Thermostability of Hybrid Thermoelectric Materials Consisting of Poly(Ni-ethenetetrathiolate), Polyimide and Carbon Nanotubes
Abstract
:1. Introduction
2. Materials and Experimental Processes
2.1. Materials
2.2. Preparation of Nano-PETT
2.3. Fabrication of Three-Component Hybrid Films: Nano-PETT/PI/SG-CNT
2.4. Characterization
3. Results and Discussion
3.1. Preparation and Characterization of Nano-PETT/PI/SG-CNT Hybrid Films
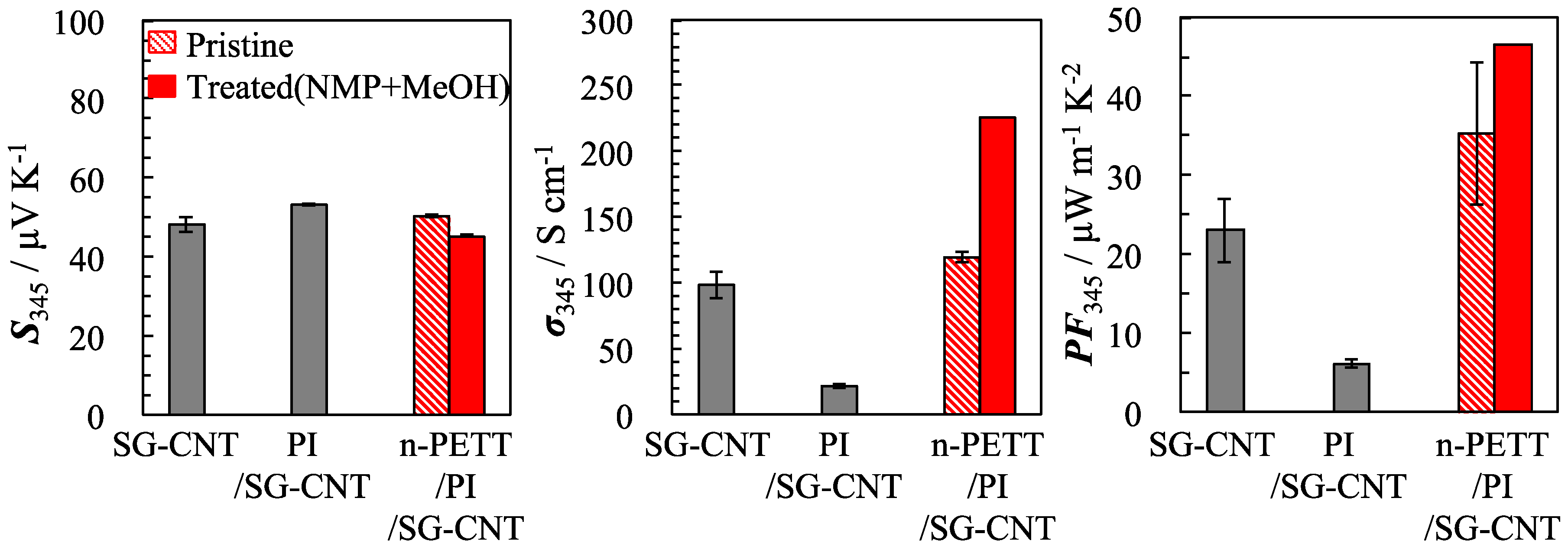
3.2. Thermoelectric Properties of Nano-PETT/PI/SG-CNT Hybrid Films
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Toshima, N. Recent progress of organic and hybrid thermoelectric materials. Synth. Met. 2017, 225, 3–21. [Google Scholar] [CrossRef]
- Goldsmid, H.J. Thermoelectric Refrigeration; Plenum Press: New York, NY, USA, 1964. [Google Scholar]
- Dresselhaus, M.S.; Chen, G.; Tang, M.Y.; Yang, R.; Lee, H.; Wang, D.; Ren, Z.; Fleurial, J.-P.; Gogna, P. New directions for low-dimensional thermoelectric materials. Adv. Mater. 2007, 19, 1043–1053. [Google Scholar] [CrossRef]
- Tritt, T.M.; Boettner, H.; Chen, L. Thermoelectrics: Direct solar thermal energy conversion. Mater. Res. Bull. 2008, 33, 366–368. [Google Scholar] [CrossRef]
- Watanabe, N.; Toshima, N. Preparation and characterization of nanomaterials of tellurium, bismuth, and bismuth telluride. Bull. Chem. Soc. Jpn. 2007, 80, 208–214. [Google Scholar] [CrossRef]
- He, M.; Qiu, F.; Lin, Z. Towards high-performance polymer-based thermoelectric materials. Energy Environ. Sci. 2013, 6, 1352–1361. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Y.; Xu, W.; Zhu, D. Organic thermoelectric materials: Emerging green energy materials converting heat to electricity directly and efficiently. Adv. Mater. 2014, 26, 6829–6851. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, Y.; Liang, Z. Solution processed organic thermoelectrics: Towards flexible thermoelectric modules. Energy Environ. Sci. 2015, 8, 401–422. [Google Scholar] [CrossRef]
- Mateeva, N.; Niculescu, H.; Schlenoff, J.; Testardi, L.R. Correlation of Seebeck coefficient and electric conductivity in polyaniline and polypyrrole. J. Appl. Phys. 1998, 83, 3111–3117. [Google Scholar] [CrossRef]
- Toshima, N. Conductive polymers as a new type of thermoelectric material. Macromol. Symp. 2002, 186, 81–86. [Google Scholar] [CrossRef]
- Hiroshige, Y.; Ookawa, M.; Toshima, N. Thermoelectric figure-of-merit of iodine-doped copolymer of phenylenevinylene with dialkoxyphenylenevinylene. Synth. Met. 2007, 157, 467–474. [Google Scholar] [CrossRef]
- Bubnova, O.; Khan, Z.U.; Malti, A.; Braun, S.; Fahlman, M.; Berggren, M.; Crispin, X. Optimization of the thermoelectric figure of merit in the conducting polymer poly(3,4-ethylenedioxythiophene). Nat. Mater. 2011, 10, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.-H.; Shao, L.; Zhang, K.; Pipe, K.P. Engineered doping of organic semi-conductors for enhanced thermoelectric efficiency. Nat. Mater. 2013, 12, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Mukaida, M.; Kirihara, K.; Naitoh, Y.; Ishida, T. Recent progress on PEDOT-based thermoelectric materials. Materials 2015, 8, 732–750. [Google Scholar] [CrossRef]
- Yu, C.; Choi, K.; Yin, L.; Grunlan, J.C. Light-weight flexible carbon nanotube based organic composites with large thermoelectric power factors. ACS Nano 2011, 5, 7885–7892. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, A.B. Systematic conductivity behavior in conducting polymers: Effects of heterogeneous disorder. Adv. Mater. 2001, 13, 927–941. [Google Scholar] [CrossRef]
- Yakuphanoglu, F.; Senkal, B.F. Electronic and thermoelectric properties of polyaniline organic semiconductor and electrical characterization of Al/PANI MIS diode. J. Phys. Chem. C 2007, 111, 1840–1846. [Google Scholar] [CrossRef]
- Xuan, Y.; Liu, X.; Desbief, S.; Leclère, P.; Fahlman, M.; Lazzaroni, R.; Berggren, M.; Cornil, J.; Crispin, X. Thermoelectric properties of conducting polymers: The case of poly(3-hexylthiophene). Phys. Rev. B 2010, 82, 115454. [Google Scholar] [CrossRef]
- Bounioux, C.; Diaz-Chao, P.; Campoy-Quiles, M.; Martin-Gonzalez, M.S.; Goni, A.R.; Yerushalmi-Rozene, R.; Muller, C. Thermoelectric composites of poly(3-hexyl thiophene) and carbon nanotubes with a large power factor. Energy Environ. Sci. 2013, 6, 918–925. [Google Scholar] [CrossRef]
- Suemori, K.; Hoshino, S.; Kamata, T. Flexible and lightweight thermoelectric generators composed of carbon nanotube–polystyrene composites printed on film substrate. Appl. Phys. Lett. 2013, 103, 153902. [Google Scholar] [CrossRef]
- Wang, L.; Yao, Q.; Bi, H.; Huang, F.; Wang, Q.; Chen, L. Large thermoelectric power factor in polyaniline/graphene nanocomposite films prepared by solution-assistant dispersing method. J. Mater. Chem. A 2014, 2, 11107–11113. [Google Scholar] [CrossRef]
- Yan, H.Y.; Kou, K.C. Enhanced thermoelectric properties in polyaniline composites with polyaniline-coated carbon nanotubes. J. Mater. Sci. 2014, 49, 1222–1228. [Google Scholar] [CrossRef]
- Zhang, B.; Sun, J.; Katz, H.E.; Fang, F.; Opila, R.L. Promising thermoelectric properties of commercial PEDOT: PSS materials and their Bi2Te3 powder composites. ACS Appl. Mater. Interfaces 2010, 2, 3170–3178. [Google Scholar] [CrossRef] [PubMed]
- See, K.C.; Feser, J.P.; Chen, C.E.; Majumdar, A.; Urban, J.J.; Segalman, R.A. Water-processable polymer−nanocrystal hybrids for thermoelectrics. Nano Lett. 2010, 10, 4664–4667. [Google Scholar] [CrossRef] [PubMed]
- Coates, N.E.; Yee, S.K.; McCulloch, B.; See, K.C.; Majumdar, A.; Segalman, R.A.; Urban, J.J. Effect of interfacial properties on polymer–nanocrystal thermoelectric transport. Adv. Mater. 2013, 25, 1629–1633. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Bi, P.; Kang, H.; Wei, W.; Liu, F.; Shi, J.; Peng, L.; Wang, Z.; Xiong, R. Low thermal conductivity and high thermoelectric figure of merit in p-type Sb2Te3/poly(3,4-ethylenedioxythiophene) thermoelectric composites. Appl. Phys. Lett. 2014, 105, 023901. [Google Scholar] [CrossRef]
- Toshima, N.; Jiravanichanun, N.; Marutani, H. Organic thermoelectric materials composed of conducting polymers and metal nanoparticles. J. Electron. Mater. 2012, 41, 1735–1742. [Google Scholar] [CrossRef]
- Yoshida, A.; Toshima, N. Gold nanoparticle and gold nanorod embedded PEDOT: PSS thin films as organic thermoelectric materials. J. Electron. Mater. 2014, 43, 1492–1497. [Google Scholar] [CrossRef]
- Oshima, K.; Shiraishi, Y.; Toshima, N. Novel nano-dispersed polymer complex, poly(nickel 1,1,2,2-ethenetetrathiolate): Preparation and hybridization for n-type of organic thermoelectric materials. Chem. Lett. 2015, 44, 1185–1187. [Google Scholar] [CrossRef]
- Pop, E.; Mann, D.; Wang, Q.; Goodson, K.; Dai, H. Thermal conductance of an individual single-wall carbon nanotube above room temperature. Nano Lett. 2006, 6, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Nakai, Y.; Honda, K.; Yanagi, K.; Kataura, H.; Kato, T.; Yamamoto, T.; Maniwa, Y. Giant Seebeck coefficient in semiconducting single-wall carbon nanotube film. Appl. Phys. Express 2014, 7. [Google Scholar] [CrossRef]
- Nonoguchi, Y.; Ohashi, K.; Kanazawa, R.; Ashiba, K.; Hata, K.; Nakagawa, T.; Adachi, C.; Tanase, T.; Kawai, T. Systematic conversion of single walled carbon nanotubes into n-type thermoelectric materials by molecular dopants. Sci. Rep. 2013, 3, 3344. [Google Scholar] [CrossRef] [PubMed]
- Toshima, N.; Oshima, K.; Anno, H.; Nishinaka, T.; Ichikawa, S.; Iwata, A.; Shiraishi, Y. Novel hybrid organic thermoelectric materials: Three-component hybrid films consisting of a nanoparticle polymer complex, carbon nanotubes, and vinyl polymer. Adv. Mater. 2015, 27, 2246–2251. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Sheng, P.; Di, C.; Jiao, F.; Xu, W.; Qui, D.; Zhu, D. Organic thermoelectric materials and devices based on p- and n-type poly(metal 1,1,2,2-ethenetetrathiolate)s. Adv. Mater. 2012, 24, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Oshima, K.; Asano, H.; Shiraishi, Y.; Toshima, N. Dispersion of carbon nanotubes by poly(Ni-ethenetetrathiolate) for organic thermoelectric hybrid materials. Jpn. J. Appl. Phys. 2016, 55. [Google Scholar] [CrossRef]
- Wei, Q.; Uehara, C.; Mukaida, M.; Kirihara, K.; Ishida, T. Measurement of in-plane thermal conductivity in polymer films. AIP Adv. 2016, 6, 045315-1–045315-6. [Google Scholar] [CrossRef]
- Wei, Q.; Mukaida, M.; Naitoh, Y.; Ishida, T. Morphological change and mobility enhancement in PEDOT: PSS by adding co-solvents. Adv. Mater. 2013, 2831–2836. [Google Scholar] [CrossRef] [PubMed]
- Oshima, K.; Inoue, J.; Sadakata, S.; Shiraishi, Y.; Toshima, N. Hybrid-type organic thermoelectric materials containing nanoparticles as a carrier transport promoter. J. Electron. Met. 2017, 46, 3207–3214. [Google Scholar] [CrossRef]
- Asano, H.; Sakura, N.; Oshima, K.; Shiraishi, Y.; Toshima, N. Development of ethenetetrathiolate hybrid thermoelectric materials consisting of cellulose acetate and semiconductor nanomaterials. Jpn. J. Appl. Phys. 2016, 55, 02BB02/1–02BB07/5. [Google Scholar] [CrossRef]
- Oshima, K.; Yanagawa, Y.; Asano, H.; Shiraishi, Y.; Toshima, N. Improvement of stability of n-type super growth CNTs by hybridization with polymer for organic hybrid thermoelectrics. Synth. Met. 2017, 225, 81–85. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Toshima, N. Oxidation of ethylene catalyzed by colloidal dispersions of poly(sodium acrylate)-protected silver nanoclusters. Colloids Surf. A 2000, 169, 59–66. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Nakayama, M.; Takagi, E.; Tominaga, T.; Toshima, N. Effect of quantity of polymer on catalysis and superstructure size of polymer-protected Pt nanoclusters. Inorg. Chem. Acta 2000, 300–302, 964–969. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Arakawa, D.; Toshima, N. pH-dependent color change of colloidal dispersions of gold nanoclusters: Effect of stabilizer. Eur. Phys. J. E 2002, 8, 377–383. [Google Scholar] [CrossRef] [PubMed]




| Pristine | Treated | |||
|---|---|---|---|---|
| MeOH | NMP | NMP + MeOH | ||
| ρ/g·cm−3 | 1.31 ± 0.06 | 1.12 ± 0.15 | 1.12 ± 0.08 | 0.85 ± 0.04 |
| α/mm2·s−1 | 0.13 ± 0.03 | 0.14 ± 0.00 | 0.13 ± 0.01 | 0.15 ± 0.00 |
| Cp/J·g−1·K−1 | 0.86 ± 0.06 | 0.87 ± 0.01 | 0.80 ± 0.02 | 0.93 ± 0.04 |
| κ/W·m−1·K−1 | 0.18 ± 0.01 | 0.14 ± 0.02 | 0.12 ± 0.01 | 0.12 ± 0.02 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oshima, K.; Sadakata, S.; Asano, H.; Shiraishi, Y.; Toshima, N. Thermostability of Hybrid Thermoelectric Materials Consisting of Poly(Ni-ethenetetrathiolate), Polyimide and Carbon Nanotubes. Materials 2017, 10, 824. https://doi.org/10.3390/ma10070824
Oshima K, Sadakata S, Asano H, Shiraishi Y, Toshima N. Thermostability of Hybrid Thermoelectric Materials Consisting of Poly(Ni-ethenetetrathiolate), Polyimide and Carbon Nanotubes. Materials. 2017; 10(7):824. https://doi.org/10.3390/ma10070824
Chicago/Turabian StyleOshima, Keisuke, Shifumi Sadakata, Hitoshi Asano, Yukihide Shiraishi, and Naoki Toshima. 2017. "Thermostability of Hybrid Thermoelectric Materials Consisting of Poly(Ni-ethenetetrathiolate), Polyimide and Carbon Nanotubes" Materials 10, no. 7: 824. https://doi.org/10.3390/ma10070824
APA StyleOshima, K., Sadakata, S., Asano, H., Shiraishi, Y., & Toshima, N. (2017). Thermostability of Hybrid Thermoelectric Materials Consisting of Poly(Ni-ethenetetrathiolate), Polyimide and Carbon Nanotubes. Materials, 10(7), 824. https://doi.org/10.3390/ma10070824





