1. Introduction
Anaerobic digestion of organic waste or agricultural residues can be performed in technically simple digesters. Users of such digesters can benefit from the obtained biogas as fuel for cooking while the digestate can be applied as fertilizer [
1,
2,
3]. In China and India, more than 30 million household digesters have been built up to 2012 [
4]. Floating drum and fixed dome digesters are among the most common models [
5], and are designed for treating liquid substrates, typically manure or diluted dung. Dissemination of domestic biogas plant is therefore often limited to regions with good water availability and to households with stabled cattle [
6,
7]. A successful operation of a domestic anaerobic digester depends on the availability and ease of supply of substrates and water. The net benefits for the users/owners can depend strongly on the workload associated with the digester in particular with water supply [
3]. In Africa, only about 26,000 digesters are in operation [
5], while many households are facing problems with energy supply as the availability of traditional fuels such as firewood is declining [
8]. The development and dissemination of domestic digesters with a reduced water demand and a process independent of stabled livestock could help to bring the benefits of anaerobic digestion to regions which until now have been excluded from domestic biogas applications.
Solid state anaerobic digestion (SSAD) has been considered as an alternative process in order to meet the above mentioned constraints [
9]. SSAD is defined as an anaerobic digestion (AD) process where the substrate is stackable, typically with a solid content above 20% [
10]. With SSAD, the process water demand can be reduced significantly when compared to a liquid AD process. Further, in SSAD digesters floating layer problems are avoided, which allows the use of fibrous substrates. Additional benefits of SSAD are a safe and easy handling of digestate as the solid consistency simplifies storage and distribution on fields [
11]. SSAD also allows retaining nitrogen by establishing a permanent liquid phase in the bottom of the digester [
12]. Thus, nitrogen deficiency can also be avoided when using substrates with a high C:N ratio and thereby reducing the dependency on nitrogen-rich substrates such as animal manure. Moreover, the higher dry matter content in SSAD digesters allows treating the same amount of biomass in a smaller digester.
However, SSAD implies some drawbacks when compared to liquid AD: liquid substrates allow continuously run processes with a steady gas flow. On the contrary, a single SSAD digester cannot provide a steady gas flow as the use of solid substrates requires batch mode operation. Thus, parallel operation of several off-set run digesters is necessary in order to obtain an even gas production. Moreover, the low water content in SSAD can limit mass transfer of intermediate compounds in the digester, which can significantly slow down the digestion process [
13]. Additionally, the high risk of irreversible acidification requires a high inoculum to substrate (I/S) ratio [
14]. In order to accelerate mass transfer and reduce the risk of acidification SSAD is often operated in two-stage digesters and/or with leachate recirculation [
15,
16].
In this study, yam peelings, which are an abundant organic residue in Ghana, were used as a model substrate for SSAD in lab scale batch digesters. The aim of this work was to explore the possibilities of operating SSAD in technically simple household digesters, which can operate reliably without process features such as leachate recirculation or two-stage digestion. For this, different inoculation strategies were tested with respect to their effect on biogas yield, production rate and process acidification. The inoculation strategies included bottom inoculation (Bot), with solid seeding material in the bottom of the digester and substrate placed on top, multi-layered inoculation (Lay), where seeding material and substrate were distributed in alternating layers and completely mixed inoculation (Mix). Moreover, the effect of cow dung addition was tested in combination with the different inoculation strategies. An application scenario based on the experimental results of this study exemplifies a respective implementation concept which is compared to a conventional fixed dome digester.
2. Materials and Methods
2.1. Substrate and Inoculum
Ghanaian origin white yams (Dioscorea rotundata) obtained from a local grocery store were peeled manually. The peelings had an average particle size of 2.5 cm and accounted for 12% of the original vegetable mass. The peelings were soaked in cold water for 1 h in order to provide sufficient humidity for the SSAD process. Prior to soaking, the maximum water holding capacity of the peelings was determined and a corresponding volume of soaking water was applied to avoid draining water and loss of soluble compounds.
Fresh cow dung was collected from a cattle farm in Jutland, Denmark. Wheat straw (Triticum avaestivum L.) was harvested in Skærbæk, Denmark, chopped to approximately 5 cm pieces and dried (90% total solids (TS)). The wheat straw was pre-inoculated using digested municipal sewage sludge as seeding material. The sludge was collected from a local waste water plant (Mølleåværket, Lyngby, Denmark) where it was treated in an anaerobic digester at 37 °C. For pre-inoculation, 1 kg of wheat straw was incubated with 3 L of sludge at 28 °C for 30 days.
2.2. Biomethane Potential Test
Biomethane potentials were determined in batch trials. Triplicates of each sample were distributed in 1000 mL serum bottles in amounts of 1.0 g volatile solids (VS). The bottles were inoculated with 100 g of inoculum (digested sewage sludge). Water was added to each bottle to reach a total active volume of 200 mL. For subtraction of methane produced by the inoculum, vials only containing inoculum and water were also set up. In order to obtain anaerobic conditions the headspace of the flasks was flushed for 40 s with a gas mixture of N2/CO2 (80%/20%). All bottles were closed with butyl rubber septa and incubated for a period of 60 days. Incubation temperature was set to 28 °C, to mimic the conditions in a digester operated at ambient temperature in Ghana.
2.3. Experimental Set-up of the SSAD Experiments
The SSAD experiments were carried out in triplicates and performed in 1000 mL PVC (Polyvinyl chloride) bottles sealed with silicon stoppers with a custom made gas outlet. The bottles were incubated in a temperature controlled water bath at 28 °C (see
Section 2.2). The incubation times were 89 days and 61 days for the first and the second run, respectively. The duration of the second run was shortened due to a faster conversion. Gas production was measured in an automatic methane potential test system (Bioprocess Control, Lund, Sweden) equipped with a panel of 15 liquid displacement units.
2.4. Experimental Design of the SSAD Experiments
The SSAD experiments were designed to test different inoculation strategies applicable in low-tech batch digestion and were carried out in two consecutive runs. The first run comprised five series applying different combinations of yam peelings, pre-inoculated wheat straw and/or cow dung. The consecutive second run involved four series in which yam peelings were incubated using digestate from the first run as seeding material. Pre-inoculated wheat straw and digestate from the first run are referred to as solid inoculum (SI) in the first and second run, respectively.
2.4.1. Stratified and Mixed Inoculation Strategies
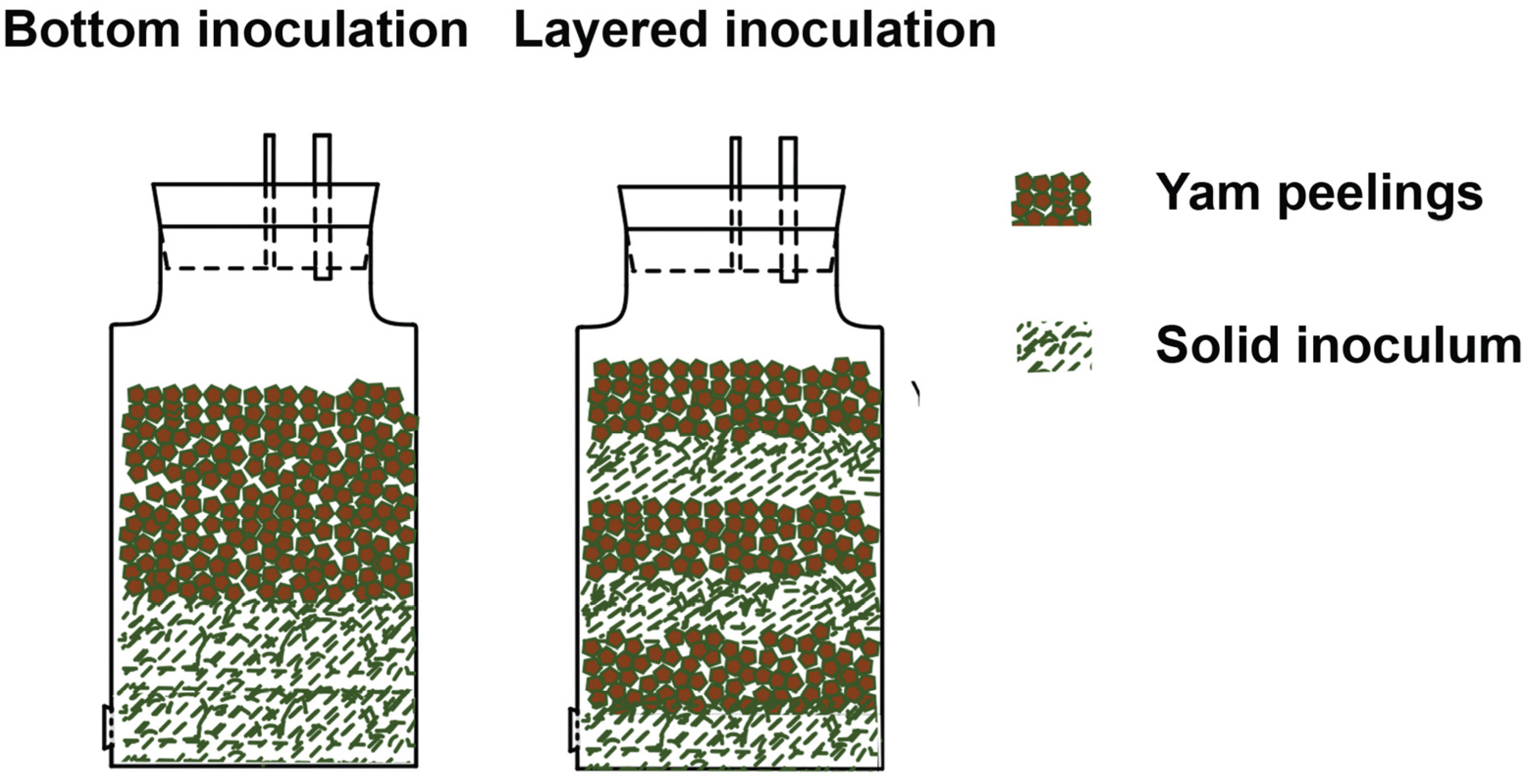
Both runs included bottom inoculation, multi-layered inoculation and mixed inoculation. Bottom inoculation featured a bed of SI placed in the bottom of the digester, with yam peelings as substrate placed on top of the bed (see
Figure 1). Both layers had a height of 6 cm. In the case of multi-layered inoculation the digesters were loaded with alternating layers of SI and substrate, each with approximately 2 cm thickness. In the case of mixed inoculation, the yam peelings were completely mixed with SI (or dung) prior to incubation.
Figure 1.
Bottom inoculation and multi-layered inoculation in the SSAD experiment.
Figure 1.
Bottom inoculation and multi-layered inoculation in the SSAD experiment.
2.4.2. Inocula, Cow Dung Addition and Batch Loads
In the first run, pre-digested wheat straw was used as SI for bottom (
Bot) and multi-layered (
Lay) inoculation (
Table 1). Both strategies were additionally tested with cow dung as a supportive biomass in addition to SI (
Bot + Dung and
Lay + Dung). The dung was mixed with yam peelings prior to incubation. The first run also involved one series in which no SI was applied (
MixDung), instead, dung served as seeding material.
In the second run, digestate from the first run was applied as inoculum. Digestate taken from the bottom of Bot and Bot + Dung served as SI in Bot II and Bot II (Dung), respectively. Lay II (Dung) was inoculated with digestate from Lay + Dung which consisted of a mixture of digestion residues from all layers. The mixed inoculation (Mix II (Dung)) was carried out with digestate from Bot + Dung. The second run did not involve addition of dung. Labels with “dung” in parentheses state that the applied SI originated from a first run series with dung addition. The residues from Lay and MixDung were not used as inoculum in the second run as the material was inactive due to acidification during the first run.
Table 1.
Inoculation strategies and batch loads in the SSAD experiment.
Table 1.
Inoculation strategies and batch loads in the SSAD experiment.
| Label | Inoculation Strategy | Inoculum Material | Number of Layers | Yam Peels (g) a | Inoculum (g) a | Dung (g) a | TS (%) | S/I Ratio (TS Based) |
|---|
| First run |
| Bot | Bottom inoculation | Inoculated straw | 2 | 205 | 170 | - | 18.7 | 2.0 |
| Lay | Layered inoculation | Inoculated straw | 6 | 205 | 170 | - | 18.6 | 2.0 |
| Bot + Dung | Bottom inoculation with dung addition | Inoculated straw | 2 | 205 | 170 | 125 | 17.6 | 2.0 |
| Lay + Dung | Layered inoculation with dung addition | Inoculated straw | 6 | 205 | 170 | 125 | 17.6 | 2.0 |
| MixDung | Substrate premixed with dung, no additional inoculum | Cow dung | 1 | 205 | - | 343 | 17.5 | 0.9 b |
| Second run |
| Bot II | Bottom inoculation with adapted digestate | Digestate from Bot | 2 | 205 | 170 | - | 18.0 | 2.3 |
| Bot II (Dung) | Bottom inoculation with adapted digestate | Digestate from Bot + Dung | 2 | 205 | 170 | - | 18.3 | 2.1 |
| Lay II (Dung) | Layered inoculation with adapted digestate | Digestate from Lay + Dung | 6 | 205 | 170 | - | 17.8 | 2.3 |
| Mix II (Dung) | Substrate pre-mixed with adapted digestate | Digestate from Bot + Dung | 1 | 205 | 170 | - | 18.3 | 2.1 |
2.5. Analytical Methods
TS and VS were determined using standard methods [
17]. The accumulating methane production in the biomethane potential test was measured by gas chromatography (GC). For that, 0.5 mL gas samples were taken from the headspace of the batch flasks using a syringe with pressure lock. The pressure lock was closed after the needle of the syringe had penetrated the septum, making it possible to sample a fixed volume of gas at the actual pressure in the headspace of the batch flasks. From the fixed volume sampled and the measured mass of methane in the sample, the amount of methane in the headspace of the flasks was calculated. The gas samples were analyzed with a Shimadzu 8A gas chromatograph (Shimadzu, Kyoto, Japan) featuring a 2 m Porapak-Q column. The gas chromatograph was run with nitrogen carrier at 130 °C and employed a flame ionization detector. The volume of the total gas production in the SSAD batch reactors was measured by an automated metering system based on the principle of liquid displacement (Bioprocess Control) [
18]. CH
4 and CO
2 concentrations in the produced biogas were determined by gas chromatography using a Mikrolab ML GC 82 (Mikrolab, Aarhus, Denmark) with thermal conductivity detection using helium carrier and a Porapak-Q column at 70 °C. For GC analysis, 1.0 mL samples were taken from the headspace of the SSAD reactors with a gas tight syringe. Biogas and methane production is given in normalized liters (at 273 K (
TStandard) and 1013 mbar (
PStandard)). For normalization of the measured volume of methane (
Vm), ambient temperature (
Tm) and pressure (
Pm) were recorded at every measurement. The volume of methane in norm liters (
Vn) was calculated according to:
For volatile fatty acids (VFA) determination, acetate, propionate, butyrate, isobuyrate, valerate and isovalerate were measured and summarized as total VFA. 1 mL samples were acidified with 3 M sulphuric acid until a pH below 2.5 was obtained, centrifuged at 3920× g for 20 min, and analyzed on HP 6890 GC system (Hewlett-Packard, Palo Alto, CA, USA) equipped with flame ionization detector. The pH was measured with a Meterlab pHM210 meter (Radiometer Analytical, Lyon, France). For determination of chemical oxygen demand (COD), total N and P, biomass samples were diluted with 400 mL H2O/g TS and homogenized in a wet mill. The measurements were carried out with a Hach Lange DR 2800 spectrometer (Hach Lange, Düsseldorf, Germany) using Hach Lange LCK test kits 514, 338 and 350, for COD, total N and total P, respectively.
3. Results and Discussion
3.1. Biomass Characterization
The characteristics of the used biomasses (cow dung, solid inoculum and yam peelings) are shown in
Table 2 together with the biomethane potential (BMP). Both solid inoculum and yam peelings are above the favorable COD/N range for AD processes of 20–30 [
19], while cow dung had COD/N ratio below that range. The BMP of yam peelings is considered sufficiently high with respect to its feasibility as substrate for biogas production [
20]. The BMP of the solid inoculum indicates that a considerable amount of convertible solids in the straw was still present after the incubation with sewage sludge.
Table 2.
Biomass characteristics.
Table 2.
Biomass characteristics.
| Parameter | Cow Dung | Solid Inoculum | Yam Peelings |
|---|
| TS (%) | 14.4 ± 0.2 | 13.6 ± 0.5 | 22.8 ± 0.2 |
| VS (%) a | 11.8 ± 0.2 | 12.6 ± 0.6 | 22.12 ± 0.2 |
| COD (g O2/kg) | 155 ± 7.8 | 142 ± 1.4 | 240 ± 3.4 |
| Total N (g/kg TS) | 62.3 ± 0.6 | 22.0 ± 0.8 | 19.1 ± 0.6 |
| Total P (g/kg TS) | 20.8 ± 1.25 | 7.35 ± 0.7 | 3.18 ± 0.2 |
| COD/N ratio | 17 | 47 | 55 |
| BMP (mL CH4/g VS) | 218 ± 22 | 151 ± 18 | 271 ± 17 |
3.2. Performance of the First SSAD Run
The objective of the first SSAD run was to evaluate the effect of the different inoculation strategies on the conversion dynamics and methane yields when using non-adapted inoculum in an initial process start-up. For that, the accumulative methane production in the SSAD batches was monitored and compared. As illustrated in
Figure 2, initial cow dung addition increased the methane yield significantly (compare
Bot and
Bot + Dung). A further increase could be achieved by layered inoculation with dung addition;
Bot + Dung and
Lay + Dung achieved methane yields of 116 ± 7 mL/g VS and 143 ± 4 mL/g VS, respectively. Layered inoculation aimed at increasing the degradation rate by increasing the contact area between seeding material and substrate. The substantial yield increase suggests that this approach supported the digestion process significantly when combined with the addition of dung. However, in the absence of dung (
Bot and
Lay), the AD performance was poor and layered inoculation had no significant effect on the yield.
Figure 2.
Accumulative methane yields from SSAD of yam peelings in the first run (A) and second run (B).
Figure 2.
Accumulative methane yields from SSAD of yam peelings in the first run (A) and second run (B).
After finalizing the incubation experiments each layer was subjected for further chemical studies (
Table 3). The pH in
Bot was below the range (6.5 to 8.5) in which methanogenic activity can occur [
21]. Further, dissolved COD and VFA concentrations indicate that hydrolysis and acidification have taken place, but further conversion to biogas failed as the methane yield was negligible.
The analysis of
Bot + Dung indicates that the top layer, which consisted of yam peelings and dung, was also subjected to acidification. On the contrary, the bottom layer had a favorable pH (7.69) and low dissolved COD and VFA concentrations, which suggests that methanogenic activity had only taken place in the bottom layer of
Bot + Dung. Nonetheless, the VS concentration in the top layer was reduced by 32% (data not shown), despite of the low pH. This was possibly achieved by a mass transfer between the layers, where hydrolyzed substrate from the top percolated into the active bottom layer, where it could be converted to biogas. The increased stability of the pH in
Bot + Dung suggests that dung also increased the buffer capacity [
22]. However, the effect of dung was only evident in the bottom layer, although dung was added to the top layer. Thus, it can be assumed that dung had partially percolated into the bottom layer.
Table 3.
Accumulative methane yields and chemical analysis of layers in the batch reactors.
Table 3.
Accumulative methane yields and chemical analysis of layers in the batch reactors.
| Inoculation strategy | CH4 Yield (mL/g VS) | Layer a | pH | Dissolved COD (g/L) | Total VFA (g/L) |
|---|
| First run |
| Bot | 51 ± 18 | Top (yam) | 4.46 | 16.05 ± 1.3 | 14.1 ± 1.6 |
| Bottom (SI) | 6.03 | 10.09 ± 0.7 | 8.6 ± 0.4 |
| Lay | 53 ± 9 | 6 (Yam) | 4.48 | 18.3 ± 2.0 | 13.8 ± 1.1 |
| 5 (SI) | 4.67 | 22.8 ± 3.1 | 17.8 ± 1.9 |
| 4 (Yam) | 4.64 | 19.3 ± 0.9 | 15.8 ± 0.7 |
| 3 (SI) | 4.72 | 18.6 ± 4.2 | 15.8 ± 2.6 |
| 2 (Yam) | 4.83 | 22.4 ± 2.5 | 15.4 ± 1.3 |
| 1 (SI) | 4.8 | 30.0 ± 4.0 | 14.5 ± 3.6 |
| Bot + Dung | 116 ± 7 | Top (yam) | 5.4 | 17.7 ± 0.6 | 15.3 ± 0.4 |
| Bottom (SI) | 7.69 | 5.0 ± 0.2 | 2.51 ± 0.3 |
| Lay + Dung | 143 ± 4 | 6 (Yam) | 8.32 | 21.31 ± 1.5 | 3.64 ± 0.3 |
| 5 (SI) | 8.41 | 3.44 ± 0.7 | 0.46 ± 0.04 |
| 4 (Yam) | 8.47 | 19.92 ± 1.4 | 2.90 ± 0.3 |
| 3 (SI) | 8.33 | 6.92 ± 1.1 | 2.44 ± 0.5 |
| 2 (Yam) | 8.42 | 3.44 ± 0.3 | 1.13 ± 0.2 |
| 1 (SI) | 7.90 | 6.16 ± 0.7 | 1.88 ± 0.3 |
| MixDung | 9 ± 7 | (mix) | 5.07 | 21.8 ± 6.7 | 20.2 ± 8.6 |
| Second run |
| Bot II | 140 ± 16 | Top (yam) | 7.76 | 26.1 ±2.6 | 4.75 ± 0.3 |
| Bottom (SI) | 6.86 | 27.2 ± 1.8 | 5.06 ± 0.3 |
| Bot II (Dung) | 142 ± 5 | Top (yam) | 7.95 | 10.8 ± 1.1 | 2.21 ± 0.4 |
| Bottom (SI) | 7.28 | 23.9 ± 1.3 | 9.50 ± 0.8 |
| Lay II (Dung) | 161 ± 4 | 6 (Yam) | 7.90 | 24.38 ± 2.6 | 3.24 ± 1.1 |
| 5 (SI) | 7.88 | 12.33 ± 0.9 | 1.34 ± 0.2 |
| 4 (Yam) | 7.90 | 21.56 ± 1.9 | 2.87 ± 0.3 |
| 3 (SI) | 7.70 | 7.88 ± 0.6 | 2.16 ± 0.3 |
| 2 (Yam) | 7.76 | 6.02 ± 0.4 | 2.30 ± 0.2 |
| 1 (SI) | 7.88 | 4.41 ± 0.9 | 1.22 ± 0.4 |
| Mix II (Dung) | 8 ± 4 | Top (mix) | 4.73 | 40.9 ± 7.3 | 22.5 ± 4.1 |
| Bottom (mix) | 4.75 | 51.1 ± 9.4 | 29.2 ± 6.6 |
Without the addition of dung, layered inoculation (Lay) did not improve the conditions for AD in the first run. In the Lay set-up, all layers had a pH below 5. Acidification in the inoculation layers was more severe than in Bot. The thinner layers have a larger contact area/volume ratio which can facilitate penetration by acids from neighboring yam peeling layers. Presumably, layered inoculation made the process more sensitive to acidification, when compared to bottom inoculation. In the contrary, the combination of layered inoculation with the addition of dung (Lay + Dung) showed a superior ability to prevent acidification, with a pH range between 7.9 and 8.5. Further, low VFA concentrations were observed throughout all layers, suggesting the presence of sufficient acetogenic and methanogenic activity. However, relatively high concentrations of dissolved COD were found in the layers 4 (yam) and 6 (yam) of Lay + Dung. Together with the low VFA concentrations, this points out that the hydrolysis rate in these layers was significantly higher than the acidogenesis rate.
The significant effect of dung addition in
Bot + Dung and
Lay + Dung can be attributed to a stimulation of bacterial growth by a more favorable COD/N ratio and the high concentration of micronutrients typically found in dung [
15,
19]. Furthermore, it can be assumed that anaerobic microorganisms present in fresh cow dung have supported the digestion process. Accordingly, dung had a multilateral role as substrate, nutrient supply and additional inoculum.
However, in the batch series without the addition of solid inoculum (MixDung) biogas production was marginal. This indicates that the anaerobic activity of dung alone was too low to initiate an AD process in a mixture with yam peelings. The objective of MixDung was to investigate if an initial start-up of SSAD is possible without using pre-inoculated material, so that a process start-up could be independent of inoculum supply from an external functional digester. The poor performance of MixDung suggests that the application of active digestate as inoculum is indispensible and cannot be replaced by dung.
3.4. Comparison by Production Rate
The highest methane production rates (given in mL methane per L reactor working volume and day) were observed in the series with layered inoculation, where methane production started instantly. During the first 10 days
Lay Dung and
Lay II (Dung) produced on average 408 mL/L/d and 610 mL/L/d, respectively (
Table 4). Hereafter, the methane production continued on a comparatively high level until around day 40, where the production significantly decreased. The bottom inoculated series
Bot + Dung,
Bot II and Bot II (Dung) showed a lower but more even production rate throughout the incubation period, when compared to the layered inoculated series. The higher production rate maxima in the second run indicate a higher maximum metabolic rate which suggests that microbial enrichment took place in the reused inocula.
Table 4.
Average and maximum methane production rates a in different phases during the SSAD experiments in mL/L/d.
Table 4.
Average and maximum methane production rates a in different phases during the SSAD experiments in mL/L/d.
| Inoculation strategy | Day |
|---|
| 0 to 10 | 11 to 40 | 41 to 60 | 61 to 89 | Maximum |
|---|
| First run |
| Bot | 124 | 93 | 86 | 49 | 188 |
| Lay | 211 | 125 | 84 | 22 | 265 |
| Bot + Dung | 288 | 317 | 245 | 74 | 402 |
| Lay + Dung | 408 | 478 | 151 | 82 | 583 |
| MixDung | 56 | 35 | 7 | 7 | 104 |
| Second run |
| Bot II | 248 | 373 | 174 | - | 426 |
| Bot II (Dung) | 203 | 390 | 249 | - | 459 |
| Lay II (Dung) | 610 | 394 | 80 | - | 870 |
| Mix II (Dung) | 129 | 0 | 8 | - | 192 |
For conventional liquid AD in domestic digesters, a wide range of production rates from 140 to 500 mL/L/d has been reported in the literature [
25,
26,
27]. Comparability is limited due to different operating conditions, substrates and digester models. The rates obtained in this study are in the higher end or slightly above this range. As SSAD is characterized by a 2–4 times higher solid concentration than in a typical domestic liquid digester, significantly higher production rates are theoretically possible and the results of this study suggest that substantially higher rates could be achieved if limitations as e.g., slow mass transfer in dry medium can be overcome by technical means. However, the aim of this study was primarily to test simple process set-ups rather than exploiting the margins of process efficiency. Further, the production rates achieved in this study suggest that appropriate inoculation strategies can make SSAD sufficiently efficient to make more complex measures as leachate recirculation or two-stage digestion dispensable.
3.5. Application Scenario and Comparison to a Fixed Dome Digester
A brief scenario for a household scale implementation of SSAD based on the results of
Lay II (Dung) was developed in order to compare an SSAD application with a conventional fixed-dome digester fed with cow dung, serving as reference scenario. The comparison aims at displaying the different requirements of the two scenarios that are necessary to produce a specific daily methane demand. For comparability, a target daily methane production was defined. In literature, the daily methane demand for cooking has been estimated in the range of 0.6 to 1.5 m
3 CH
4/day for one-family households in low-income rural regions [
4,
25,
26]. Accordingly, the target daily methane production was set to 1 m
3 and the two scenarios were dimensioned to meet this target.
The data of the reference scenario is adopted from a fixed-dome plug flow domestic digester fed with cow dung as substrate [
25] in semi-continuous operation and comprises a digester volume of 4 m
3 to meet the target daily gas production. The SSAD scenario is based on batch mode operation, which does not supply a steady gas flow throughout the incubation time of a single batch digester. Therefore, the scenario features several digesters with time-delayed start-up so that a pseudo-steady gas production can be obtained from all digesters together. The number and volume of the digesters, as well as the time-delay between start-ups of single digesters depends on gas production dynamics. For the SSAD scenario based on the gas production pattern of
Lay II (Dung), a set of five 0.5 m
3 digesters was chosen. When started-up one-by-one in intervals of 8 day and an incubation time of 40 days, a pseudo steady-state gas production of 944 ± 24 L CH
4/d is achieved from day 35.
Figure 3 illustrates the overlapping methane production rates of the single SSAD batch digesters and their combined production rate.
Figure 3.
Methane production pattern of five off-set run batch digesters and their cumulative production rate.
Figure 3.
Methane production pattern of five off-set run batch digesters and their cumulative production rate.
The required digester volume, water and substrate demand are significantly reduced in the SSAD scenario, when compared to a fixed-dome digester (
Table 5). The significant differences are caused by the different yields of the used substrates and a higher solid content in the SSAD digesters. It should be noted that methane yield and solid content are lowered if the addition of dung is necessary to maintain a stable AD process. Thus, the assumption of dispensing dung in this comparison favors the SSAD scenario. However, the results of the second experimental run of this study suggest that the previous assumption is valid for at least one subsequent cycle after initial start-up. Tests with a high number of successive incubation cycles are necessary to determine if dung addition to SSAD of yam peelings is dispensable in the long run.
Table 5.
Comparison of SSAD and fixed dome scenario.
Table 5.
Comparison of SSAD and fixed dome scenario.
| Parameter | SSAD a | Fixed Dome b |
|---|
| Target daily methane supply (m3) | 1 | 1 |
| Process mode | multiple batch | continuous |
| Total digester volume (m3) | 5 × 0.5 | 4 |
| SRT c/HRT d (d) | 40 | 55 |
| Feeding frequency (d) | 8 | 1 |
| Substrate per feed (kg fresh mass) | 149 | - |
| Daily substrate demand (kg fresh mass) | 18.6 | 38 |
| Daily dry matter demand (kg TS/d) | 2.3 | 4.2 |
| Daily water demand (L/d) | 7 e | 38 |
3.6. Operational Considerations
In regions where water is scarce, SSAD can be more suitable than liquid AD. Further, a reduced or eliminated demand for dung could lower the dependency of domestic biogas applications on the availability of stabled livestock. However, the need of dung addition will also depend on handling and properties of the digestate reused for inoculation. For example, accumulation of nutrient rich drainage water in the bottom of the digesters was observed by the end of the SSAD experiments (data not shown). An operational practice which retains the draining water in the digester when emptying/feeding can therefore help to recycle nitrogen, phosphorus and trace elements and thereby reduce the necessity of supplying nutrients by e.g., dung addition.
The choice between SSAD and liquid AD is also strongly dependent on factors as e.g., installation costs, required labor for digester operation, reliability of gas supply and the simplicity of operation and maintenance. Stackable digestate, as obtained from SSAD, will presumably reduce and simplify the workload related to the reuse of digestate as fertilizer on the fields. On the contrary, manual emptying of the digester and handling of digestate for subsequent reuse can imply a more complex procedure than for liquid AD, especially when layered inoculation is considered. Therefore, bottom inoculation could be favored to layered inoculation, as the start of a new batch cycle only requires the replacement of the upper half of the digester content with new substrate. Additionally, SSAD requires digesters with wide openings for adding or removing biomass, which involves additional challenges with respect to reactor design and gas-tightness. Conclusively, further studies should address technical aspect in pilot scale to assess practical and technical constraints and challenges when operating SSAD in low-tech digesters.
4. Conclusions
The tested inoculation strategies had a significant impact on the performance of SSAD of yam peels in batch digesters. Layered inoculation increased the methane yield significantly compared to bottom inoculation. The addition of cow dung was essential for the start-up of the process. Without dung addition, both bottom and layered inoculation were subjected to severe acidification. Dung as sole inoculum with pre-mixed inoculation failed to establish an anaerobic conversion process. The reuse of digestate from AD of yam peelings as inoculum for a consecutive incubation indicated that microbial adaptation took place, making cow dung addition unnecessary. This suggests that the addition of cow dung as supportive biomass can accelerate the start-up phase of a batch digester, but does not further improve the process performance in consecutive batch cycles. Accordingly, SSAD could presumably be employed in households where the lack of sufficient livestock excrements excludes the operation of a conventional animal dung based domestic digester. Further, a relatively low water process demand supports the application of SSAD in regions were water scarcity makes liquid-based processes unfavorable. In many African countries where household biogas digesters are rare, SSAD could potentially help providing biogas technology and its benefits to more people. However, the results of this study were obtained in two successive lab-scale incubations with specific substrate and process conditions. Studies with several successive incubation cycles using varying process conditions and substrate types are necessary to assess the suitability of the respective inoculation strategies for household-scale applications.