrides. Angew. Chem. Int. End. 2009, 48, 6659–6663. [Google Scholar] [CrossRef]
- Černý, R.; Severa, G.; Ravnsbæk, D.B.; Filinchuk, Y.; D’Anna, V.; Hagemann, H.; Haase, D.; Jensen, C.M.; Jensen, T.R. NaSc(BH4)4: A Novel Scandium-Based Borohydride. J. Phys. Chem. C 2010, 114, 1357–1364. [Google Scholar] [CrossRef]
- Severa, G.; Hagemann, H.; Longhini, M.; Kaminski, J.W.; Wesolowski, T.A.; Jensen, C.M. Thermal Desorption, Vibrational Spectroscopic, and DFT Computational Studies of the Complex Manganese Borohydrides Mn(BH4)2 and [Mn(BH4)4]2−. J. Phys. Chem. C 2010, 114, 15516–15521. [Google Scholar] [CrossRef]
- Fang, Z.Z.; Kang, X.D.; Wang, P.; Li, H.-W.; Orimo, S. Unexpected Dehydrogenation Behavior of LiBH4/Mg(BH4)2 Mixture Associated with the In Situ Formation of Dual-Cation Borohydride. J. Alloys Compd. 2010, 491, L1–L4. [Google Scholar] [CrossRef]
- Lee, J.Y.; Ravnsbæk, D.; Lee, Y.-S.; Kim, Y.; Cerenius, Y.; Shim, J.-H.; Jensen, T.R.; Hur, N.H.; Cho, Y.W. Decomposition Reactions and Reversibility of the LiBH4-Ca(BH4)2 Composite. J. Phys. Chem. C 2009, 113, 15080–15086. [Google Scholar] [CrossRef]
- Černý, R.; Ravnsbæk, D.B.; Severa, G.; Filinchuk, Y.; D’Anna, V.; Hagemann, H.; Haase, D.; Skibsted, J.; Jensen, C.M.; Jensen, T.R. Structure and Characterization of KSc(BH4)4. J. Phys. Chem. C 2010, 114, 19540–19549. [Google Scholar] [CrossRef]
- Vajo, J.J.; Olson, G.L. Hydrogen Storage in Destabilized Chemical Systems. Scripta Mater. 2007, 56, 829–834. [Google Scholar] [CrossRef]
- NIST Webbook. Available online: http://webbook.nist.gov/chemistry (accessed on 12 January 2011).
- Vajo, J.J.; Skeith, S.L.; Mertens, F. Reversible Storage of Hydrogen in Destabilized LiBH4. J. Phys. Chem. B 2005, 109, 3719–3722. [Google Scholar] [CrossRef] [PubMed]
- Aoki, M.; Miwa, K.; Noritake, T.; Kitahara, G.; Nakamori, Y.; Orimo, S.; Towata, S. Destabilization of LiBH4 by Mixing with LiNH2. Appl. Phys. A 2005, 80, 1409–1412. [Google Scholar] [CrossRef]
- Johnson, S.R.; Anderson, P.A.; Edwards, P.P.; Gameson, I.; Prendergast, J.W.; Al-Mamouri, M.; Book, D.; Harris, I.R.; Speight, J.D.; Walton, A. Chemical Activation of MgH2, a New route to Superior Hydrogen Storage Materials. Chem. Commun. 2005, 22, 2823–2825. [Google Scholar] [CrossRef]
- Pinkerton, F.E.; Meisner, G.P.; Meyer, M.S.; Balogh, M.P.; Kundrat, M.D. Hydrogen Desorption Exceeding Ten Weight Percent from the New Quaternary Hydride Li3BN2H8. J. Phys. Chem. B 2005, 109, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Alapati, S.V.; Johnson, J.K.; Sholl, D.S. Identification of Destabilized Metal Hydrides for Hydrogen Storage Using First Principles Calculations. J. Phys. Chem. B 2006, 110, 8769–8776. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.W.; Shim, J.-H.; Lee, B.-J. Thermal Destabilization of Binary and Complex Metal Hydrides by Chemical Reaction: A Thermodynamic Analysis. Comput. Coupling Phase Diagr. Thermochem. 2006, 30, 65–59. [Google Scholar] [CrossRef]
- Yu, X.B.; Grant, D.M.; Walker, G.S. A New Dehydrogenation Mechanism for Reversible Multicomponent Borohydride Systems—the Role of Li-Mg Alloys. Chem. Commun. 2006, 37, 3906–3908. [Google Scholar] [CrossRef]
- Nakamori, Y.; Ninomiya, A.; Kitahara, G.; Aoki, M.; Noritake, T.; Miwa, K.; Kojima, Y.; Orimo, S. Dehydriding Reactions of Mixed Complex Hydrides. J. Power Sources. 2006, 155, 447–455. [Google Scholar] [CrossRef]
- Meisner, G.P.; Scullin, M.L.; Balogh, M.P.; Pinkerton, F.E.; Meyer, M.S. Hydrogen Release from Mixtures of Lithium Borohydride and Lithium Amide: A Phase Diagram Study. J. Phys. Chem. B 2006, 110, 4189–4192. [Google Scholar] [CrossRef]
- Noritake, T.; Aoki, M.; Towata, S.; Ninimiya, A.; Nakamori, Y.; Orimo, S. Crystal Structure Analysis of Novel Complex Hydrides Formed by Combination of LiBH4 an LiNH2. Appl. Phys. A 2006, 83, 277–279. [Google Scholar] [CrossRef]
- Pinkerton, F.E.; Meyer, M.S.; Meisner, G.P.; Balogh, M.P. Improved Hydrogen Release from LiB0.33N0.67H2.67 with Novel Metal Additives. J. Phys. Chem. B 2006, 110, 7967–7974. [Google Scholar] [CrossRef] [PubMed]
- Chater, P.A.; David, W.I.F.; Johnson, S.R.; Edwards, P.P.; Anderson, P.A. Synthesis and Crystal Structure of Li4BH4(NH2)3. Chem. Commun. 2006, 23, 2439–2441. [Google Scholar] [CrossRef]
- Noritake, T.; Aoki, M.; Towata, S.; Ninomiya, A.; Nakamori, Y.; Orimo, S. Crystal Structure Analysis of Novel Complex Hydrides Formed by the Combination of LiBH4 and LiNH2. Appl. Phys. A 2006, 83, 277–279. [Google Scholar] [CrossRef]
- Alapati, S.V.; Johnson, J.K.; Sholl, D.S. Stability Analysis of Doped Materials for Reversible Hydrogen Storage in Destabilized Metal Hydrides. Phys. Rev. B 2007, 76, 104108. [Google Scholar] [CrossRef]
- Yang, J.; Sudik, A.; Wolverton, C. Destabilizing LiBH4 with a Metal (M = Mg, Al, Ti, V, Cr, or Sc) or Metal Hydride (MH2 = MgH2, TiH2, CaH2). J. Phys. Chem. 2007, 111, 19134–19140. [Google Scholar]
- Siegel, D.J.; Wolverton, C.; Ozoliņš, V. Thermodynamics Guidelines for the Prediction of Hydrogen Storage Reactions and Their Application to Destabilized Hydride Mixtures. Phys. Rev. B 2007, 76, 134102. [Google Scholar] [CrossRef]
- Bösenberg, U.; Doppiu, S.; Mosegaard, L.; Barkhordarian, G.; Eigen, N.; Borgschulte, A.; Jensen, T.R.; Cerenius, Y.; Gutfleisch, O.; Klassen, T.; Dornheim, M.; Bormann, R. Hydrogen Sorption Properties of MgH2–LiBH4 Composites. Acta Mater. 2007, 55, 3951–3958. [Google Scholar] [CrossRef]
- Kang, X.-D.; Wang, P.; Ma, L.-P.; Cheng, H.-M. Reversible Hydrogen Storage in LiBH4 Destabilized by Milling with Al. Appl. Phys. A 2007, 89, 963–966. [Google Scholar] [CrossRef]
- Pinkerton, F.E.; Meyer, M.S. Reversible Hydrogen Storage in the Lithium Borohydride—Calcium Hydride Coupled System. J. Alloys Compd. 2008, 464, L1–L4. [Google Scholar] [CrossRef]
- Wolverton, C.; Siegel, D.J.; Akbarzadeh, A.R.; Ozoliņš, V. Discovery of Novel Hydrogen Storage Materials: An Atomci Scale Computional Approach. J. Phys.: Condens. Matter 2008, 20, 064228. [Google Scholar] [CrossRef]
- Sudik, A.; Yang, J.; Halliday, D.; Wolverton, C. Hydrogen Storage Properties in (LiNH2)2-LiBH4-(MgH2)x Mixture (x = 0.0–1.0). J. Phys. Chem. C 2008, 112, 4384–4390. [Google Scholar] [CrossRef]
- Yang, J.; Sudik, A.; Siegel, D.J.; Halliday, D.; Drews, A.; Carter, R.O., III; Wolverton, C.; Lewis, G.J.; Sachtler, J.W.A.; Low, J.J.; Faheem, S.A.; Lesch, D.A.; Ozoliņš, V. A Self-Catalyzing Hydrogen-Storage Material. Angew. Chem. Int. Ed. 2008, 47, 882–887. [Google Scholar] [CrossRef]
- Alapati, S.V.; Johnson, J.K.; Sholl, D.S. Large-Scale Screening of Metal Hydride Mixtures for High-Capacity Hydrogen Storage from First-Principle Calculations. J. Phys. Chem. C 2008, 112, 5258–5262. [Google Scholar] [CrossRef]
- Purewal, J.; Hwang, S.-J.; Bowman, R.C., Jr.; Rönnebro, E.; Fultz, B.; Ahn, C. Hydrogen Sorption Behavior of the ScH2-LiBH4 System: Experimental Assesment of Chemical Destabilization Effects. J. Phys. Chem. C 2008, 112, 8481–8485. [Google Scholar] [CrossRef]
- Jin, S.-A.; Lee, Y.-S.; Shim, J.-H.; Cho, Y.W. Reversible Hydrogen Storage in LiBH4-MgH2 (M = Ce, Ca). J. Phys. Chem. C 2008, 112, 9520–9524. [Google Scholar] [CrossRef]
- Wan, X.; Markmaitree, T.; Osborn, W.; Shaw, L.L. Nanoengineering-Enabled Solid-State Hydrogen Uptake and Release in the LiBH4 Plus MgH2 System. J. Phys. Chem. C 2008, 112, 18232–18243. [Google Scholar] [CrossRef]
- Langmi, H.W.; McGrady, G.S. Ternary Nitrides for Hydrogen Storage: Li-B-N, Li-Al-N and Li-Ga-N Systems. J. Alloys Compd. 2008, 466, 287–292. [Google Scholar] [CrossRef]
- Jin, S.-A.; Shim, J.-H.; Cho, Y.W.; Yi, K.-W.; Zabara, O.; Fichtner, M. Reversible Hydrogen Storage in LiBH4-Al-LiH Composite Powder. Scripta Mater. 2008, 58, 963–965. [Google Scholar] [CrossRef]
- Fan, M.-Q.; Sun, L.-X.; Zhang, Y.; Xu, F.; Zhang, J.; Chu, H.-L. The Catalytic Effect of Additive Nb2O5 on the Reversible Hydrogen Storage Performances of LiBH4-MgH2 Composite. Int. J. Hydrogen Energy. 2008, 33, 74–80. [Google Scholar] [CrossRef]
- Tang, W.S.; Wu, G.T.; Liu, T.; Wee, A.T.S.; Yong, C.K.; Xiong, Z.; Hor, T.S.; Chen, P. Cobalt-Catalyzed Hydrogen Desorption from the LiNH2-LiBH4 System. Dalton Trans. 2008, 18, 2395–2399. [Google Scholar] [CrossRef] [PubMed]
- Bösenberg, U.; Vainio, U.; Pranzas, P.K.; Bellosta von Colbe, J.M.; Goerigk, G.; Welter, E.; Dornheim, M.; Schreyer, A.; Bormann, R. On the Chemical State and Distribution of Zr- and V-Based Additives in Reactive Hydride Composites. Nanotechnology 2009, 20, 204003. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.J.; Ma, L.P.; Fang, Z.Z.; Kang, X.D.; Wang, P. Improved Hydrogen Storage Properties of Li-Mg-B-H System by Milling with Titanium Trifluoride. Energy Environ. Sci. 2009, 2, 120–123. [Google Scholar] [CrossRef]
- Du, A.J.; Smith, S.C.; Yao, X.D.; Sun, C.H.; Li, L.; Lu, G.Q. First Principle Study of Hydrogen of MgB2: An Important Step toward Reversible Hydrogen Storage in the Coupled LiBH4/MgH2 System. J. Nanosci. Nanotechnol. 2009, 9, 4388–4391. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hirano, S. Improving the Hydrogen Reaction Kinetics of Complex Hydrides. Adv. Mater. 2009, 21, 1–6. [Google Scholar]
- Graetz, J.; Chaudhuri, S.; Salguero, T.T.; Vajo, J.J.; Meyer, M.S.; Pinkerton, F.E. Local bonding and atomic environments in Ni-catalyzed complex hydrides. Nanotechnology 2009, 20, 204007. [Google Scholar] [CrossRef] [PubMed]
- Sudik, A.; Yang, J.; Siegel, D.J.; Wolverton, C.; Carter, R.O., III; Drews, A.R. Impact of Stoichiometry on the Hydrogen Storage Properties of LiNH2-LiBH4-MgH2 Ternary Composites. J. Phys. Chem. C 2009, 113, 2004–2013. [Google Scholar] [CrossRef]
- Walker, G.S.; Grant, D.M.; Price, T.C.; Yu, X.B.; Legrand, V. High Capacity Multicomponent Hydrogen Storage Materials: Investigation of the Effect of Stoichimetry and Decomposition Conditions on the Cycling Behaviour of LiBH4–MgH2. J. Power Sources 2009, 194, 1128–1134. [Google Scholar] [CrossRef]
- Mao, J.F.; Guo, Z.P.; Liu, H.K.; Yu, X.B. Reversible Hydrogen Storage in Titanium-Catalyzed LiAlH4-LiBH4. J. Alloys. Compd. 2009, 487, 434–438. [Google Scholar] [CrossRef]
- Puszkiel, J.A.; Gennari, F.C. Reversible Hydrogen Storage in Metal-Doped Mg-LiBH4 Composites. Scripta Mater. 2009, 60, 667–670. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, Q.; Chu, H.L.; Zhang, J.; Sun, L.; Sun, J.; Wen, Z. Hydogen De/Resorption Properties of the LiBH4-MgH2-Al System. J. Phys. Chem. C 2009, 113, 21964–21969. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, MI.; Wang, K.; Udovic, T.J.; Rush, J.J.; Yildirim, T.; Bendersky, L.A.; Gross, A.F.; van Atta, S.L.; Vajo, J.J.; Pinkerton, F.E.; Meyer, M.S. Size Effects on the Hydrogen Storage Properties of Nanoscaffolded Li3BN2H8. Nanotechnology 2009, 20, 204002. [Google Scholar] [CrossRef] [PubMed]
- Pinkerton, F.E.; Meyer, M.S. Hydrogen Desorption Behavior of Nickel-Chloride-Catalyzed Stoichiometric Li4BN3H10. J. Phys. Chem. C 2009, 113, 11172–11176. [Google Scholar] [CrossRef]
- Kim, J.W.; Friedrichs, O.; Ahn, J.-P.; Kim, D.H.; Kim, S.C.; Remhof, A.; Chung, H.-S.; Lee, J.; Shim, J.-H.; Cho, Y.W.; Züttel, A.; Oh, K.H. Microstructural Change of 2LiBH4/Al with Hydrogen Sorption cycling: Separation of Al and B. Scripta Mater. 2009, 60, 1089–1092. [Google Scholar] [CrossRef]
- Friedrichs, O.; Kim, J.W.; Remhof, A.; Buchter, F.; Borgschulte, A.; Wallacher, D.; Cho, Y.W.; Fichtner, M.; Oh, K.H.; Züttel, A. The Effect of Al on the Hydrogen Sorption Mechanism of LiBH4. Phys. Chem. Chem. Phys. 2009, 11, 1515–1520. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, D.; Shi, Q.; Boothroyd, C.B.; Vegge, T. Reversibility of Al/Ti Modified LiBH4. J. Phys. Chem. C 2009, 113, 14059–14066. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, Q.; Zhang, J.; Liu, S.-S.; Sun, L.-X. The Dehydrogenation Reactions and Kinetics of 2LiBH4-Al Composite. J. Phys. Chem. C 2009, 113, 18424–18430. [Google Scholar] [CrossRef]
- Singer, J.P.; Meyer, M.S.; Speer, R.M., Jr.; Fischer, J.E.; Pinkerton, F.E. Determination o the Phase Behavior of (LiNH2)c(LiBH4)1−c Quaternary Hydride through in Situ X-ray Diffraction. J. Phys. Chem. C 2009, 113, 18927–18934. [Google Scholar] [CrossRef]
- Li, W.; Vajo, J.J.; Cumberland, R.W.; Liu, P.; Hwang, S.-J.; Kim, C.; Bowman, R.C., Jr. Hydrogenation of Magnesium Nickel Boride for Reversible Hydrogen Storage. Phys. Chem. Lett. 2010, 1, 69–72. [Google Scholar] [CrossRef]
- Wang, P.-J.; Fang, Z.-Z.; Ma, L.-P.; Kang, X.-D.; Wang, P. Effect of Carbon Addition on Hydrogen Storage Behaviors of Li-Mg-B-H System. Int. J. Hydrogen Energy 2010, 35, 3072–3075. [Google Scholar] [CrossRef]
- Shim, J.-H.; Lim, J.-H.; Rather, S.-U.; Lee, Y.-S.; Reed, D.; Kim, Y.; Book, D.; Cho, Y.W. Effect of Hydrogen Back Pressure on Dehydrogenation Behavior of LiBH4-Based Reactive Hydride Composites. Phys. Chem. Lett. 2010, 1, 59–63. [Google Scholar] [CrossRef]
- Deprez, E.; Muñoz-Márquez, M.A.; Roldán, M.A.; Prestipino, C.; Javier Palomares, F.; Bonatto Minella, C.; Bösenberg, U.; Dornheim, M.; Bormann, R.; Fernández, A. Oxidation State and Local Structure of Ti-Based Additives in the Reactive Hydride Composite 2 LiBH4 + MgH2. J. Phys. Chem. 2010, 114, 3309–3317. [Google Scholar]
- Bösenberg, U.; Kim, J.W.; Gosslar, D.; Eigen, N.; Jensen, T.G.; Bellosta von Colbe, J.M.; Zhou, Y.; Dahms, M.; Kim, D.H.; Günther, R.; Cho, Y.W.; Oh, K.H.; Klassen, T.; Bormann, R.; Dornheim, M. Role of Additives in LiBH4-MgH2 Reactive Hydride Composites for Sorption Kinetics. Acta Mater. 2010, 58, 3381–3389. [Google Scholar] [CrossRef]
- Price, T.C.; Grant, D.M.; Legrand, V.; Walker, G.S. Enhanced Kinetics for the LiBH4:MgH2 Multi-Component Hydrogen Storage System–The Effects of Stoichiometry and Decomposition Environment on Cycling Behaviour. Int. J. Hydrogen Energy 2010, 35, 4154–4161. [Google Scholar] [CrossRef]
- Somer, M.; Acar, S.; Koz, C.; Kokal, I.; Höhn, P.; Cardoso-Gil, R.; Aydemir, U.; Akselrud, L. α- and β-Na2[BH4][NH2]: Two Modifications of a Complex Hydride in the System NaNH2-NaBH4; Syntheses, Crystal Structures, Thermal Analyses, Mass and Vibrational Spectra. J. Alloys Compd. 2010, 491, 98–105. [Google Scholar] [CrossRef]
- Yu, X.B.; Guo, Y.H.; Sun, D.L.; Yang, Z.X.; Ranjbar, A.; Guo, Z.P.; Liu, H.K.; Dou, S.X. A Combined Hydrogen Storage System of Mg(BH4)2-LiNH2 with Favorable Dehydrogenation. J. Phys. Chem. C 2010, 114, 4733–4737. [Google Scholar] [CrossRef]
- Mao, J.; Guo, Z.; Leng, H.; Wu, Z.; Guo, Y.; Yu, X.; Liu, H. Reversible Hydrogen Storage in Destabilized LiAlH4−MgH2−LiBH4 Ternary-Hydride System Doped with TiF3. J. Phys. Chem. C 2010, 114, 11643–11649. [Google Scholar] [CrossRef]
- Ravnsbæk, D.B.; Jensen, T.R. Tuning hydrogen storage properties and reactivity: Investigation of the LiBH4-NaAlH4 sytem. J. Phys Chem. Sol. 2010, 71, 1144–1149. [Google Scholar] [CrossRef]
- Mauron, P.; Bielmann, M.; Remhof, A.; Züttel, A.; Shim, J.-K.; Cho, Y.W. Stability of the LiBH4/CeH2 Composite System Determined by Dynamic pcT Measurements. J. Phys. Chem. C 2010, 114, 16801–16805. [Google Scholar] [CrossRef]
- Bösenberg, U.; Ravnsbæk, D.B.; Hagemann, H.; D’Anna, V.; Minella, C.B.; Pistidda, C.; van Beek, W.; Jensen, T.R.; Bormann, R.; Dornheim, M. Pressure and Temperature Influence on the Desorption Pathway of the LiBH4-MgH2 Composite System. J. Phys. Chem. C 2010, 114, 15212–15217. [Google Scholar] [CrossRef]
- Zeng, L.; Miyaoka, H.; Ichikawa, T.; Kojima, Y. Superior Hydrogen Exchange Effect in the MgH2-LiBH4 System. J. Phys. Chem. C 2010, 114, 13132–13135. [Google Scholar] [CrossRef]
- Crosby, K.; Shaw, L.L. Dehydriding and re-hydriding properties of high-energy ball milled LiBH4 + MgH2 mixture. Int. J. Hydrogen Energy 2010, 35, 7519–7529. [Google Scholar] [CrossRef]
- Choudhury, P.; Bhethanabotla, V.R.; Stefanakos, E. First principles study to identify the reversible reaction step of a multinary hydrogen storage “Li-Mg-B-N-H” system. Int. J. Hydrogen Energy 2010, 35, 9002–9011. [Google Scholar] [CrossRef]
- Shaw, L.L.; Wan, X.; Hu, J.Z.; Kwak, J.H.; Yang, Z. Solid-State Hydriding Mechanism in the LiBH4-MgH2 System. J. Phys. Chem. C 2010, 114, 8089–8098. [Google Scholar] [CrossRef]
- Garroni, S.; Milanese, C.; Girella, A.; Marini, A.; Mulas, G.; Menéndez, E.; Pistidda, C.; Dornheim, M.; Suriñach, S.; Baró, M.D. Sorption Properties of NaBH4/MH2 (M = Mg, Ti) Powder System. Int. J. Hydrogen. Energy 2010, 35, 5434–5441. [Google Scholar] [CrossRef]
- Gennari, F.C.; Puszkiel, J.A. Enhanced Hydrogen Sorption Kinetics of Mg50Ni-LiBH4. J. Power Sources. 2010, 195, 3266–3274. [Google Scholar] [CrossRef]
- Hattrick-Simpers, J.R.; Maslar, J.E.; Niemann, M.U.; Chium, C.; Srinivasan, S.S.; Stefanakos, E.K.; Bendersky, L.A. Raman Spectroscopic Observation of Dehydrogenation in Ball-Milled LiNH2-LiBH4-MgH2 Nanoparticles. Int. J. Hydrogen Energy. 2010, 35, 6323–6331. [Google Scholar] [CrossRef]
- Barkhordarian, G.; Klassen, T.; Dornheim, M.; Bormann, R. Unexpected Kinetic Effect of MgB2 in Reactive Composites Containing Complex borohydrides. J. Alloys Compd. 2007, 440, L18–L21. [Google Scholar] [CrossRef]
- Barkhordarian, G.; Jensen, T.R.; Doppiu, S.; Bo1senberg, U.; Borgschulte, A.; Gremaud, R.; Cerenius, Y.; Dornheim, M.; Klassen, T.; Bormann, R. Formation of Ca(BH4)2 from Hydrogenation of CaH2 + MgB2 Composite. J. Phys. Chem. C 2008, 112, 2743–2749. [Google Scholar] [CrossRef]
- Hu, J.Z.; Kwak, J.H.; Y, Z.; Wan, X.; Shaw, L.L. Direct Observation of Ion Exchange in Mechanically Activated LiH + MgB2 System Using Ultrahigh Field Nuclear Magnetic Resonance Spectroscopy. Appl. Phys. Lett. 2009, 94, 141905. [Google Scholar] [CrossRef]
- Gosalawit-Utke, R.; Bellosta von Colbe, J.M.; Dornheim, M.; Jensen, T.R.; Cerenius, Y.; Minella, C.B.; Peschke, M.; Bormann, R. LiF−MgB2 System for Reversible Hydrogen Storage. J. Phys. Chem. C 2010, 114, 10291–10296. [Google Scholar] [CrossRef]
- Gonzalez-Silveira, M.; Gremaud, R.; Schreuders, H.; van Setten, M.J.; Batyrev, E.; Rougier, A.; Dupont, L.; Bardají, E.G.; Lohstroh, W.; Dam, B. In-Situ Deposition of Alkali and Alkaline Earth Hydride Thin Films To Investigate the Formation of Reactive Hydride Composites. J. Phys. Chem. C 2010, 114, 13895–13901. [Google Scholar] [CrossRef]
- Rongeat, C.; D’Anna, V.; Hagemann, H.; Borgschulte, A.; Züttel, A.; Schultz, L.; Gutfleisch, O. Effect of Additives on the Synthesis and Reversibility of Ca(BH4)2. J. Alloys Compd. 2010, 493, 281–287. [Google Scholar] [CrossRef]
- Kim, Y.; Reed, D.; Lee, Y.-S.; Shim, J.-H.; Han, H.N.; Book, D.; Cho, Y.W. Hydrogenation Reaction of CaH2-CaB6-Mg Mixture. J. Alloys Compd. 2010, 492, 597–600. [Google Scholar] [CrossRef]
- Bogdanović, B.; Schwickardi, M. Ti-doped alkali metal aluminium hydrides as potential novel reversible hydrogen storage materials. J. Alloy. Compd. 1997, 253–254, 1–9. [Google Scholar] [CrossRef]
- Au, M.; Jurgensen, A. Modified Lithium Borohydrides for Reversible Hydrogen Storage. J. Phys. Chem. B 2006, 110, 7062–7067. [Google Scholar] [CrossRef] [PubMed]
- Au, M.; Jurgensen, A.; Zeigler, K. Modified Lithium Borohydrides for Reversible Hydrogen Storage (2). J. Phys. Chem. B 2006, 110, 26482–26487. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.B.; Wu, Z.; Chen, Q.R.; Li, Z.L.; Weng, B.C.; Huang, T.S. Improved Hydrogen Storage Properties of LiBH4 Destabilized by Carbon. Appl. Phys. Lett. 2007, 90, 034106. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.-S.; Wang, A.-Q.; Sun, L.-X.; Fan, M.-Q.; Chu, H.-L.; Sun, J-C.; Zhang, T. LiBH4 Nanoparticles Supported by Disordered Mesoporous Carbon: Hydrogen Storage Performances and Destabilization Mechanisms. Int. J. Hydrogen Energy. 2007, 32, 3976–3980. [Google Scholar] [CrossRef]
- Varin, R.A.; Chiu, C.; Wronski, Z.S.; Calka, A. The Effects of Oxidized and Oxide-Free Boron on the Mg-B-H Nanohydrides Transformation in the Nearly Nanosized Powders. Solid State Phenom. 2007, 128, 47–52. [Google Scholar] [CrossRef]
- Mosegaard, L.; Møller, B.; Jørgensen, J.-E.; Filinchuk, Y.; Cerenius, Y.; Hanson, J.; Dimasi, E.; Besenbacher, F.; Jensen, T.R. Reactivity of LiBH4: In Situ Synchrotron Radian Powder X-ray Diffraction Study. J. Phys. Chem. C 2008, 112, 1299–1303. [Google Scholar] [CrossRef]
- Yin, L.; Wang, P.; Fang, Z.Z.; Cheng, H. Thermodynamically Tuning LiBH4 by Fluorine Anion Doping for Hydrogen Storage: A Density functional Study. Chem. Phys. Lett. 2008, 450, 318–321. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.-S.; Fan, M.-Q.; Liu, S.-S.; Chu, H.-L.; Zhang, Y.-H.; Gao, X-Y.; Sun, L.-X. Enhanced Hydrogen Storage Performance of LiBH4-SiO2-TiF3 Composite. J. Phys. Chem. C 2008, 112, 4005–4110. [Google Scholar] [CrossRef]
- Fang, Z.Z.; Kang, X.D.; Dai, H.B.; Zhang, M.J.; Wang, P.; Cheng, H.M. Reversible Dehydrogenation of LiBH4 Catalyzed by As-Prepared Sing-Walled Carbon Nanotubes. Scripta Mater. 2008, 58, 922–925. [Google Scholar] [CrossRef]
- Xu, J.; Yu, X.B.; Zou, Z.Q.; Li, Z.L.; Wu, Z.; Akins, L.D.; Yang, H. Enhanced Dehydrogenation of LiBH4 Catalyzed by Carbon-Supported Pt Nanoparticles. Chem. Commun. 2008, 44, 5740–5742. [Google Scholar] [CrossRef]
- Fang, Z.-Z.; Kang, X.-D.; Wang, P.; Cheng, H.-M. Improved Reversible Dehydrogenation of Lithium Borohydride by Milling with As-Prepared Single-Walled Carbon Nanotubes. J. Phys. Chem. C 2008, 112, 17023–17029. [Google Scholar] [CrossRef]
- Au, M.; Jurgensen, A.R.; Spencer, W.A.; Anto, D.A.; Pinkerton, F.E.; Hwang, S.-J.; Kim, C.; Bowman, R.C., Jr. Stability and Reversibility of Lithium Borohydrides Doped by Metal Halides and Hydrides. J. Phys. Chem. C 2008, 112, 18661–18671. [Google Scholar] [CrossRef]
- Yu, X.B.; Grant, D.M.; Walker, G.S. Dehydrogenation of LiBH4 Destabilized with Various Oxides. J. Phys. Chem. C 2009, 113, 17945–17949. [Google Scholar] [CrossRef]
- Arnbjerg, L.M.; Ravnsbæk, D.B.; Filinchuk, Y.; Vang, R.T.; Cerenius, Y.; Besenbacher, F.; Jørgensen, J.-E.; Jakobsen, H.J.; Jensen, T.R. Structure and Dynamics for LiBH4-LiCl Solid Solutions. Chem. Mater. 2009, 21, 5772–5782. [Google Scholar] [CrossRef]
- Guo, Y.H.; Yu, X.B.; Gao, L.; Xia, G.L.; Guo, Z.P.; Liu, H.K. Significantly Improved Dehydrogenation of LiBH4 Destabilized by TiF3. Energy Environ. Sci. 2010, 3, 465–470. [Google Scholar] [CrossRef]
- Fang, Z.Z.; Kang, X.D.; Wang, P. Improved Hydrogen Storage Properties of LiBH4 by Mechanical Milling with Various Carbon Additives. J. Int. Hydrogen Energy 2010, 35, 8247–8252. [Google Scholar] [CrossRef]
- Zhang, B.J.; Liu, B.H. Hydrogen Desorption from LiBH4 destabilized by chlorides of transition metal Fe, Co, and Ni. Int. J. Hydrogen Energy 2010, 35, 7288–7294. [Google Scholar] [CrossRef]
- Gutowska, A.; Li, L.; Shin, Y.; Wang, C.M.; Li, X.S.; Linehan, J.C.; Smith, R.S.; Kay, B.D.; Schmid, B.; Shaw, W.; Gutowski, M.; Autrey, T. Nanoscaffold Mediates Hydrogen Release and the Reactivity of Ammonia Borane. Angew. Chem. Int. Ed. 2005, 44, 3578. [Google Scholar] [CrossRef]
- Gross, A.F.; Vajo, J.J.; van Atta, S. L.; Olson, G.L. Enhanced Hydrogen Storage Kinetics of LiBH4 in Nanoporous Carbon Scaffolds. J. Phys. Chem. C 2008, 112, 5651–5657. [Google Scholar] [CrossRef]
- Fang, Z.Z.; Wang, P.; Rufford, T.E.; Kang, X.D.; Lu, G.Q.; Cheng, H.M. Kinetic- and Thermodynamic-Based Improvements of Lithium Borohydride Incorporated Into Activated Carbon. Acta Mater. 2008, 56, 6257–6263. [Google Scholar] [CrossRef]
- Cahen, S.; Eymery, J.-B.; Janot, R.; Tarascon, J.-M. Improvement of the LiBH4 Hydrogen Desorption by Inclusion into Mesoporous Carbons. J. Power Sources 2009, 189, 902–908. [Google Scholar] [CrossRef]
- Ingleson, M.J.; Barrio, J.P.; Bacsa, J.; Steiner, A.; Darling, G.R.; Jones, J.T.A.; Khimyak, Y.Z.; Rosseinsky, M.J. Magnesium Borohydride Confined in a Metal–Organic Framework: A Preorganized System for Facile Arene Hydroboration. Angew. Chem. Int. Ed. 2009, 48, 2012–2016. [Google Scholar] [CrossRef]
- Fichtner, M.; Zhao-Karger, Z.; Hu, J.; Roth, A.; Weidler, P. The Kinetic Properties of Mg(BH4)2 Infiltrated in Activated Carbon. Nanotechnology 2009, 20, 204029. [Google Scholar] [CrossRef] [PubMed]
- Ngene, P.; Adelhelm, P.; Beale, A.M.; de Jong, K.P.; de Jongh, P.E. LiBH4/SBA-15 Nanocomposites Prepared by Melt infiltration under Hydrogen pressure: Synthesis and Hydrogen Sorption Properties. J. Phys. Chem. C 2010, 114, 6163–6168. [Google Scholar] [CrossRef]
- Shane, D.T.; Corey, R.L.; McIntosh, C.; Rayhel, L.H.; Bowman, R.C., Jr.; Vajo, J.J.; Gross, A.F.; Conradi, M.S. LiBH4 in Carbon Aerogel Nanoscaffolds: An NMR Study of Atomic Motions. J. Phys. Chem. C 2010, 114, 4008–4014. [Google Scholar] [CrossRef]
- Liu, X.; Peaslee, D.; Jost, C.Z.; Majzoub, E.H. Controlling the Decomposition Pathway of LiBH4 via Confinement in Highly Ordered Nanoporous Carbon. J. Phys. Chem. C 2010, 114, 14036–14041. [Google Scholar] [CrossRef]
- Ngene, P.; van Zwienen, M.R.; de Jongh, P.E. Reversibility of the hydrogen desorption from LiBH4: A synergetic effect of nanoconfinement and Ni addition. Chem. Comm. 2010, 46, 8201–5203. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.K.; Bösenber, U.; Gosalawit, R.; Dornheim, M.; Cerenius, Y.; Besenbacher, F.; Jensen, T.R. A Reversible Nanoconfined Chemical Reaction. ACS NANO 2010, 4, 3903–3908. [Google Scholar] [CrossRef] [PubMed]
- Sartori, S.; Knudsen, K.D.; Zhao-karger, Z.; Bardaji, E.G.; Muller, J.; Fichtner, M.; Hauback, B.C.; Sartori, S.; Knudsen, K.D.; Zhao-karger, Z.; Bardaji, E.G.; Muller, J.; Fichtner, M.; Hauback, B.C. Nanoconfined Magnesium Borohydride for Hydrogen Storage Application Investigation by SANS and SAXS. J. Phys. Chem. C 2010, 114, 18785–18789. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, H.-W.; Yan, Y.; Orimo, S.-i.; Züttel, A.; Jensen, C.M. Recent Progress in Metal Borohydrides for Hydrogen Storage. Energies 2011, 4, 185-214. https://doi.org/10.3390/en4010185
Li H-W, Yan Y, Orimo S-i, Züttel A, Jensen CM. Recent Progress in Metal Borohydrides for Hydrogen Storage. Energies. 2011; 4(1):185-214. https://doi.org/10.3390/en4010185
Chicago/Turabian StyleLi, Hai-Wen, Yigang Yan, Shin-ichi Orimo, Andreas Züttel, and Craig M. Jensen. 2011. "Recent Progress in Metal Borohydrides for Hydrogen Storage" Energies 4, no. 1: 185-214. https://doi.org/10.3390/en4010185
APA StyleLi, H.-W., Yan, Y., Orimo, S.-i., Züttel, A., & Jensen, C. M. (2011). Recent Progress in Metal Borohydrides for Hydrogen Storage. Energies, 4(1), 185-214. https://doi.org/10.3390/en4010185





