Fabrication of Nanosized Island-Like CdO Crystallites-Decorated TiO2 Rod Nanocomposites via a Combinational Methodology and Their Low-Concentration NO2 Gas-Sensing Behavior
Abstract
:1. Introduction
2. Materials and Methods
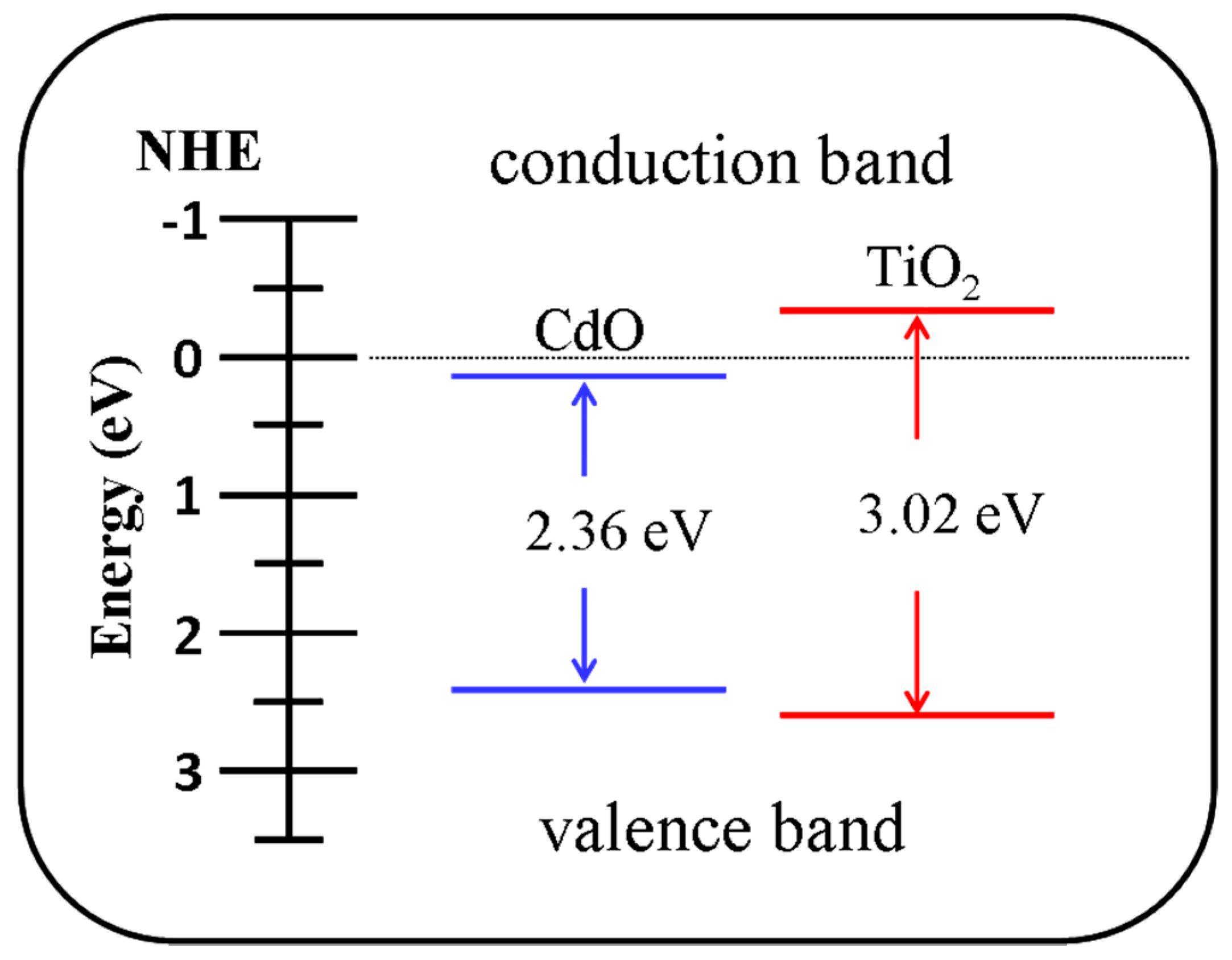
3. Result and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liang, Y.C.; Lee, C.M. Cosputtering crystal growth of zinc oxide-based composite films: From the effects of doping to phase on photoactivity and gas sensing properties. J. Appl. Phys. 2016, 120, 135306–135309. [Google Scholar] [CrossRef]
- Liang, Y.C.; Liao, W.K.; Deng, X.S. Synthesis and substantially enhanced gas sensing sensitivity of homogeneously nanoscale Pd- and Au-particle decorated ZnO nanostructures. J. Alloys Compd. 2014, 599, 87–92. [Google Scholar] [CrossRef]
- Guillén, M.G.; Gámez, F.; Suárez, B.; Queirós, C.; Silva, A.M.G.; Barranco, Á.; Sánchez-Valencia, J.R.; Pedrosa, J.M.; Lopes-Costa, T. Preparation and Optimization of Fluorescent Thin Films of Rosamine-SiO2/TiO2 Composites for NO2 Sensing. Materials 2017, 10, 124. [Google Scholar] [CrossRef]
- Arafat, M.M.; Dinan, B.; Akbar, S.A.; Haseeb, A.S.M.A. Gas Sensors Based on One Dimensional Nanostructured Metal-Oxides: A Review. Sensors 2012, 12, 7207–7258. [Google Scholar] [CrossRef] [PubMed]
- Bian, H.; Ma, S.; Sun, A.; Xu, X.; Yang, G.; Gao, J.; Zhang, Z.; Zhu, H. Characterization and acetone gas sensing properties of electrospun TiO2 nanorods. Superlattices Microstruct. 2015, 81, 107–113. [Google Scholar] [CrossRef]
- Kılınç, N.; Şennik, E.; Işık, M.; Ahsen, A.Ş.; Öztürk, O.; Öztürk, Z.Z. Fabrication and gas sensing properties of C-doped and un-doped TiO2 nanotubes. Ceram. Int. 2014, 40, 109–115. [Google Scholar] [CrossRef]
- Francioso, L.; Taurino, A.M.; Forleo, A.; Siciliano, P. TiO2 nanowires array fabrication and gas sensing properties. Sens. Actuators B 2008, 130, 70–76. [Google Scholar] [CrossRef]
- Yoo, S.; Akbar, S.A.; Sandhage, K.H. Nanocarving of titania (TiO2): A novel approach for fabricating chemical sensing platform. Ceram. Int. 2004, 30, 1121–1126. [Google Scholar] [CrossRef]
- Miller, D.R.; Akbar, S.A.; Morris, P.A. Nanoscale metal oxide-based heterojunctions for gas sensing: A review. Sens. Actuators B 2014, 204, 250–272. [Google Scholar] [CrossRef]
- Lou, Z.; Deng, J.; Wang, L.; Wang, R.; Fei, T.; Zhang, T. A class of hierarchical nanostructures: ZnO surfacefunctionalized TiO2 with enhanced sensing properties. RSC Adv. 2013, 3, 3131–3136. [Google Scholar] [CrossRef]
- Wang, X.; Sang, Y.; Wang, D.; Ji, S.; Liu, H. Enhanced gas sensing property of SnO2 nanoparticles by constructing the SnO2–TiO2 nanobelt heterostructure. J. Alloys Compd. 2015, 639, 571–576. [Google Scholar] [CrossRef]
- Wu, H.; Kan, K.; Wang, L.; Zhang, G.; Yang, Y.; Li, H.; Jing, L.; Shen, P.; Li, L.; Shi, K. Electrospinning of mesoporous p-type In2O3/TiO2 composite nanofibers for enhancing NOx gas sensing properties at room temperature. Cryst. Eng. Commun. 2014, 16, 9116–9124. [Google Scholar] [CrossRef]
- Chandiramouli, R.; Jeyaprakash, B.G. Review of CdO thin films. Solid State Sci. 2013, 16, 102–110. [Google Scholar] [CrossRef]
- Yakuphanoglu, F. Nanocluster n-CdO thin film by sol–gel for solar cell applications. Appl. Surf. Sci. 2010, 257, 1413–1419. [Google Scholar] [CrossRef]
- Velusamy, P.; Babu, R.R.; Ramamurthi, K.; Elangovan, E.; Viegas, J.; Dahlem, M.S.; Arivanandhan, M. Characterization of spray pyrolytically deposited high mobility praseodymium doped CdO thin films. Ceram. Int. 2016, 42, 12675–12685. [Google Scholar] [CrossRef]
- Ueda, N.; Maeda, H.; Hosono, H.; Kawazoe, H. Band-gap widening of CdO thin films. J. Appl. Phys. 1998, 84, 6174–6177. [Google Scholar] [CrossRef]
- Bulakhe, R.N.; Lokhande, C.D. Chemically deposited cubic structured CdO thin films: Use in liquefied petroleum gas sensor. Sens. Actuators B 2014, 200, 245–250. [Google Scholar] [CrossRef]
- Rajesh, N.; Kannan, J.C.; Leonardi, S.G.; Neri, G.; Krishnakumar, T. Investigation of CdO nanostructures synthesized by microwave assisted irradiation technique for NO2 gas detection. J. Alloys Compd. 2014, 607, 54–60. [Google Scholar] [CrossRef]
- Chandiramoulia, R.; Jeyaprakash, B.G. Operating temperature dependent ethanol and formaldehyde detection of spray deposited mixed CdO and MnO2 thin films. RSC Adv. 2015, 5, 43930–43940. [Google Scholar] [CrossRef]
- Ban, S.G.; Liu, X.H.; Ling, T.; Dong, C.K.; Yang, J.; Du, X.W. CdO nanoflake arrays on ZnO nanorod arrays for efficient detection of diethyl ether. RSC Adv. 2016, 6, 2500–2503. [Google Scholar] [CrossRef]
- Gao, H.; Staruch, M.; Jain, M.; Gao, P.X.; Shimpi, P.; Guo, Y.; Cai, W.; Lin, H.J. Structure and magnetic properties of three-dimensional (La, Sr)MnO3 nanofilms on ZnO nanorod arrays. Appl. Phys. Lett. 2011, 98, 123105. [Google Scholar] [CrossRef]
- Baba, K.; Lazzaroni, C.; Nikravech, M. ZnO and Al doped ZnO thin films deposited by Spray Plasma: Effect of the growth time and Al doping on microstructural, optical and electrical properties. Thin Solid Films 2015, 595, 129–135. [Google Scholar] [CrossRef]
- Liang, Y.C.; Lung, T.W. Growth of Hydrothermally Derived CdS-Based Nanostructures with Various Crystal Features and Photoactivated Properties. Nanoscale Res. Lett. 2016, 11, 264. [Google Scholar] [CrossRef] [PubMed]
- Peia, Z.; Weng, S.; Liu, P. Enhanced photocatalytic activity by bulk trapping and spatial separation of charge carriers: A case study of defect and facet mediated TiO2. Appl. Catal. B 2016, 180, 463–470. [Google Scholar] [CrossRef]
- Golestani-Fard, F.; Hashemi, T.; Mackenzie, K.J.D.; Hogarth, C.A. Formation of cadmium stannates studied by electron spectroscopy. J. Mater. Sci. 1983, 18, 3679–3685. [Google Scholar] [CrossRef]
- Moholkar, A.V.; Agawane, G.L.; Sim, K.U.; Kwon, Y.B.; Choi, D.S.; Rajpure, K.Y.; Kim, J.H. Temperature dependent structural, luminescent and XPS studies of CdO:Ga thin films deposited by spray pyrolysis. J. Alloys Compd. 2010, 506, 794–799. [Google Scholar] [CrossRef]
- Tshabalala, Z.P.; Shingange, K.; Dhonge, B.P.; Ntwaeaborwa, O.M.; Mhlongo, G.H.; Motaung, D.E. Fabrication of ultra-high sensitive and selective CH4 room temperature gas sensing of TiO2 nanorods: Detailed study on the annealing temperature. Sens. Actuator B 2017, 238, 402–419. [Google Scholar] [CrossRef]
- Devi, L.G.; Nithya, P.M.; Abraham, C.; Kavitha, R. Influence of surface metallic silver deposit and surface fluorination on the photocatalytic activity of rutile TiO2 for the degradation of crystal violet a cationic dye under UV light irradiation. Mater. Today Commun. 2017, 10, 1–13. [Google Scholar] [CrossRef]
- Liang, Y.C.; Lee, C.M.; Lo, Y.J. Reducing gas-sensing performance of Ce-doped SnO2 thin films through a cosputtering method. RSC Adv. 2017, 7, 4724–4734. [Google Scholar] [CrossRef]
- Liang, Y.C.; Cheng, Y.R. Combinational physical synthesis methodology and crystal features correlated with oxidizing gas detection ability of one-dimensional ZnO–VOx crystalline hybrids. CrystEngCommun 2015, 17, 5801–5807. [Google Scholar] [CrossRef]
- Liang, Y.C.; Lina, T.Y.; Lee, C.M. Crystal growth and shell layer crystal feature-dependent sensing and photoactivity performance of zinc oxide–indium oxide core–shell nanorod heterostructures. CrystEngCommun 2015, 17, 7948–7955. [Google Scholar] [CrossRef]
- Liang, Y.C.; Liu, S.L.; Hsia, H.Y. Physical synthesis methodology and enhanced gas sensing and photoelectrochemical performance of 1D serrated zinc oxide-zinc ferrite nanocomposites. Nanoscale Res. Lett. 2015, 10, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Guo, F.; Liu, S.; Yang, B.; Jiang, Y.; Qi, L.; Fan, X. Both oxygen vacancies defects and porosity facilitated NO2 gas sensing response in 2D ZnO nanowalls at room temperature. J. Alloys Compd. 2016, 682, 352–356. [Google Scholar] [CrossRef]
- Zou, C.W.; Wang, J.; Xie, W. Synthesis and enhanced NO2 gas sensing properties of ZnO nanorods/TiO2 nanoparticles heterojunction composites. J. Colloid Interface Sci. 2016, 478, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gao, J.; Wu, B.; Kan, K.; Xu, S.; Xie, Y.; Li, L.; Shim, K. Designed Synthesis of In2O3 Beads@TiO2–In2O3 Composite Nanofibers for High Performance NO2 Sensor at Room Temperature. ACS Appl. Mater. Interfaces 2015, 7, 27152–27159. [Google Scholar] [CrossRef] [PubMed]
- Epifani, M.; Comini, E.; Díaz, R.; Force, C.; Siciliano, P.; Fagli, G. TiO2 colloidal nanocrystals surface modification by V2O5 species: Investigation by Ti MAS-NMR and H2, CO and NO2 sensing properties. Appl. Surf. Sci. 2015, 351, 1169–1173. [Google Scholar] [CrossRef]
- Zhao, P.X.; Tang, Y.; Mao, J.; Chen, Y.X.; Song, H.; Wang, J.W.; Song, Y.; Liang, Y.Q.; Zhang, X.M. One-Dimensional MoS2-Decorated TiO2 nanotube gas sensors for efficient alcohol sensing. J. Alloys Compd. 2016, 674, 252–258. [Google Scholar] [CrossRef]
- Arafat, M.M.; Haseeb, A.S.M.A.; Akbar, S.A.; Quadir, M.Z. In-situ fabricated gas sensors based on one dimensional core-shell TiO2-Al2O3 nanostructures. Sens. Actuator B 2017, 238, 972–984. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, B.; Zhang, J.; Cui, H.; Pan, Y.; An, H.; Zhai, J. Factors influencing the photocatalytic activity of rutile TiO2 nanorods with different aspect ratios for dye degradation and Cr(VI) photoreduction. Phys. Chem. Chem. Phys. 2015, 17, 18670–18676. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, R.; Shankar, H.; Prakash, T.; Narayanan, V.; Stephen, A. ZnO/CdO composite nanorods for photocatalytic degradation of methylene blueunder visible light. Mater. Chem. Phys. 2011, 125, 277–280. [Google Scholar] [CrossRef]









| Materials | Synthesis Method | NO2 Sensing Condition | Response | Response Time/Recovery Time | Response Definition | Ref. |
|---|---|---|---|---|---|---|
| TiO2−In2O3 | Electrospinning | 2.9 ppm/RT | 3.5 | 8 s/56 s | (Rg–Ra)/Ra (N-type behavior) | [35] |
| TiO2–V2O5 | Sol–gel and solvothermal methods | 2 ppm/200 °C | 0.8 | N/A | (Rg–Ra)/Ra (N-type behavior) | [36] |
| TiO2–MoS2 | Anodization and hydrothermal methods | 100 ppm/150 °C | 1.1 | N/A | Ra/Rg (P-type behavior) | [37] |
| TiO2–Al2O3 | Thermal oxidation | 1000 ppm/650 °C | 1.9 | 180 s/180 s | Rg/Ra (N-type behavior) | [38] |
| TiO2–CdO | Hydrothermal and sputtering methods | 5 ppm/275 °C | 7.41 | 53 s/341 s | Rg/Ra (N-type behavior) | Present work |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.-C.; Xu, N.-C.; Wang, C.-C.; Wei, D.-H. Fabrication of Nanosized Island-Like CdO Crystallites-Decorated TiO2 Rod Nanocomposites via a Combinational Methodology and Their Low-Concentration NO2 Gas-Sensing Behavior. Materials 2017, 10, 778. https://doi.org/10.3390/ma10070778
Liang Y-C, Xu N-C, Wang C-C, Wei D-H. Fabrication of Nanosized Island-Like CdO Crystallites-Decorated TiO2 Rod Nanocomposites via a Combinational Methodology and Their Low-Concentration NO2 Gas-Sensing Behavior. Materials. 2017; 10(7):778. https://doi.org/10.3390/ma10070778
Chicago/Turabian StyleLiang, Yuan-Chang, Nian-Cih Xu, Chein-Chung Wang, and Da-Hua Wei. 2017. "Fabrication of Nanosized Island-Like CdO Crystallites-Decorated TiO2 Rod Nanocomposites via a Combinational Methodology and Their Low-Concentration NO2 Gas-Sensing Behavior" Materials 10, no. 7: 778. https://doi.org/10.3390/ma10070778
APA StyleLiang, Y.-C., Xu, N.-C., Wang, C.-C., & Wei, D.-H. (2017). Fabrication of Nanosized Island-Like CdO Crystallites-Decorated TiO2 Rod Nanocomposites via a Combinational Methodology and Their Low-Concentration NO2 Gas-Sensing Behavior. Materials, 10(7), 778. https://doi.org/10.3390/ma10070778






