1. Introduction
Concrete carbonation is one of the main causes for the deterioration of reinforced concrete (RC) structures. This phenomenon is caused by the penetration of atmospheric CO
2 into the concrete, resulting in decreased basicity [
1,
2,
3,
4,
5,
6]. Concrete carbonation is a complex physicochemical process that takes place upon prolonged exposure to atmospheric CO
2. The concentration of CO
2 is variable and depends on the different climatic and atmospheric conditions. Generally, 0.03% CO
2 is present in normal air while in industrially polluted environment, it could reach around 0.3% and at such concentrations, CO
2 can penetrate easily if there is no protection applied on the concrete cover [
7,
8]. This process destroys the passive film that surrounds the reinforcement bars exposing them to corrosive attack [
9,
10,
11,
12,
13,
14].
The carbonation of concrete is one of the most disruptive processes that affects the durability of concrete and causes a significant reduction in its service life [
15,
16].
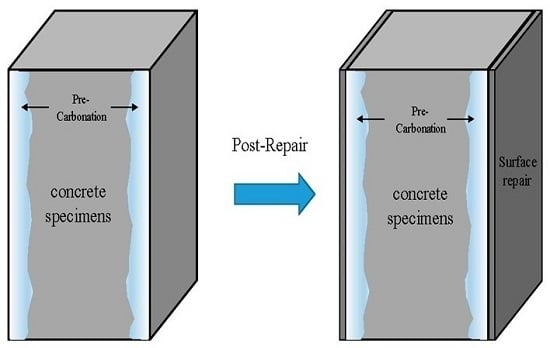
Reinforcement bars can be inflated due to corrosion, which causes the concrete to crack, resulting in decreased strength of the member and degradation of its structural durability. This problem has necessitated the repair of many RC structures in recent times. However, there has been almost no study on post-repair concrete carbonation. Many researchers have proposed carbonation estimation equations, which enable the prediction of the carbonation depth. Such equations were based on results obtained under conditions of indoor accelerated carbonation and exposure experiments, which were performed on concrete that had not undergone repair. Such tests have also been used to develop equations to evaluate the carbonation depth from which the endurance life of concrete can be predicted.
The reduction in durability of RC structures can be caused by a destruction of concrete or the corrosion of the reinforcement bars or a combination of both [
17].
In the report by RILEM, it was stated that a high amount of CH and CSH in concrete might reduce the penetration of CO
2 [
18], but until now it is a controversial topic. It has also been reported that both the type of filler and the cement paste composition play major roles.
A prognosis durability model has been discussed by Czarnecki and Woyciechowski (2013) for the repair of RC structures that suffered carbonation and chloride attack [
19].
Neves et al. [
20] examined the carbonation resistance of concrete by measuring its gas permeability. Kuosa et al. [
21] presented an equation to predict the carbonation caused only by freezing and thawing, from which the endurance life of the concrete could be evaluated. Duprat et al. [
22] conducted accelerated carbonation tests on unrepaired concretes for a probabilistic durability prediction. Kashef-Haghighi et al. [
23] developed a mathematical model of CO
2 absorption based on an accelerated carbonation test on concrete without repair materials. Silva et al. [
24] investigated the carbonation process in recycled aggregate concretes, while Hills et al. [
25] statistically analyzed the carbonation rate in concretes containing no repair materials. Also, the Korea Concrete Institute (KCI) [
26] predicted the rate of carbonation as a function of the water/binder (W/B) ratio.
While the previous studies mentioned above have predicted the carbonation of only concrete without considering repair materials, there have been several other works which have also taken repair materials into consideration in their studies. The accelerated carbonation test for the durability of concrete was mentioned in the Chinese National Standard GB/T 50082-2009 titled “Standard for test methods of long-term performance and durability of ordinary concrete” [
27]. The Architectural Institute of Japan (AIJ) [
28] also assigned coefficients to different types of repair materials for the prediction of carbonation. In addition, Köliö et al. [
29] investigated the corrosion of a reinforcement bar induced by the carbonation of a concrete repair material additive. However, the carbonation prediction equations derived in these studies only considered the carbonation in the original concrete, or carbonation in a concrete to which a repair material had already been applied.
Liu et al. have proposed a mechanism to understand the effect of carbonation and chloride aerosol attack in ordinary Portland cement concrete using 20% CO
2 [
30]. On the other hand, Mohammed et al have investigated the 100% carbonation of self-compacting concrete and found that the replacement of limestone powder (LP) increased the depth of carbonation during the accelerated test, whereas there was no effect if fly ash (FA) or a combination of fly ash and silica fume (FA + SF) was used [
31]. Since there are only a few reports on the accelerated carbonation in post-repair carbonated concrete, especially with respect to experimental and analytical data, there is a need to investigate this phenomenon. Therefore, in the present study, we have focused on concrete structures in which carbonation was already underway. Carbonated building structures were simulated by accelerated carbonation as a sequel to surface repair. The carbonation coefficients for different repair materials were compared by conducting accelerated carbonation tests using 20% CO
2 [
31,
32]. From the values of carbonation coefficients obtained for the different repair materials, the progress of carbonation was predicted using the prediction equation for post-repair carbonation. The reliability of the predicted carbonation depths was examined using both the Finite Difference Method (FDM) and the Finite Element Method (FEM).