Stability Proxies for Water-in-Oil Emulsions and Implications in Aqueous-based Enhanced Oil Recovery
Abstract
:1. Introduction

Evidence of Emulsions Conformance Effects: Heavy-Oil EOR

2. Stabilizing Agents
2.1. Introduction
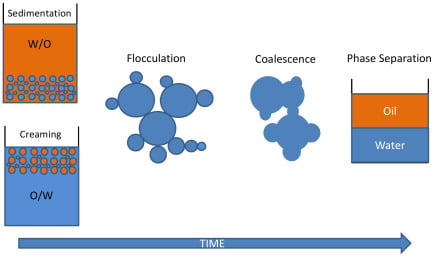
2.2. What Controls Emulsion Stability?
2.2.1. Solid-Stabilized Emulsions: Pickering Emulsions
- Composition of the oleic phase. This includes asphaltene content and polarity of this fraction of the oil phase. Resins and waxes can also play an important role.
- Particle type. Hydrophobicity, size distribution and concentration appear to impact emulsion stability.
- Phase volume fractions. The general observation is that the most abundant phase usually becomes the continuous phase. Phase inversion occurs at a given phase concentration. In oil producing wells, as watercut reaches high values, O/W emulsions become more frequently encountered [38].
- Salinity of the brine phase. It is apparent that the overall Total Dissolved Solid concentration (TDS) impacts stability. However, the concentration of divalent cations appears to impact the stability of Pickering emulsions even more so that monovalent cations.
- pH of the aqueous phase. Yan and Masliyah [39] show that pH changes the wettability of clay particles and alters the droplet size. The value of pH also alters the surface charge of particles.
- Temperature.
- Stress conditions (pressure).
2.2.2. Organic Crude Oil Components
2.2.2.1. Asphaltenes
2.2.2.2. Resins
2.2.2.3. Organic Acids
3. Stability Proxies
3.1. Droplet Size Distribution Based Methods
3.1.1. Nuclear Magnetic Resonance (NMR)
- I.
- Gaussian phase distribution model (GPD)This method was first formulated by Murday and Cotts [68] and assumes diffusion following the gradient pulses is Gaussian phase distributed. The signal attenuation for the spherical cavity has a complex relationship with the radius of the sphere (droplet radius).
- II.
- Short Gradient Pulse (SGP) modelThis model assumes that the duration of the applied magnetic field gradient is zero. In the equations describe magnetic responses, the radius of the emulsion droplet is the only free parameter and all other parameters are determined in the experiment. Since emulsions are generally polydispersed, echo signals coming from different droplet sizes are superimposed. This can be solved by assuming the distribution function for droplet size. The measured signal in this case can be defined by the following integral:
3.1.2. Near-Infrared Spectroscopy (NIR)
3.1.3. Acoustic and Electro-Acoustic Techniques
- Viscous losses to shear waves generated by the particles oscillating in the acoustic pressure field. This is important mostly for particles smaller than the wavelength of ultrasound.
- Thermal mechanism. This is thermodynamic in nature and relates to resonance of sound waves in particles, which converts acoustic energy to thermal and causes attenuation of sound waves. This is important for deformable, soft particles, including emulsion droplets.
- Scattering mechanisms due to diffraction, reflection and refraction phenomena when sound waves interact with particles. This mechanism does not convert acoustic energy into other kinds of energy, but due to changes in direction, a portion of the wave cannot reach the transducer and as a result cannot be detected. At higher concentration particle-particle interaction and multiple scattering have to be considered.
- Intrinsic absorption of ultrasound by particles and medium due to interaction at the molecular level, which is not related to the particles size.
- Structural mechanism causes the acoustic attenuation in some structural network of particles.
- Electrokinetic mechanisms due to the interaction of ultrasound with electrical double layer of particles. Its contribution to acoustic attenuation is negligible, but this is the basis for electroacoustic.
3.1.4. Microscopy
- The resolution of the microscope, which refers to the ability to discriminate between two closely spaced points in the field of view of the microscope. Light waves emanating from two neighboring points can interfere with one another to produce a diffraction pattern of alternate light and dark bands especially when the distance between the points is comparable with the wavelength of the light being used. If these two points are the opposite edges of a particle, then the particle appears as an object of indeterminate shape surrounded by a dark rings [85]. Hence, the operational size domain is limited by the wavelength of the applied light and known as the Rayleigh limit [54]. The resolving power, dp, of a microscope can be calculated by:where n0 the refractive index of the medium and θ is the half angle subtended by the microscope objective at the focal plane. n0sinθ is known as the numerical aperture (N.A.). Applying a light with the lower wavelength or increasing the refractive index or θ, decrease dp, and improve resolution. Usually, visible light is used but by filling the region between the lens and the sample with the transparent oil (immersion oil) instead of air, the refractive index increases. Using wide angle lenses is also helpful, but the angle is limited by other optical problems like spherical and chromatic aberration [85].
- Optical properties of the sample. Sample must be transparent and the continuous phase and the droplets must have different optical properties to make them optically distinguishable such as different refractive indices and different colors [54].
3.1.5. Light Scattering and Diffraction
3.1.6. Small Angle Neutron Scattering
3.1.7. Differential Scanning Calorimetry
3.2. Phase Separation Based Methods
3.3. Electro-Coalescence
4. Discussion
| Basis | Method | Pros | Cons | Separation Step | EOR Applicability |
|---|---|---|---|---|---|
| Size Distribution | NMR (Nuclear Magnetic Resonance) | Easy to implement and fast. Non-invasive and non-destructive. Rich in useful information such as diffusion and compositional analysis. | Significant preparation steps are needed and interpretation can be complex. An external driving force is required for stiff emulsions, e.g., centrifugal force. | Coalescence | Applicable |
| NIR (Near-infrared Spectroscopy) | Simple and fast. | Reference and calibration techniques are required. Insensitive to minor constituents. An external driving force is required for stiff emulsions, e.g., centrifugal force. | Coalescence | Applicable | |
| Acoustic and Electro-Acoustic | Relatively simple to operate. It is not sensitive to optical density. | Large amount of sample and a complex interpretation model. An external driving force is required for stiff emulsions, e.g., centrifugal force. | Coalescence | Applicable if significant volume of sample is available. | |
| Microscopy | Direct observation | TEM and SEM are too complex. Optical microscopy is time consuming, except for automated systems. Dark emulsions are problematic. An external driving force is required for stiff emulsions, e.g., centrifugal force. | Coalescence | Applicable if automated methods are developed, but unless contrast techniques are used (fluorescence), not good for dark emulsions. | |
| Light Scattering and diffraction | DLS requires small sample volume. Both are simple. Fast and reliable. | DLS works well for small drop size and mostly for oil-in-water. LD needs a special solvent for W/O. An external driving force is required for stiff emulsions, e.g., centrifugal force. | Coalescence | DLS can be used for microemulsions (O/W). Light diffraction works well with macroemulsions. | |
| SANS (Small Angle Neutron Scattering) | Good for interfacial studies | It provides specific surface area rather than size. Complex technique. It requires specialized facility and preparation time. | Coalescence | Too complex to be practical. | |
| DSC | Differential Scanning Calorimetry | Simple to set up and relatively fast. Small sample volume. | A drop radius-freezing temp. calibration curves is needed. An external driving force is required for stiff emulsions, e.g., centrifugal force. | Coalescence | Not applicable as phase behavior (e.g., surfactants) depends on temperature |
| Gravity | Bottle Tests (Phase Separation) | Simple to implement and some standard techniques are available, but not directly as proxy of stability | Requires extreme accelerations (forces) for stiff emulsions, not attainable in traditional systems. Inaccurate and requires relatively large sample volume. | Phase Separation | Applicable, if emulsions can be broken. |
| Electric Cond. | Electro-coalescence | Relatively fast. Small sample volume can be used. It can provide significant volume of information. Very stiff emulsions can be studied. | Information on water content and size distribution may be needed to compare emulsions. | Coalescence | Applicable, but the technique is not as developed as other methods. |
5. Concluding Remarks
Acknowledgements
References and Notes
- Kim, Y.H.; Wasan, D.T.; Breen, P.J. A study of dynamic interfacial mechanisms for demulsification of water-in-oil emulsions. Colloids Surf. A 1995, 95, 235–247. [Google Scholar] [CrossRef]
- Li, M.; Lin, M.; Wu, Z.; Crhisty, A.A. The influence of NaOH on the stability of parafinnic crude oil emulsion. Fuel 2004, 84, 183–187. [Google Scholar] [CrossRef]
- Hirasaki, G.J.; Miller, C.A.; Raney, O.G.; Poindexter, M.K.; Nguyen, D.T.; Hera, J. Separation of produced emulsions from surfactant enhanced oil recovery processes. Energy Fuels 2011, 25, 555–561. [Google Scholar] [CrossRef]
- Huang, J.S.; Varadaraj, R. Colloid and interface science in the oil industry. Curr. Opin. Colloid Interface Sci. 1996, 1, 535–539. [Google Scholar] [CrossRef]
- Mandal, A.; Samanta, A.; Bera, A.; Ojha, K. Characterization of oil-water emulsion and its use in enhanced oil recovery. Ind. Eng. Chem. Res. 2010, 49, 12756–12761. [Google Scholar] [CrossRef]
- Bryan, J.; Kantzas, A. Enhanced Heavy-Oil Recovery by Alkali-Surfactant Flooding (SPE 110738). In Proceedings of the SPE Annual Technical Conference and Exhibition, Anaheim, CA, USA, 11–14 November, 2007.
- Bryan, J.; Mai, A.; Kantzas, A. Investigation into the Processes Responsible for Heavy Oil Recovery by Alkali-Surfactant Flooding (SPE 113993). In Proceedings of the SPE/DOE Improved Oil Recovery Symposium, Tulsa, OK, USA, 19–23 April, 2008.
- Bryan, J.; Kantzas, A. Potential for alkali-surfactant flooding in heavy oil reservoirs through oil-in-water emulsification. J. Can. Pet. Technol. 2009, 48, 37–46. [Google Scholar] [CrossRef]
- Mai, A.; Bryan, J.; Goodarzi, N.; Kantzas, A. Insights into non-thermal recovery of heavy oil. J. Can. Pet. Technol. 2009, 48, 27–35. [Google Scholar] [CrossRef]
- Zhang, H.; Dong, M.; Zhao, S. Which one is more important in chemical flooding for enhanced court heavy oil recovery, lowering interfacial tension or reducing water mobility? Energy Fuels 2010, 24, 1829–1836. [Google Scholar] [CrossRef]
- Cobos, S.; Carvalho, M.S.; Alvarado, V. Flow of oil-water emulsions through a constricted capillary. Int. J. Multiphase Flow 2009, 35, 507–515. [Google Scholar] [CrossRef]
- Liu, Q.; Dong, M.; Yue, X.; Hou, J. Synergy of alkali and surfactant emulsification of heavy oil in brine. Physicochem. Eng. Aspects 2006, 273, 219–228. [Google Scholar] [CrossRef]
- Liu, Q.; Dong, M.; Ma, S.; Tu, Y. Surfactant enhanced alkaline flooding for Western Canadian heavy oil. Physicochem. Eng. Aspects 2007, 293, 64–71. [Google Scholar]
- Lei, Z.; Yuan, S.; Song, J.; Yuan, J.; Wu, Y.-S. A Mathematical Model for Emulsion Mobilization and Its Effect on EOR During ASP Flooding (SPE 113145). In Proceedings of the SPE/DOE Improved Oil Recovery Symposium, Tulsa, Oklahoma, USA, 19–23 April, 2008.
- Zhang, Y.; Morrow, N.R. Comparison of Secondary and Tertiary Recovery with Change in Injection Brine Composition of Crude Oil/sandstone Combinations (SPE 99757). In Proceedings of the SPE/DOE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 22–26 April, 2006.
- Wang, X.; Alvarado, V. Direct current electrorheological stability determination of water-in-crude oil. J. Phys. Chem. B 2009, 113, 13811–13816. [Google Scholar] [CrossRef] [PubMed]
- Borges, B.; Rondon, M.; Sereno, O.; Asuaje, J. Breaking of water-in-crude oil emulsions. 3. Influence of salinity and water-oil ratio on demulsifier action. Energy Fuels 2009, 23, 1568–1574. [Google Scholar] [CrossRef]
- Arhuoma, M.; Yang, D.; Dong, M.; Li, H.; Idem, R. numerical simulation of displacement mechanisms for enhancing heavy oil recovery during alkaline flooding. Energy Fuels 2009, 23, 5995–6002. [Google Scholar] [CrossRef]
- Soo, H.; Radke, CJ. The flow mechanism of dilute, stable emulsions in porous-media. Ind. Eng. Chem. Fundam. 1984, 23, 342–347. [Google Scholar] [CrossRef]
- Soo, H.; Radke, C.J. A Filtration model for the flow of dilute, stable emulsions in porous-media.1. Theory. Chem. Eng. Sci. 1986, 41, 263–272. [Google Scholar] [CrossRef]
- Soo, H.; Williams, M.K.; Radke, C.J. A Filtration model for the flow of dilute, stable emulsions in Porous-Media.2. Parameter evaluation and estimation. Chem. Eng. Sci. 1986, 41, 263–272. [Google Scholar] [CrossRef]
- Kokal, S. Crude-oil emulsions: A state-of-the-art review. SPE Prod. Facil. 2005, 20, 5–13. [Google Scholar] [CrossRef]
- Kokal, S.; Wingrove, M. Emulsion Separation Index: From Laboratory to Field Case Studies (63165). In Proceedings of the SPE Annual Technical Conference and Exhibition, Dallas, TX, USA, October, 2000.
- Moradi, M.; Alvarado, V.; Huzurbazar, S.V. Effect of salinity on water-in-crude oil emulsion stability: Evaluation of drop-size distribution proxy. Energy Fuels 2011, 25, 260–268. [Google Scholar] [CrossRef]
- Pickering, S.U. Emulsions. J. Chem. Soc. 1907, 91, 2001–2021. [Google Scholar] [CrossRef]
- Alvarado, V.; Manrique, E. Enhanced Oil Recovery: Field Planning and Development Strategies; Elsevier, Gulf Publishing: Houston, TX, USA, 2010. [Google Scholar]
- Tang, G.Q.; Morrow, N.R. Influence of brine composition and fines migration on crude oil/brine/rock interactions and oil recovery. J. Pet. Sci. Eng. 1999, 24, 99–111. [Google Scholar] [CrossRef]
- Yan, Y.; Masliyah, J.H. Solids-stabilized oil-in-water emulsions: Scavenging emulsion droplets by fresh oil addition. Colloids Surf. A 1993, 75, 123–132. [Google Scholar] [CrossRef]
- Hunter, T.N.; Pugh, R.J.; Franks, G.V.; Jameson, G.J. The role of particles in stabilizing foams and emulsions. Adv. Colloid Interface Sci. 2008, 137, 57–81. [Google Scholar] [CrossRef] [PubMed]
- Arditty, S.; Schmitt, V.; Giermanska-Kahn, J.; Leal-Calderon, F. Materials based on solid-stabilized emulsions. J. Colloid Interface Sci. 2004, 275, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Binks, B.P. Particles as surfactants–similarities and differences. Curr. Opin. Colloid Interface Sci. 2000, 7, 21–41. [Google Scholar] [CrossRef]
- Yekeler, M.; Ulusoy, U.; Hicyilmaz, C. Effect of particle shape and rougness of talk mineral ground by different mills on the wettability and flotability. Powder Technol. 2004, 140, 68–78. [Google Scholar] [CrossRef]
- Langevin, D.; Poteau, S.; Hénaul, I.; Argillier, J.F. Crude oil emulsion properties and their application to heavy oil transportation. Revue de l'Institut Français du Pétrole 2004, 59, 511–521. [Google Scholar] [CrossRef]
- Moran, K.; Yeung, A.; Masliyah, J. The viscoplastic properties of crude oil-water interfaces. Chem. Eng. Sci. 2006, 61, 6016–6028. [Google Scholar] [CrossRef]
- Yaghi, B. Rheology of oil-in-water emulsions containing fine particles. J. Pet. Sci. Eng. 2003, 40, 103–110. [Google Scholar] [CrossRef]
- Sztukowski, D.M.; Yarranton, H.W. Oilfield solids and water-in-oil emulsion stability. J. Colloid Interface Sci. 2005, 285, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Tambe, D.E.; Sharma, M.M. Factors controlling the stability of colloid-stabilized emulsions. J. Colloid Interface Sci. 1993, 157, 244–253. [Google Scholar] [CrossRef]
- Janssen, P.H.; van den Broek, W.M.G.T.; Harris, C.K. Laboratory study investigating emulsion formation in the near-wellbore region of a high water-cut oil well. SPE J. 2001, 6, 71–79. [Google Scholar] [CrossRef]
- Yan, N.; Masliyah, J.H. Effect of pH on adsorption and desorption of clay particles at oil-water interface. J. Colloid Interface Sci. 1996, 181, 20–27. [Google Scholar] [CrossRef]
- McLean, J.D.; Kilpatrick, P.K. Effects of asphaltene solvency on stability of water-in-crude-oil emulsions. J. Colloid Interface Sci. 1997, 189, 242–253. [Google Scholar] [CrossRef]
- Grutters, M.; van Dijk, M.; Dubey, S.; Adamski, R.; Gelin, F.; Cornelisse, P. Asphaltene induced W/O emulsion: False or true? J. Dispersion Sci. Technol. 2007, 28, 357–360. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, Z.; Xu, Z.; Masliyah, J. Bitumen-clay interactions in aqueous media studied by zeta potential distribution measurement. J. Colloid Interface Sci. 2002, 252, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Sztukowski, D.M.; Yarranton, H.W. Characterization and interfacial behavior of oil sands solids implicated in emulsion stability. J. Dispersion Sci. Technol. 2004, 25, 299–310. [Google Scholar] [CrossRef]
- Tsamantakis, C.; Masliyah, J.; Yeung, A.; Gentzis, T. The behavior of micro-bitumen drops in aqueous clay environments. J. Colloid Interface Sci. 2005, 288, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Ngai, T.; Auweter, H.; Behrens, S.H. Environmental responsiveness of microgel particles and particle-stabilized emulsions. Macromolecules 2006, 39, 8171–8177. [Google Scholar] [CrossRef]
- Kruglyakov, P.M.; Nushtayeva, A.V. Investigation of the influence of capillary pressure on stability of a thin layer emulsion stabilized by solid particles. Colloids Surf. A 2005, 263, 330–335. [Google Scholar] [CrossRef]
- Yang, F.; Niu, Q.; Lan, Q.; Sun, D. Effect of dispersion ph on the formation and stability of pickering emulsions stabilized by layered double hydroxides particles. J. Colloid Interface Sci. 2007, 306, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Thieme, J.; Abend, S.; Lagaly, G. Aggregation in pickering emulsions. Colloid Polym. Sci. 1999, 277, 257–260. [Google Scholar] [CrossRef]
- Horozov, T.S.; Binks, B.P. Particle-stabilized emulsions: A bilayer or a bridging monolayer? Angew. Chem. Int. Ed. 2006, 45, 773–776. [Google Scholar] [CrossRef]
- Yarranton, H.W.; Sztukowske, D.M.; Urrutia, P. Effect of interfacial rheology on model emulsion coalescence I. Interfacial rheology. J. Colloid Interface Sci. 2007, 310, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.R. Crude oil waxes, emulsions, and asphaltenes. In Crude Oil Waxes, Emulsions & Asphaltenes; Pennwell Books: Houston, TX, USA, 1997. [Google Scholar]
- Sjöblom, J. Encyclopedic Handbook of Emulsion Technology; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Tan, X.; Fenniri, H.; Gray, M.R. Water enhances the aggregation of model asphaltenes in solution via hydrogen bonding. Energy Fuels 2009, 23, 3687–3693. [Google Scholar] [CrossRef]
- McAuliffe, C.D. Crude-oil-in-water emulsions to improve fluid flow in an oil reservoir. J. Pet. Technol. 1973, 25, 721–726. [Google Scholar] [CrossRef]
- Czarnecki, J.; Moran, K. On the stabilization mechanism of water-in-oil emulsions in petroleum systems. Energy Fuels 2005, 19, 2074–2079. [Google Scholar] [CrossRef]
- Ali, M.F.; Alqam, M.H. The role of asphaltenes, resins and other solids in the stabilization of water in oil emulsions and its effects on oil production in saudi oil fields. Fuel 2000, 79, 1309–1316. [Google Scholar] [CrossRef]
- Xu, Y.; Dabros, T.; hamza, H.; Shelfantook, W.E. Destabilization of Water in Bitumen Emulsion by Washing with Water. Pet. Sci. Technol. 1999, 17, 1051. [Google Scholar] [CrossRef]
- Yang, X.; Verruto, V.J.; Kilpatrick, P.K. Dynamic asphaltene-resin exchange at the oil/water interface: Time-dependent W/O emulsion stability for asphaltene/resin model oils. Energy Fuels 2007, 21, 133–1349. [Google Scholar]
- Brandal, Ø.; Hanneseth, A.-M.D.; Sjöblom, J. Interactions between synthetic and indigenous naphthenic acids and divalent cations across oil-water interfaces: Effects of addition of oil-soluble non-ionic surfactants. Colloid Polym. Sci. 2005, 284, 124–133. [Google Scholar]
- Mohammed, M.A.; Sorbie, K.S. Naphthenic acid extraction and characterization from napthenate field deposits and crude oils using ESMS and APCI-MS. Colloids Surf. A 2009, 349, 1–18. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Sorbie, K.S. Thermodynamic modelling of calcium naphthenate formation: Model predictions and experimental results. Colloids Surf. A 2010, 369, 1–10. [Google Scholar] [CrossRef]
- Wang, X.Y.; Brandvik, A.; Alvarado, V. Probing interfacial water-in-crude oil emulsion stability controls using electrorheology. Energy Fuels 2010, 24, 6359–6365. [Google Scholar] [CrossRef]
- Abraham, R.J.; Fisher, J.; Loftus, P. Introduction to NMR Spectroscopy; John Wiley and Sons: Hoboken, NJ, USA, 1988. [Google Scholar]
- Sjöblom, J.; Aske, N.; Auflem, I.H.; Brandal, Ø.; Havre, T.E.; Saether, O.; Westvik, A.; Johnsen, E.E.; Kallevik, H. Our current understanding of water-in-crude oil emulsions. Recent characterization techniques and high pressure performance. Adv. Colloid Interface Sci. 2003, 100, 399–473. [Google Scholar] [CrossRef]
- Tanner, J.E.; Stejskal, E.O. Restricted diffusion of protons in colloidal systems by the pulsed field gradient method. J. Phys. Chem. 1968, 49, 1768–1777. [Google Scholar] [CrossRef]
- Johns, M.L. NMR studies of emulsions. Curr. Opin. Colloid Interface Sci. 2009, 14, 178–183. [Google Scholar] [CrossRef]
- Packer, K.J.; Rees, C. Pulsed NMR studies of restricted diffusion. I. Droplet size distributions in emulsions. J. Colloid Interface Sci. 1972, 40, 206–218. [Google Scholar] [CrossRef]
- Murday, J.S.; Cotts, J.M. Self-diffusion coefficient of liquid lithium. J. Chem. Phys. 1968, 48, 4938–4945. [Google Scholar] [CrossRef]
- Peña, A.A.; Hirasaki, G.J. Enhanced characterization of oilfield emulsions via NMR diffusion and transverse relaxation experiments. Adv. Colloid Interface Sci. 2003, 105, 103–150. [Google Scholar] [CrossRef] [PubMed]
- Garasanin, T.; Cosgrove, T.; Marteaux, L.; Kretschmer, A.; Goodwin, A.; Zick, K. NMR self-diffusion studies on PDMS oil-in-water emulsion. Langmuir 2002, 18, 10298–10304. [Google Scholar] [CrossRef]
- Balinov, B.; Urdahl, O.; Söderman, O.; Sjöblom, J. Characterization of water-in-crude oil emulsions by the NMR self-diffusion technique. Colloids Surf. A 1994, 82, 173–181. [Google Scholar] [CrossRef]
- Aichele, C.P.; Flaum, M.; Jiang, T.; Hirasaki, G.J.; Chapman, W.G. Water in oil emulsion droplet size characterization using a pulsed field gradient with diffusion editing (PFG-DE) NMR technique. J. Colloid Interface Sci. 2007, 315, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Merkus, H.G. Particle size Measurements; Springer: Berlin, Germany, 2009. [Google Scholar]
- Siesler, H.W.; Ozaki, Y.; Kawata, S.; Heis, H.M. Near-Infrared Spectroscopy; Wiley-VCH: Hoboken, NJ, USA, 2002. [Google Scholar]
- Araujo, A.M.; Santos, L.M.; Fortuny, M.; Melo, R.; Coutinho, R.C.C.; Santos, A.F. Evaluation of water content and average droplet size in water-in-crude oil emulsions by means of near-infrared spectroscopy. Energy Fuels 2008, 22, 3450–3458. [Google Scholar] [CrossRef]
- Bohren, C.F.; Huffman, D.R. Absorption and Scattering of Light by Small Particles; Wiley-VCH: Weinheim, Germany, 2004; Chapter 11; pp. 287–324. [Google Scholar]
- Collins, E.A. Emulsion Polymerization and Emulsion Polymers; Lovell, P.A., El-Aasser, M.S., Eds.; John Wiley and Sons: Chichester, UK, 1997; Chapter 12; pp. 385–436. [Google Scholar]
- Kallevik, H.; Hansen, S.B.; Sæther, Ø.; Kvalheim, O.M.; Sjöblom, J. Crude oil model emulsion characterised by means of near infrared spectroscopy and multivariate techniques. J. Dispersion Sci. Technol. 2000, 21, 245–262. [Google Scholar] [CrossRef]
- Paso, K.; Kallevik, H.; Sjöblom, J. Measurement of wax appearance temperature using near-infrared (NIR) scattering. Energy Fuels 2009, 23, 4988–4994. [Google Scholar] [CrossRef]
- Kallevik, H.; Hansen, S.B.; Saether, O.; Kvalheim, O.M.; Sjöblom, J. Crude oil model emulsion characterised by means of near infrared spectroscopy and multivariate techniques. J. Dispersion Sci. Technol. 2000, 21, 245–262. [Google Scholar] [CrossRef]
- Dukhin, A.S.; Goetz, P.J. Acoustic and electroacoustic spectroscopy for characterizing concentrated dispersions and emulsions. Adv. Colloid Interface Sci. 2001, 92, 73–132. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, E.E.; Huang, H.; Babchin, A.J.; Chow, R.S. Electroacoustic method for monitoring the coalescence of water-in-oil emulsions. Colloids Surf. 1990, 46, 177–192. [Google Scholar] [CrossRef]
- Marques, L.C.D.; de Oliveira, J.F.; Gonzalez, G. Investigation on the role of asphaltenes as stabilizers for toluene-in-water miniemulsions using an electrosonic analysis (ESA) method. J. Dispersion Sci. Technol. 1997, 18, 477–488. [Google Scholar] [CrossRef]
- Boxall, J.A.; Koh, C.A.; Sloan, E.D.; Sum, A.K.; Wu, D.T. Measurement and calibration of droplet size distributions in water-in-oil emulsions by particle video microscope and a focused beam reflectance method. Ind. Eng. Chem. Res. 2010, 49, 1412–1418. [Google Scholar] [CrossRef]
- Hunter, J. Foundations of Colloid Science, 2nd ed.; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Binks, B.P.; Rocher, A. Effects of temperature on water-in-oil emulsions stabilised solely by wax microparticles. J. Colloid Interface Sci. 2009, 335, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Eley, D.D.; Hey, M.J.; Symonds, J.D. Emulsions of water in asphaltene-containing oils 1. Droplet size distribution and emulsification rates. Colloids Surf. 1988, 32, 87–101. [Google Scholar] [CrossRef]
- Nandi, A.; Agterof, W.G.M.; van den Ende, D.; Mellema, J. Investigation of the effect of a simple salt on the kinetics of gravity induced coalescence for a viscosity matched emulsion system. Colloids Surf. A 2003, 213, 199–208. [Google Scholar] [CrossRef]
- Gelot, A.; Friesen, W.; Hamza, H.A. Emulsification of oil and water in the presence of finely divided solids and surface-active agents. Colloids Surf. 1984, 12, 271–303. [Google Scholar] [CrossRef]
- Sullivan, A.P.; Zaki, N.N.; Sjoblom, J.; Kilpatrick, P.K. The stability of water-in-crude and model oil emulsions. Can. J. Chem. Eng. 2007, 85, 793–807. [Google Scholar] [CrossRef]
- Jokela, P.; Fletcher, P.D.I.; Aveyard, R.; Lu, J.-R. The use of computerized microscopic image analysis to determine emulsion droplet size distributions. J. Colloid Interface Sci. 1990, 134, 417–426. [Google Scholar] [CrossRef]
- Mason, S.L.; May, K.; Hartland, S. Drop size and concentration profile determination in petroleum emulsion separation. Colloids Surf. A 1995, 96, 85–92. [Google Scholar] [CrossRef]
- De Oliveira, M.C.K.; Carvalho, R.M.; Carvalho, A.B.; Couto, B.C.; Faria, F.R.D.; Cardoso, R.L.P. Waxy crude oil emulsion gel: Impact on flow assurance. Energy Fuels 2009, 24, 2287–2293. [Google Scholar] [CrossRef]
- Cazabat, A.M.; Langevin, D.; Pouchelon, A. Light-scattering study of water-oil microemulsions. J. Colloid Interface Sci. 1980, 73, 1–12. [Google Scholar] [CrossRef]
- Novales, B.; Papineau, P.; Sire, A.; Axelos, M.A.V. Characterization of emulsions and suspensions by video image analysis. Colloids Surf. A 2003, 221, 81–89. [Google Scholar] [CrossRef]
- Miller, D.J.; Böhm, R. Optical studies of coalescence in crude oil emulsions. J. Pet. Sci. Eng. 1993, 9, 1–8. [Google Scholar] [CrossRef]
- Willis, B.T.M.; Carlile, C.J. Experimental Neutron Scattering; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Verruto, V.J.; Kilpatrick, P.K. Water-in-model oil emulsion studied by small-angle neutron scattering: Interfacial film thickness and composition. Langmuir 2008, 24, 12807–12822. [Google Scholar] [CrossRef] [PubMed]
- Gawrys, K.L.; Blankenship, G.A.; Kilpatrick, P.K. Solvent entrainment in and flocculation of asphaltenic aggregates probed by small-angle neutron scattering. Langmuir 2006, 22, 4487–4497. [Google Scholar] [CrossRef] [PubMed]
- Gawrys, K.L.; Blankenship, G.A.; Kilpatrick, P.K. On the distribution of chemical properties and aggregation of solubility fractions in asphaltenes. Langmuir 2006, 22, 4487–4497. [Google Scholar] [CrossRef] [PubMed]
- Verruto, V.J.; Kilpatrick, P.K. Preferential solvent partitioning within asphaltenic aggregates dissolved in binary solvent mixtures. Energy Fuels 2007, 21, 1217–1225. [Google Scholar] [CrossRef]
- Jestin, J.; Simon, S.B.; Zupancic, L.; Barre, L. A small angle neutron scattering study of the adsorbed asphaltene layer in water-in-hydrocarbon emulsions: Structural description related to stability. Langmuir 2007, 23, 10471–10478. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, P.A.; Gilbert, E.P.; White, E.P.J. High internal phase water-in-oil emulsions studied by small-angle neutron scattering. J. Phys. Chem. B 2000, 104, 7012–7022. [Google Scholar] [CrossRef]
- Höhne, G.; Hemminger, W.; Flammersheim, H.J. Differential Scanning Calorimetry; Springer: Berlin, Germany, 1996. [Google Scholar]
- Clausse, D.; Gomez, F.; Dalmazzone, C.; Noik, C. A method for the characterization of emulsion, Thermogranulometry: Application to water-in-crude oil emulsion. J. Colloid Interface Sci. 2005, 287, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Dalmazzone, C.; Noik, C.; Clausse, D. Application of DSC for emulsified system characterization. Oil Gas Sci. Technol. 2009, 64, 543–555. [Google Scholar] [CrossRef]
- Abivin, P.; Henaut, I.; Chaudemanche, C.; Argillier, J.F.; Chinesta, F.; Maan, M. Dispersed systems in heavy crude oils. Oil Gas Sci. Technol. 2009, 64, 557–570. [Google Scholar] [CrossRef]
- Follotec, A.L.; Pezron, I.; Noik, C.; Dalmazzone, C.; Metlas-Komunjer, L. Triblock copolymer as destabilizer of water-in-crude oil emulsion. Colloid Surf. A 2010, 365, 162–170. [Google Scholar] [CrossRef]
- Frising, T.; Noïk, C.; Dalmazzone, C. The liquid/liquid sedimentation process: From droplet coalescence to technologically enhanced water/oil emulsion gravity separators: A review. J. Dispersion Sci. Technol. 2006, 27, 1035–1057. [Google Scholar] [CrossRef]
- Hemmingsen, P.V.; Silset, A.; Hannisdal, A; Sjöblom, J. Emulsions of heavy crude oils. I: Influence of viscosity, temperature, and dilution. J. Dispersion Sci. Technol. 2005, 26, 615–627. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Alvarado, V.; Wang, X.; Moradi, M. Stability Proxies for Water-in-Oil Emulsions and Implications in Aqueous-based Enhanced Oil Recovery. Energies 2011, 4, 1058-1086. https://doi.org/10.3390/en4071058
Alvarado V, Wang X, Moradi M. Stability Proxies for Water-in-Oil Emulsions and Implications in Aqueous-based Enhanced Oil Recovery. Energies. 2011; 4(7):1058-1086. https://doi.org/10.3390/en4071058
Chicago/Turabian StyleAlvarado, Vladimir, Xiuyu Wang, and Mehrnoosh Moradi. 2011. "Stability Proxies for Water-in-Oil Emulsions and Implications in Aqueous-based Enhanced Oil Recovery" Energies 4, no. 7: 1058-1086. https://doi.org/10.3390/en4071058
APA StyleAlvarado, V., Wang, X., & Moradi, M. (2011). Stability Proxies for Water-in-Oil Emulsions and Implications in Aqueous-based Enhanced Oil Recovery. Energies, 4(7), 1058-1086. https://doi.org/10.3390/en4071058