Hard Chrome-Coated and Fullerene-Doped Metal Surfaces in Orthopedic Bearings
Abstract
:1. Introduction
2. Results
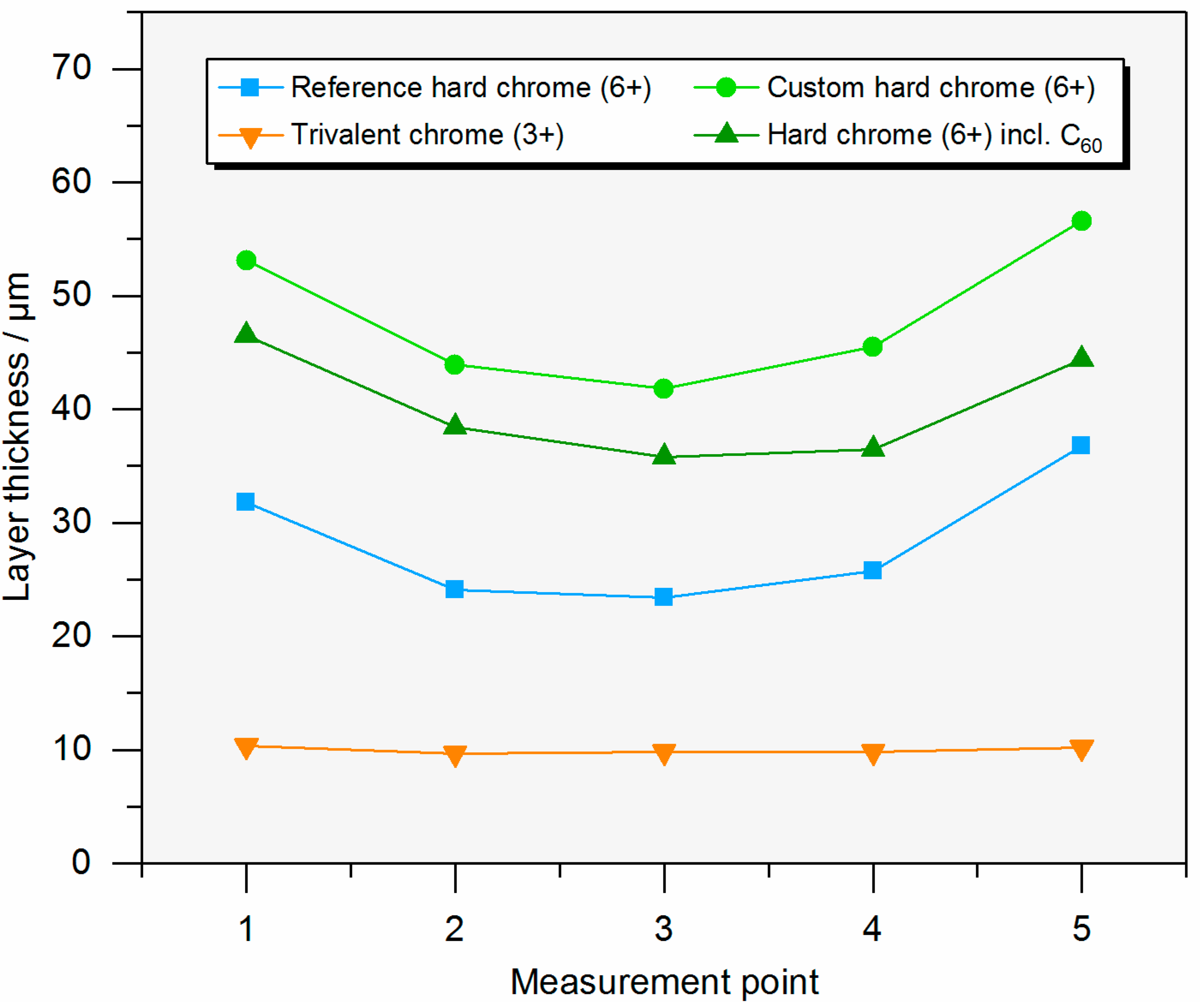
2.1. Characterization of the Coatings
2.2. Detection of the Fullerenes
2.3. Wear Testing
3. Discussion
4. Materials and Methods
4.1. Electrolytes and Parameters
- (a)
- CoCr: uncoated and polished CoCr which serves as a clinical reference;
- (b)
- Reference hard chrome (6+): hard chrome coating based on an industrially available standard electrolyte with a CrO3 content of 280 g/L;
- (c)
- Custom hard chrome (6+): hard chrome coating based on a reduced CrO3 content of 50 g/L;
- (d)
- Hard chrome (6+) incl. C60: same as (c) but including 0.75 g/L of HTAB and 0.375 g/L of fullerenes (C60);
- (e)
- Trivalent chrome (3+) incl. C60: industrially available trivalent chromium electrolyte (pristine trivalent chrome (3+) without C60 used for coating characterization).
4.2. Experimental Setup for Electrodeposition
4.3. Characterization of the Coatings
4.4. Wear and Friction Analysis
4.5. Statistics
5. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Willert, H.G.; Bertram, H.; Buchhorn, G.H. Osteolysis in Alloarthroplasty of the Hip: The Role of Ultra-High Molecular Weight Polyethylene Wear Particles. Clin. Orthop. Relat. Res. 1990, 258, 95–107. [Google Scholar] [CrossRef]
- Harris, W.H. The problem is osteolysis. Clin. Orthop. Relat. Res. 1995, 311, 46–53. [Google Scholar]
- National Joint Registry. National Joint Registry for England, Wales, Northern Ireland and the Isle of Man; 13th Annual Report; National Joint Registry: Hemel Hempstead, UK, 2016. [Google Scholar]
- Rieker, C.B.; Schön, R.; Konrad, R.; Liebentritt, G.; Gnepf, P.; Shen, M.; Roberts, P.; Grigoris, P. Influence of the Clearance on In-Vitro Tribology of Large Diameter Metal-on-Metal Articulations Pertaining to Resurfacing Hip Implants. Orthop. Clin. N. Am. 2005, 36, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Affatato, S.; Leardini, W.; Jedenmalm, A.; Ruggeri, O.; Toni, A. Larger diameter bearings reduce wear in metal-on-metal hip implants. Clin. Orthop. Relat. Res. 2007, 456, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Dieppe, P.; Vernon, K.; Porter, M.; Blom, A.W. Failure rates of stemmed metal-on-metal hip replacements: Analysis of data from the National Joint Registry of England and Wales. Lancet 2012, 379, 1199–1204. [Google Scholar] [CrossRef]
- Australian Orthopaedic Association. Supplementary Report: Metal on Metal Bearing Surface Conventional Hip Arthroplasty; National Joint Replacement Registry: Adelaide, Australia, 2016. [Google Scholar]
- Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR). The Safety of Metal-on-Metal Joint Replacements with a Particular Focus on Hip Implants; EU Commission Final Opinion; EU Commission: Brussels, Belgium, 2014. [Google Scholar]
- Gunther, K.P.; Schmitt, J.; Campbell, P.; Delaunay, C.P.; Drexler, H.; Ettema, H.B.; Garcia-Cimbrelo, E.; Hannemann, F.; Hartmann, A.; Huberti, H.; et al. Consensus statement “Current evidence on the management of metal-on-metal bearings”-April 16, 2012. Hip Int. 2013, 23, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Medicines and Healthcare products Regulatory Agency (MHRA). Medical Device Alert: All Metal-on-Metal (MoM) Hip Replacements; Medicines and Healthcare products Regulatory Agency (MHRA): London, UK, 2012.
- US Food and Drug Administration (FDA). Safety Communication: Metal-on-Metal Hip Implants; US Food and Drug Administration (FDA): Silver Spring, MD, USA, 2013.
- Seppanen, M.; Karvonen, M.; Virolainen, P.; Remes, V.; Pulkkinen, P.; Eskelinen, A.; Liukas, A.; Makela, K.T. Poor 10-year survivorship of hip resurfacing arthroplasty. Acta Orthop. 2016, 87, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Amstutz, H.C.; Le Duff, M.J. Failure rates of stemmed metal-on-metal hip replacements. Lancet 2012, 380. [Google Scholar] [CrossRef]
- Moroni, A.; Miscione, M.T.; Orsini, R.; Micera, G.; Mosca, S.; Sinapi, F.; De Girolamo, L.; Banfi, G. Clinical and radiographic outcomes of the Birmingham Hip Resurfacing arthroplasty at a minimum follow-up of 10 years: Results from an independent centre. Hip Int. 2017, 27, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Issa, K.; Palich, A.; Tatevossian, T.; Kapadia, B.H.; Naziri, Q.; Mont, M.A. The outcomes of hip resurfacing compared to standard primary total hip arthroplasty in Men. BMC Musculoskelet. Disord. 2013, 14. [Google Scholar] [CrossRef] [PubMed]
- Al-Hajjar, M.; Fisher, J.; Williams, S.; Tipper, J.L.; Jennings, L.M. Effect of femoral head size on the wear of metal on metal bearings in total hip replacements under adverse edge-loading conditions. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 213–222. [Google Scholar] [CrossRef] [PubMed]
- De Haan, R.; Pattyn, C.; Gill, H.S.; Murray, D.W.; Campbell, P.A.; De Smet, K. Correlation between inclination of the acetabular component and metal ion levels in metal-on-metal hip resurfacing replacement. J. Bone Jt. Surg. Br. 2008, 90, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Haddad, F.S.; Thakrar, R.R.; Hart, A.J.; Skinner, J.A.; Nargol, A.V.F.; Nolan, J.F.; Gill, H.S.; Murray, D.W.; Blom, A.W.; Case, C.P. Metal-on-metal bearings: The evidence so far. J. Bone Jt. Surg. Br. 2011, 93, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Jin, Z.; Roberts, P.; Grigoris, P. Importance of head diameter, clearance, and cup wall thickness in elastohydrodynamic lubrication analysis of metal-on-metal hip resurfacing prostheses. Proc. Inst. Mech. Eng. H 2006, 220, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Jameson, S.S.; Baker, P.N.; Mason, J.; Porter, M.L.; Deehan, D.J.; Reed, M.R. Independent predictors of revision following metal-on-metal hip resurfacing: A retrospective cohort study using National Joint Registry data. J. Bone Jt. Surg. Br. 2012, 94, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Amstutz, H.C.; Le Duff, M.J. Hip resurfacing: History, current status, and future. Hip Int. 2015, 25, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Van Der Straeten, C.; De Smet, K.A. Current expert views on metal-on-metal hip resurfacing arthroplasty. Consensus of the 6th advanced Hip resurfacing course, Ghent, Belgium, May 2014. Hip Int. 2016, 26, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, B.P.; Perry, K.I.; Taunton, M.J.; Mabry, T.M.; Abdel, M.P. Diagnosis of adverse local tissue reactions following metal-on-metal hip arthroplasty. Curr. Rev. Musculoskelet. Med. 2016, 9, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Pandit, H.; Glyn-Jones, S.; McLardy-Smith, P.; Gundle, R.; Whitwell, D.; Gibbons, C.L.; Ostlere, S.; Athanasou, N.; Gill, H.S.; Murray, D.W. Pseudotumours associated with metal-on-metal hip resurfacings. J. Bone Jt. Surg. Br. 2008, 90, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Hannemann, F.; Lutzner, J.; Seidler, A.; Drexler, H.; Gunther, K.P.; Schmitt, J. Metal ion concentrations in body fluids after implantation of hip replacements with metal-on-metal bearing—Systematic review of clinical and epidemiological studies. PLoS ONE 2013, 8, e70359. [Google Scholar] [CrossRef] [PubMed]
- Buran, R. Herstellung und Eigenschaften von Hartchromschichten mit Feststoffeinlagerungen. In Berichtsband Verbundwerkstoffe und Werkstoffverbunde; DGM Informationsgesellschaft: Oberursel, Germany, 1993. [Google Scholar]
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Rasovic, I. Water-soluble fullerenes for medical applications. Mater. Sci. Technol. 2016, 33, 777–794. [Google Scholar] [CrossRef]
- Zhu, J.; Ji, Z.; Wang, J.; Sun, R.; Liu, Y.; Wang, Z.; Li, A.; Ma, J.; Wang, T.; Jia, G.; et al. Tumor-inhibitory effect and immunomodulatory activity of fullerol C60(OH)x. Small 2008, 4, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- Goodarzi, S.; Da Ros, T.; Conde, J.; Sefat, F.; Mozafari, M. Fullerene: Biomedical engineers get to revisit an old friend. Mater. Today 2017, in press. [Google Scholar] [CrossRef]
- Trpkovic, A.; Todorovic-Markovic, B.; Trajkovic, V. Toxicity of pristine versus functionalized fullerenes: Mechanisms of cell damage and the role of oxidative stress. Arch. Toxicol. 2012, 86, 1809–1827. [Google Scholar] [CrossRef] [PubMed]
- Naota, M.; Shimada, A.; Morita, T.; Inoue, K.; Takano, H. Translocation pathway of the intratracheally instilled C60 fullerene from the lung into the blood circulation in the mouse: Possible association of diffusion and caveolae-mediated pinocytosis. Toxicol. Pathol. 2009, 37, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Morimoto, Y.; Ogami, A.; Myojyo, T.; Tanaka, I.; Shimada, M.; Wang, W.N.; Endoh, S.; Uchida, K.; Nakazato, T.; et al. Gene expression profiles in rat lung after inhalation exposure to C60 fullerene particles. Toxicology 2009, 258, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, N.; Matsumoto, K.; Endoh, S.; Maru, J.; Nakanishi, J. In vitro and in vivo genotoxicity tests on fullerene C60 nanoparticles. Toxicol. Lett. 2009, 191, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Gharbi, N.; Pressac, M.; Hadchouel, M.; Szwarc, H.; Wilson, S.R.; Moussa, F. Fullerene is a powerful antioxidant in vivo with no acute or subacute toxicity. Nano Lett. 2005, 5, 2578–2585. [Google Scholar] [CrossRef] [PubMed]
- Zogovic, N.S.; Nikolic, N.S.; Vranjes-Djuric, S.D.; Harhaji, L.M.; Vucicevic, L.M.; Janjetovic, K.D.; Misirkic, M.S.; Todorovic-Markovic, B.M.; Markovic, Z.M.; Milonjic, S.K.; et al. Opposite effects of nanocrystalline fullerene (C(60)) on tumour cell growth in vitro and in vivo and a possible role of immunosupression in the cancer-promoting activity of C(60). Biomaterials 2009, 30, 6940–6946. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, N.; Vranjes-Ethuric, S.; Jankovic, D.; Ethokic, D.; Mirkovic, M.; Bibic, N.; Trajkovic, V. Preparation and biodistribution of radiolabeled fullerene C60 nanocrystals. Nanotechnology 2009, 20. [Google Scholar] [CrossRef] [PubMed]
- Bullard-Dillard, R.; Creek, K.E.; Scrivens, W.A.; Tour, J.M. Tissue Sites of Uptake of 14C-Labeled C60. Bioorg. Chem. 1996, 24, 376–385. [Google Scholar] [CrossRef]
- Ai, J.; Biazar, E.; Jafarpour, M.; Montazeri, M.; Majdi, A.; Aminifard, S.; Zafari, M.; Akbari, H.R.; Rad, H.G. Nanotoxicology and nanoparticle safety in biomedical designs. Int. J. Nanomed. 2011, 6, 1117–1127. [Google Scholar]
- Kolosnjaj, J.; Szwarc, H.; Moussa, F. Toxicity studies of fullerenes and derivatives. Adv. Exp. Med. Biol. 2007, 620, 168–180. [Google Scholar] [PubMed]
- Fortner, J.D.; Lyon, D.Y.; Sayes, C.M.; Boyd, A.M.; Falkner, J.C.; Hotze, E.M.; Alemany, L.B.; Tao, Y.J.; Guo, W.; Ausman, K.D.; et al. C60 in water: Nanocrystal formation and microbial response. Environ. Sci. Technol. 2005, 39, 4307–4316. [Google Scholar] [CrossRef] [PubMed]
- Leslie, I.J.; Williams, S.; Brown, C.; Anderson, J.; Isaac, G.; Hatto, P.; Ingham, E.; Fisher, J. Surface engineering: A low wearing solution for metal-on-metal hip surface replacements. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 90, 558–565. [Google Scholar] [CrossRef] [PubMed]
- De Villiers, D.; Traynor, A.; Collins, S.N.; Banfield, S.; Housden, J.; Shelton, J.C. Chromium nitride coating for large diameter metal-on-polyethylene hip bearings under extreme adverse hip simulator conditions. Wear 2015, 328–329, 363–368. [Google Scholar] [CrossRef]
- Royle, M.; Lu, F.; Fox, A.; Housden, J.; Leyland, A.; Ellison, P.; Collins, S.; Lali, F.V.; Hart, A.J.; Shelton, J.C. CrN-coated metal-on-metal hip bearings have the potential to reduce wear and cobalt ion release. In Proceedings of the 2011 Annual Meeting of the Orthopedic Research Society, Long Beach, CA, USA, 13–15 January 2011. [Google Scholar]
- Williams, S.; Isaac, G.; Hatto, P.; Stone, M.H.; Ingham, E.; Fisher, J. Comparative wear under different conditions of surface-engineered metal-on-metal bearings for total hip arthroplasty. J. Arthroplast. 2004, 19, 112–117. [Google Scholar] [CrossRef]
- Romankiewicz, K.; Stollorz, K.; Metzner, M. Eigenschaftsmodifikation von chrombasierten Schichtsystemen durch modularen Einbau mikro- und nanoskaliger Dispersionspartikel. Galvanotechn 2009, 100, 2460–2468. [Google Scholar]
- Heister, E.; Brunner, E.W.; Dieckmann, G.R.; Jurewicz, I.; Dalton, A.B. Are Carbon Nanotubes a Natural Solution? Applications in Biology and Medicine. ACS Appl. Mater. Interfaces 2013, 5, 1870–1891. [Google Scholar] [CrossRef] [PubMed]
- Uo, M.; Akasaka, T.; Watari, F.; Sato, Y.; Tohji, K. Toxicity evaluations of various carbon nanomaterials. Dent. Mater. J. 2011, 30, 245–263. [Google Scholar] [CrossRef] [PubMed]
- Hannemann, F.; Hartmann, A.; Schmitt, J.; Lützner, J.; Seidler, A.; Campbell, P.; Delaunay, C.P.; Drexler, H.; Ettema, H.B.; García-Cimbrelo, E.; et al. European multidisciplinary consensus statement on the use and monitoring of metal-on-metal bearings for total hip replacement and hip resurfacing. Orthop. Traumatol. Surg. Res. 2013, 99, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Gruebl, A.; Marker, M.; Brodner, W.; Giurea, A.; Heinze, G.; Meisinger, V.; Zehetgruber, H.; Kotz, R. Long-term follow-up of metal-on-metal total hip replacement. J. Orthop. Res. 2007, 25, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Sonntag, R.; Reinders, J.; Kretzer, J.P. What’s next? Alternative materials for articulation in total joint replacement. Acta Biomater. 2012, 8, 2434–2441. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P. Clinical and diagnostic challenges of metal implant allergy using the example of orthopaedic surgical implants: Part 15 of the Series Molecular Allergology. Allergo J. Int. 2014, 23, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, M.T.; Pietrabissa, R. The in-vivo wear performance of prosthetic femoral heads with titanium nitride coating. Biomaterials 2000, 21, 907–913. [Google Scholar] [CrossRef]
- Harman, M.K.; Banks, S.A.; Hodge, W.A. Wear analysis of a retrieved hip implant with titanium nitride coating. J. Arthroplast. 1997, 12, 938–945. [Google Scholar] [CrossRef]
- Jaffe, W.L.; Strauss, E.J.; Cardinale, M.; Herrera, L.; Kummer, F.J. Surface Oxidized Zirconium Total Hip Arthroplasty Head Damage Due to Closed Reduction: Effects on Polyethylene Wear. J. Arthroplast. 2009, 24, 898–902. [Google Scholar] [CrossRef] [PubMed]
- Saikko, V.; Calonius, O. Slide track analysis of the relative motion between femoral head and acetabular cup in walking and in hip simulators. J. Biomech. 2002, 35, 455–464. [Google Scholar] [CrossRef]
- Kretzer, J.; Krachler, M.; Reinders, J.; Jakubowitz, E.; Thomsen, M.; Heisel, C. Determination of Low Wear Rates in Metal-on-Metal Hip Joint Replacements Based on Ultra Trace Element Analysis in Simulator Studies. Tribol. Lett. 2010, 37, 23–29. [Google Scholar] [CrossRef]












| Parameter | CoCr (Uncoated) | Reference Hard Chrome (6+) | Custom Hard Chrome (6+) | Trivalent Chrome (3+) |
|---|---|---|---|---|
| Layer thickness in µm 1 | - | 23 | 35–40 | 10 |
| Current efficiency η in % | - | 10 | ~13.5 | ~14 |
| Microhardness in HV (Vickers hardness) | 475–540 HV0.025 | 900–950 HV0.025 | 900–950 HV0.025 | 850–900 HV0.005 2 |
| Number of cracks in cracks/cm | - | ~70 | ~100–120 | ~300 |
| Surface roughness Ra in µm | 0.03 | 0.05 | 0.06 | 0.1 |
| CoCr vs. CoCr | CoCr vs. Reference Hard Chrome (6+) | CoCr vs. Custom Hard Chrome (6+) | CoCr vs. Hard Chrome (6+) Incl. C60 | CoCr vs. Trivalent Chrome (3+) Incl. C60 |
|---|---|---|---|---|
| 9.37 ± 9.14 | 2.31 ± 1.40 | 6.41 ± 5.18 | 10.72 ± 9.99 | 1.92 ± 1.12 |
| Parameter | Custom Hard Chrome (6+) 1 | Reference Hard Chrome (6+) 2 | Trivalent Chrome (3+) |
|---|---|---|---|
| Temperature in °C | 55 | 55 | 35 |
| Current density in A/dm 2 | 25 | 40 | 10 |
| pH | <1 | <1 | 2.6 (2.3–2.7) |
| Time in min | 180 | 90 | 45 |
| # | Pin | Plate | Comment |
|---|---|---|---|
| 1 | CoCr | CoCr | Uncoated reference |
| 2 | CoCr | Reference hard chrome (6+) 1 | Industrial hard chrome coating (CrO3: 280 g/L) vs. uncoated pin |
| 3 | CoCr | Custom hard chrome (6+) 1 | Chromium coating (CrO3: 50 g/L) vs. uncoated pin |
| 4 | CoCr | Hard chrome (6+) 1 incl. C60 | Chromium coating (CrO3: 50 g/L) with fullerenes vs. uncoated pin |
| 5 | CoCr | Trivalent chrome (3+) 2 incl. C60 | Trivalent chrome coating with fullerenes vs. uncoated pin |
| 6 | Hard chrome (6+) 1 incl. C60 | Hard chrome (6+) 1 incl. C60 | Chromium coating (CrO3: 50 g/L) with fullerenes, both pin and plate coated |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sonntag, R.; Feige, K.; Dos Santos, C.B.; Kretzer, J.P. Hard Chrome-Coated and Fullerene-Doped Metal Surfaces in Orthopedic Bearings. Materials 2017, 10, 1449. https://doi.org/10.3390/ma10121449
Sonntag R, Feige K, Dos Santos CB, Kretzer JP. Hard Chrome-Coated and Fullerene-Doped Metal Surfaces in Orthopedic Bearings. Materials. 2017; 10(12):1449. https://doi.org/10.3390/ma10121449
Chicago/Turabian StyleSonntag, Robert, Katja Feige, Claudia Beatriz Dos Santos, and Jan Philippe Kretzer. 2017. "Hard Chrome-Coated and Fullerene-Doped Metal Surfaces in Orthopedic Bearings" Materials 10, no. 12: 1449. https://doi.org/10.3390/ma10121449
APA StyleSonntag, R., Feige, K., Dos Santos, C. B., & Kretzer, J. P. (2017). Hard Chrome-Coated and Fullerene-Doped Metal Surfaces in Orthopedic Bearings. Materials, 10(12), 1449. https://doi.org/10.3390/ma10121449





