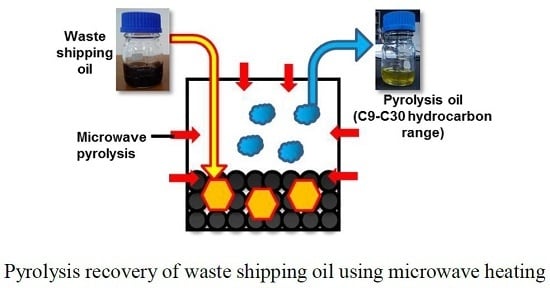
Pyrolysis Recovery of Waste Shipping Oil Using Microwave Heating
Abstract
:1. Introduction
2. Methods
2.1. Materials
2.2. Experimental Details on Microwave Pyrolysis of Waste Shipping Oil
2.3. Analytical Methods
2.3.1. Elemental Analysis
2.3.2. Hydrocarbon Content
2.3.3. Calorific Value
3. Results and Discussion
3.1. Temperature Profile and Heating Performance During Microwave Pyrolysis of Waste Shipping Oil
3.2. Pyrolysis Product Distribution
3.3. Chemical Composition of Pyrolysis Oil
3.4. Elemental Composition of Pyrolysis Oil
3.5. Calorific Value, Mass Yield and Energy Yield of Pyrolysis Oil
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- IMO (International Maritime Organization), Maritime Knowledge Centre. International Shipping Facts and Figures—Information Resources on Trade, Safety, Security, Environment. 2012. Available online: http://www.imo.org/en/KnowledgeCentre/ShipsAndShippingFactsAndFigures/Documents/International%20Shipping%20-%20Facts%20and%20Figures.pdf (accessed on 25 July 2016).
- Singh, A.; Asmath, H.; Chee, C.L.; Darsan, J. Potential oil spill risk from shipping and the implications for management in the Caribbean Sea. Mar. Pollut. Bull. 2015, 93, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Maritime Connectors, Tanker Incidents. 2013. Available online: http://maritime-connector.com/tanker-incidents/ (accessed on 25 July 2016).
- Tsai, W.-T. An analysis of used lubricant recycling, energy utilization and its environmental benefit in Taiwan. Energy 2011, 36, 4333–4339. [Google Scholar] [CrossRef]
- Lam, S.S.; Liew, R.K.; Jusoh, A.; Chong, C.T.; Ani, F.N.; Chase, H.A. Progress in waste oil to sustainable energy, with emphasis on pyrolysis techniques. Renew. Sustain. Energy Rev. 2016, 53, 741–753. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Chiueh, P.-T.; Kuan, W.-H.; Lo, S.-L. Microwave pyrolysis of lignocellulosic biomass: Heating performance and reaction kinetics. Energy 2016, 100, 137–144. [Google Scholar] [CrossRef]
- Salema, A.A.; Ani, F.N. Pyrolysis of oil palm empty fruit bunch biomass pellets using multimode microwave irradiation. Bioresour. Technol. 2012, 125, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, F.; Mat, R.; Ani, F.N. A review on microwave assisted pyrolysis of coal and biomass for fuel production. Renew. Sustain. Energy Rev. 2014, 39, 555–574. [Google Scholar] [CrossRef]
- Lam, S.S.; Liew, R.K.; Lim, X.Y.; Ani, F.N.; Jusoh, A. Fruit waste as feedstock for recovery by pyrolysis technique. Int. Biodeterior. Biodegrad. 2016, 113, 325–333. [Google Scholar] [CrossRef]
- Lam, S.S.; Liew, R.K.; Cheng, C.K.; Chase, H.A. Catalytic microwave pyrolysis of waste engine oil using metallic pyrolysis char. Appl. Catal. B Environ. 2015, 176–177, 601–617. [Google Scholar] [CrossRef]
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renew. Sustain. Energy Rev. 2016, 55, 467–481. [Google Scholar] [CrossRef]
- Yin, C. Microwave-assisted pyrolysis of biomass for liquid biofuels production. Bioresour. Technol. 2012, 120, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Undri, A.; Abou-Zaid, M.; Briens, C.; Berruti, F.; Rosi, L.; Bartoli, M.; Frediani, M.; Frediani, P. Bio-oil from pyrolysis of wood pellets using a microwave multimode oven and different microwave absorbers. Fuel 2015, 153, 464–482. [Google Scholar] [CrossRef]
- Undri, A.; Rosi, L.; Frediani, M.; Frediani, P. Upgraded fuel from microwave assisted pyrolysis of waste tire. Fuel 2014, 115, 600–608. [Google Scholar] [CrossRef]
- Lam, S.S.; Russell, A.D.; Lee, C.L.; Chase, H.A. Microwave-heated pyrolysis of waste automotive engine oil: Influence of operation parameters on the yield, composition, and fuel properties of pyrolysis oil. Fuel 2012, 92, 327–339. [Google Scholar] [CrossRef]
- Lam, S.S.; Russell, A.D.; Chase, H.A. Microwave pyrolysis, a novel process for recycling waste automotive engine oil. Energy 2010, 35, 2985–2991. [Google Scholar] [CrossRef]
- Lam, S.S.; Russell, A.D.; Chase, H.A. Pyrolysis Using Microwave Heating: A Sustainable Process for Recycling Used Car Engine Oil. Ind. Eng. Chem. Res. 2010, 49, 10845–10851. [Google Scholar] [CrossRef]
- Mohamed, B.A.; Kim, C.S.; Ellis, N.; Bi, X. Microwave-assisted catalytic pyrolysis of switchgrass for improving bio-oil and biochar properties. Bioresour. Technol. 2016, 201, 121–132. [Google Scholar] [CrossRef] [PubMed]



| Chemical Compositions | SO a | WSO a | Pyrolysis Oil a | ||
|---|---|---|---|---|---|
| 400 °C | 500 °C | 600 °C | |||
| Alkanes | 72 | 81 | 92 | 89 | 88 |
| Cycloalkanes | - b | 0.81 | - | - | - |
| Alkenes | 9 | 5 | 5 | 8 | 6 |
| Total | 81 | 87 | 97 | 97 | 94 |
| Carbon Compounds | |||||
| C5–C10 | - | - | 24 | 39 | 27 |
| C11–C15 | - | 5 | 62 | 37 | 35 |
| C16–C20 | 12 | 42 | 11 | 21 | 30 |
| C21–C30 | 42 | 27 | - | - | 2 |
| C31–C45 | 27 | 13 | - | - | - |
| PAH | |||||
| Naphthalene | - | - | - | 1 | 2 |
| Pyrene | - | - | 1 | - | - |
| Anthracene | - | 4.88 | - | - | - |
| Elemental Analysis | SO | WSO | Pyrolysis Oil | ||
|---|---|---|---|---|---|
| 400 °C | 500 °C | 600 °C | |||
| C (%) | 84.5 | 84.3 | 82.4 | 83.3 | 82.8 |
| H (%) | 14.4 | 13.6 | 14.6 | 14.7 | 14.7 |
| N (%) | 0.9 | 0.9 | 0.9 | 0.7 | 0.6 |
| S (%) | 0.09 | 0.13 | 0.45 | 0.33 | 0.37 |
| O (%) | 0.12 | 1.08 | 1.49 | 0.91 | 1.58 |
| H/C (mol/mol) | 2.05 | 1.93 | 2.15 | 2.12 | 2.13 |
| H/O (mol/mol) | 1971 | 201 | 158 | 259 | 148 |
| O/C (mol/mol) | 0.001 | 0.01 | 0.01 | 0.01 | 0.01 |
| Temperatures | Calorific Value (MJ/kg) | Mass Yield of Pyrolysis Oil (%) | Energy Recovery of Pyrolysis Oil (%) |
|---|---|---|---|
| 400 °C | 43 | 20 | 19 |
| 500 °C | 46 | 60 | 61 |
| 600 °C | 45 | 66 | 66 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan Mahari, W.A.; Zainuddin, N.F.; Wan Nik, W.M.N.; Chong, C.T.; Lam, S.S. Pyrolysis Recovery of Waste Shipping Oil Using Microwave Heating. Energies 2016, 9, 780. https://doi.org/10.3390/en9100780
Wan Mahari WA, Zainuddin NF, Wan Nik WMN, Chong CT, Lam SS. Pyrolysis Recovery of Waste Shipping Oil Using Microwave Heating. Energies. 2016; 9(10):780. https://doi.org/10.3390/en9100780
Chicago/Turabian StyleWan Mahari, Wan Adibah, Nur Fatihah Zainuddin, Wan Mohd Norsani Wan Nik, Cheng Tung Chong, and Su Shiung Lam. 2016. "Pyrolysis Recovery of Waste Shipping Oil Using Microwave Heating" Energies 9, no. 10: 780. https://doi.org/10.3390/en9100780
APA StyleWan Mahari, W. A., Zainuddin, N. F., Wan Nik, W. M. N., Chong, C. T., & Lam, S. S. (2016). Pyrolysis Recovery of Waste Shipping Oil Using Microwave Heating. Energies, 9(10), 780. https://doi.org/10.3390/en9100780