Surface Modification of Direct-Current and Radio-Frequency Oxygen Plasma Treatments Enhance Cell Biocompatibility
Abstract
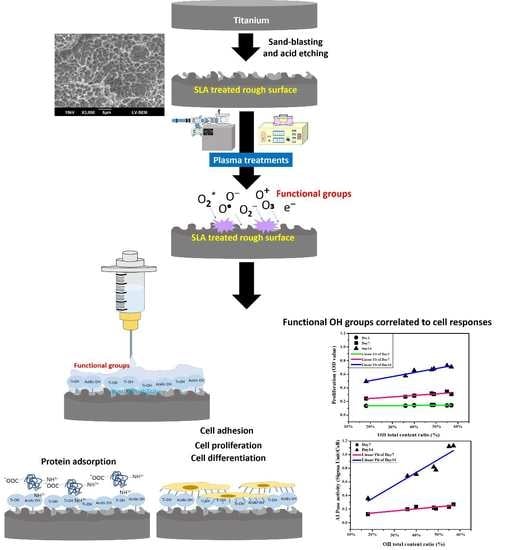
:1. Introduction
2. Results
2.1. Surface Analysis after Plasma Treatments
2.2. The Effect of Protein Adsorption
2.3. The Influence of DC and RF Plasma Treatments on Cell Bioactivity
3. Discussion
3.1. Surface Topography after Plasma Treatments
3.2. Plasma Treatments Enhance Hydrophilicity
3.3. Effect of Plasma Treatment on Functional OH Groups
3.4. Protein Adsorption of Plasma-Treated SLA Specimens
3.5. DC and RF Plasma Treatments Promote Cell Responses
4. Materials and Methods
4.1. Sample Preparation
4.2. Plasma Treatments
4.3. Specimen Characterization
4.4. Protein Adsorption
4.5. Cell Culture
4.6. Cell Morphology
4.7. Cell Proliferation
4.8. Cell Differentiation
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Branemark, P.I.; Adell, R.; Breine, U.; Hansson, B.O.; Lindstrom, J.; Ohlsson, A. Intra-osseous anchorage of dental prostheses. I. Experimental studies. Scand. J. Plast. Reconstr. Surg. 1969, 3, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Branemark, P.I.; Hansson, B.O.; Adell, R.; Breine, U.; Lindstrom, J.; Hallen, O.; Ohman, A. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand. J. Plast. Reconstr. Surg. Suppl. 1977, 16, 1–132. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Chu, P.K.; Ding, C.X. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R 2004, 47, 49–121. [Google Scholar] [CrossRef]
- Ghanem, A.; Kellesarian, S.V.; Abduljabbar, T.; Al-Hamoudi, N.; Vohra, F.; Javed, F. Role of osteogenic coatings on implant surfaces in promoting bone-to-implant contact in experimental osteoporosis: A systematic review and meta-analysis. Implant Dent. 2017. [Google Scholar] [CrossRef] [PubMed]
- Kellesarian, S.V.; Subhi, A.S.; Saleh Binshabaib, M.; Javed, F. Effect of local zoledronate delivery on osseointegration: A systematic review of preclinical studies. Acta Odontol. Scand. 2017, 75, 530–541. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.Q.; Xie, Y.T.; Pan, H.H.; Zheng, X.B.; Huang, L.P.; Ji, F.; Li, K. Plasma-sprayed titanium patterns for enhancing early cell responses. J. Therm. Spray Technol. 2016, 25, 946–958. [Google Scholar] [CrossRef]
- Vyas, N.; Sammons, R.L.; Addison, O.; Dehghani, H.; Walmsley, A.D. A quantitative method to measure biofilm removal efficiency from complex biomaterial surfaces using sem and image analysis. Sci. Rep.-UK 2016, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kang, O.L.; Ahmad, A.; Rana, U.A.; Hassan, N.H. Sol-gel titanium dioxide nanoparticles: Preparation and structural characterization. J. Nanotechnol. 2016, 10, 1–8. [Google Scholar] [CrossRef]
- Zembic, A.; Glauser, R.; Khraisat, A.; Hammerle, C.H. Immediate vs. Early loading of dental implants: 3-year results of a randomized controlled clinical trial. Clin. Oral Implants Res. 2010, 21, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.F.; Zheng, X.Y.; Zeng, G.Q.; Xu, Y.; Qu, X.H.; Zhu, M.; Lu, E.Y. Clinical efficacy of early loading versus conventional loading of dental implants. Sci. Rep.-UK 2015, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.N.; Zhang, Y.; Ma, T.; Chen, H.F.; Lin, Y. Enhanced hydrophilicity and protein adsorption of titanium surface by sodium bicarbonate solution. J. Nanomater. 2015, 1–12. [Google Scholar] [CrossRef]
- Zhao, G.; Raines, A.L.; Wieland, M.; Schwartz, Z.; Boyan, B.D. Requirement for both micron- and submicron scale structure for synergistic responses of osteoblasts to substrate surface energy and topography. Biomaterials 2007, 28, 2821–2829. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.O.; Bijelic, A.; Ziebart, T.; Koch, F.; Kammerer, P.W.; Wieland, M.; Konerding, M.A.; Al-Nawas, B. Submicron scale-structured hydrophilic titanium surfaces promote early osteogenic gene response for cell adhesion and cell differentiation. Clin. Implants Dent. R 2013, 15, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Bacakova, L.; Grausova, L.; Vacik, J.; Fraczek, A.; Blazewicz, S.; Kromka, A.; Vanecek, M.; Svorcik, V. Improved adhesion and growth of human osteoblast-like mg 63 cells on biomaterials modified with carbon nanoparticles. Diam. Relat. Mater. 2007, 16, 2133–2140. [Google Scholar] [CrossRef]
- Lee, T.M.; Chang, E.; Yang, C.Y. Attachment and proliferation of neonatal rat calvarial osteoblasts on ti6al4v: Effect of surface chemistries of the alloy. Biomaterials 2004, 25, 23–32. [Google Scholar] [CrossRef]
- Lee, T.M. Effect of passivation and surface modification on the dissolution behavior and nano-surface characteristics of ti-6al-4v in hank/edta solution. J. Mater. Sci. Mater. Med. 2006, 17, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Chsu, Y.; Hsieh, M.C.; Yang, S.P.; Su, F.C.; Lee, T.M. Effects of nano-surface properties on initial osteoblast adhesion and ca/p adsorption ability for titanium alloys. Nanotechnology 2008, 19, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Hsu, Y.C.; Su, F.C.; Lu, S.C.; Lee, T.M. Effects of passivation treatments on titanium alloy with nanometric scale roughness and induced changes in fibroblast initial adhesion evaluated by a cytodetacher. J. Biomed. Mater. Res. Part A 2009, 88, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Heredia, M.A.; Legeay, G.; Gaillard, C.; Layrolle, P. Radio frequency plasma treatments on titanium for enhancement of bioactivity. Acta Biomater. 2008, 4, 1953–1962. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Genova, T.; Tallarico, M.; Gautier, G.; Mussano, F.; Botticelli, D. Plasma of argon affects the earliest biological response of different implant surfaces: An in vitro comparative study. J. Dent. Res. 2016, 95, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.X.; Shen, H.; Shuai, K.G.; Zhang, E.W.; Bai, Y.J.; Cheng, Y.; Xiong, X.L.; Wang, S.G.; Fang, J.; Wei, S.C. Surface bioactivity modification of titanium by CO2 plasma treatment and induction of hydroxyapatite: In vitro and in vivo studies. Appl. Surf. Sci. 2011, 257, 1813–1823. [Google Scholar] [CrossRef]
- Oreffo, R.O.C.; Triffitt, J.T. In vitro and in vivo methods to determine the interactions of osteogenic cells with biomaterials. J. Mater. Sci. Mater. Med. 1999, 10, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Broggini, N.; Wieland, M.; Schenk, R.K.; Denzer, A.J.; Cochran, D.L.; Hoffmann, B.; Lussi, A.; Steinemann, S.G. Enhanced bone apposition to a chemically modified sla titanium surface. J. Dent. Res. 2004, 83, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, N.; Oates, T.W.; Vargas, A.; Alexander, P.P.; Schoolfield, J.D.; McMahan, C.A. Conventional sla and chemically modified sla implants in patients with poorly controlled type 2 diabetes mellitus—A randomized controlled trial. Clin. Oral Implants Res. 2013, 24, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Cvelbar, U.; Pejovnik, S.; Mozetie, M.; Zalar, A. Increased surface roughness by oxygen plasma treatment of graphite/polymer composite. Appl. Surf. Sci. 2003, 210, 255–261. [Google Scholar] [CrossRef]
- Alam, A.U.; Howlader, M.M.R.; Deen, M.J. The effects of oxygen plasma and humidity on surface roughness, water contact angle and hardness of silicon, silicon dioxide and glass. J. Micromech. Microeng. 2014, 24, 1–14. [Google Scholar] [CrossRef]
- Zhang, L.; He, X.S.; Chen, G.; Wang, T.; Tang, Y.J.; He, Z.B. Effects of rf power on chemical composition and surface roughness of glow discharge polymer films. Appl. Surf. Sci. 2016, 366, 499–505. [Google Scholar] [CrossRef]
- Tseng, W.Y.; Hsu, S.H.; Huang, C.H.; Tu, Y.C.; Tseng, S.C.; Chen, H.L.; Chen, M.H.; Su, W.F.; Lin, L.D. Low pressure radio-frequency oxygen plasma induced oxidation of titanium—Surface characteristics and biological effects. PLoS ONE 2013, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.Y.; Xiong, C.D.; Wang, Z.C.; Li, X.Y.; Zhang, L.F. A combination of co2 laser and plasma surface modification of poly(etheretherketone) to enhance osteoblast response. Appl. Surf. Sci. 2015, 344, 79–88. [Google Scholar] [CrossRef]
- Feng, B.; Weng, J.; Yang, B.C.; Qu, S.X.; Zhang, X.D. Characterization of surface oxide films on titanium and adhesion of osteoblast. Biomaterials 2003, 24, 4663–4670. [Google Scholar] [CrossRef]
- Kwon, J.S.; Kim, Y.H.; Choi, E.H.; Kim, K.N. The effects of non-thermal atmospheric pressure plasma jet on attachment of osteoblast. Curr. Appl. Phys. 2013, 13, S42–S47. [Google Scholar] [CrossRef]
- da Silva, J.S.P.; Amico, S.C.; Rodrigues, A.O.N.; Barboza, C.A.G.; Alves, C.; Croci, A.T. Osteoblastlike cell adhesion on titanium surfaces modified by plasma nitriding. Int. J. Oral Maxillofac. Implants 2011, 26, 237–244. [Google Scholar] [PubMed]
- Wang, R.C.C.; Hsieh, M.C.; Lee, T.M. Effects of nanometric roughness on surface properties and fibroblast’s initial cytocompatibilities of ti6al4v. Biointerphases 2011, 6, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Recek, N.; Jaganjac, M.; Kolar, M.; Milkovic, L.; Mozetic, M.; Stana-Kleinschek, K.; Vesel, A. Protein adsorption on various plasma-treated polyethylene terephthalate substrates. Molecules 2013, 18, 12441–12463. [Google Scholar] [CrossRef] [PubMed]
- Rupp, F.; Scheideler, L.; Olshanska, N.; de Wild, M.; Wieland, M.; Geis-Gerstorfer, J. Enhancing surface free energy and hydrophilicity through chemical modification of microstructured titanium implant surfaces. J. Biomed. Mater. Res. Part A 2006, 76, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Kwok, D.T.K.; Wang, W.; Wu, Z.W.; Tong, L.P.; Zhang, Y.M.; Chu, P.K. Osteoblast behavior on polytetrafluoroethylene modified by long pulse, high frequency oxygen plasma immersion ion implantation. Biomaterials 2010, 31, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Kwon, J.S.; Uhm, S.H.; Song, D.H.; Kim, Y.H.; Choi, E.H.; Kim, K.N. The effects of non-thermal atmospheric pressure plasma jet on cellular activity at sla-treated titanium surfaces. Curr. Appl. Phys. 2013, 13, S36–S41. [Google Scholar] [CrossRef]
- Rapuano, B.E.; Hackshaw, K.; Macdonald, D.E. Heat or radiofrequency plasma glow discharge treatment of a titanium alloy stimulates osteoblast gene expression in the mc3t3 osteoprogenitor cell line. J. Periodontal Implant Sci. 2012, 42, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, M.M.; Schmid, B.; Belser, U.C.; Lussi, A.; Buser, D. Early loading of non-submerged titanium implants with a sandblasted and acid-etched surface. Clin. Oral Implants Res. 2005, 16, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, S.A.; Mate Sanchez de Val, J.E.; Fernandez Dominguez, M.; de Aza Moya, P.N.; Gomez Moreno, G.; Calvo Guirado, J.L. Effects on the osseointegration of titanium implants incorporating calcium-magnesium: A resonance frequency and histomorphometric analysis in rabbit tibia. Clin. Oral Implants Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Xu, L.; Wang, Q.; Sunarso; Xu, L. Hydrothermal sterilization improves initial osteoblast responses on sandpaper-polished titanium. Materials (Basel) 2017, 10, 812. [Google Scholar] [CrossRef] [PubMed]










| O1s | F1s | C1s | Ti2p | N1s | O/Ti | |
|---|---|---|---|---|---|---|
| Control | 57.4 | ― | 19.6 | 21.5 | 1.5 | 2.6 |
| DC-30W | 58.8 | 1.7 | 16.8 | 22.5 | 0.2 | 2.6 |
| DC-40W | 59.5 | 1.8 | 17.9 | 20.3 | 0.5 | 2.9 |
| DC-50W | 57.0 | 5.1 | 18.8 | 18.9 | 0.2 | 3.0 |
| RF-50W | 55.7 | 5.8 | 19.0 | 19.2 | 0.3 | 2.9 |
| RF-100W | 51.5 | 4.4 | 26.9 | 15.8 | 1.4 | 3.3 |
| RF-200W | 47.4 | 2.7 | 36.0 | 13.0 | 0.9 | 3.7 |
| Basic Ti-OH | Acidic OH | O1s | |
|---|---|---|---|
| Control | 10.00 | 8.21 | 81.79 |
| DC-30W | 25.98 | 9.86 | 64.16 |
| DC-40W | 20.83 | 18.89 | 60.28 |
| DC-50W | 19.08 | 29.80 | 51.12 |
| RF-50W | 21.91 | 25.70 | 52.39 |
| RF-100W | 28.49 | 27.49 | 44.02 |
| RF-200W | 33.05 | 23.86 | 43.09 |
| Surface Pretreatment | Pressure | Flow Rate | Power | Nomenclature | |
|---|---|---|---|---|---|
| Control | SLA | N/A | N/A | N/A | SLA |
| DC plasma | 30 W | DC-30W | |||
| SLA | 2 × 10−1 Torr | 20 sccm | 40 W | DC-40W | |
| 50 W | DC-50W | ||||
| RF plasma | 50 W | RF-50W | |||
| SLA | 1.3 × 10−1 Torr | 20 sccm | 100 W | RF-100W | |
| 200 W | RF-200W |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chou, W.-C.; Wang, R.C.-C.; Liu, C.; Yang, C.-Y.; Lee, T.-M. Surface Modification of Direct-Current and Radio-Frequency Oxygen Plasma Treatments Enhance Cell Biocompatibility. Materials 2017, 10, 1223. https://doi.org/10.3390/ma10111223
Chou W-C, Wang RC-C, Liu C, Yang C-Y, Lee T-M. Surface Modification of Direct-Current and Radio-Frequency Oxygen Plasma Treatments Enhance Cell Biocompatibility. Materials. 2017; 10(11):1223. https://doi.org/10.3390/ma10111223
Chicago/Turabian StyleChou, Wan-Ching, Rex C.-C. Wang, Cheng Liu, Chyun-Yu Yang, and Tzer-Min Lee. 2017. "Surface Modification of Direct-Current and Radio-Frequency Oxygen Plasma Treatments Enhance Cell Biocompatibility" Materials 10, no. 11: 1223. https://doi.org/10.3390/ma10111223
APA StyleChou, W.-C., Wang, R. C.-C., Liu, C., Yang, C.-Y., & Lee, T.-M. (2017). Surface Modification of Direct-Current and Radio-Frequency Oxygen Plasma Treatments Enhance Cell Biocompatibility. Materials, 10(11), 1223. https://doi.org/10.3390/ma10111223