Synthesis and Characteristic of Xylan-grafted-polyacrylamide and Application for Improving Pulp Properties
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Xylan-g-PAM
2.3. Preparation of Cationic Fiber Fines
2.4. Characterization of Prepared Products
2.5. Preparation of Handsheet Formation and Mechanical Properties Test
3. Results and Discussion
3.1. Influence of the Synthesis Conditions on Xylan-g-PAM
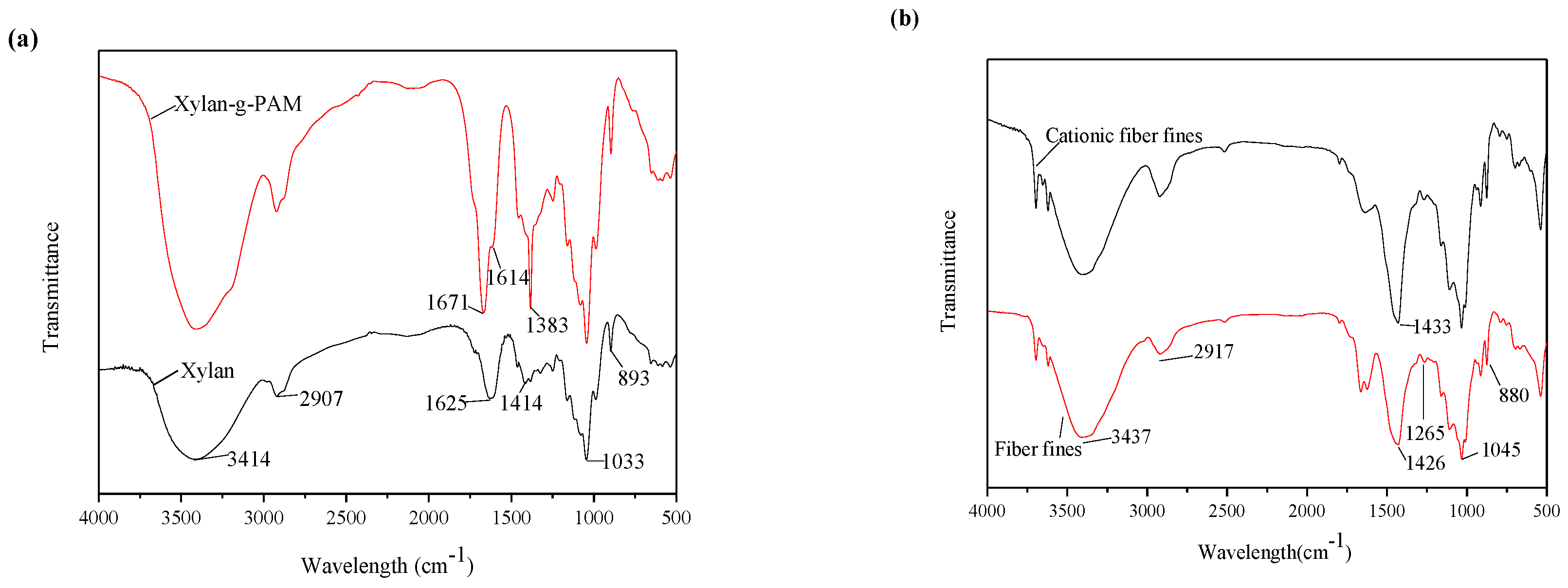
3.2. FTIR Spectra
3.3. TGA Analysis
3.4. 13C-NMR Spectra
3.5. Rheological Study
3.6. Influence of Xylan and Xylan-g-PAM on the Mechanical Properties of Handsheets of Waste Newspaper Pulp
3.7. Influence of Xylan-g-PAM with Fiber Fines or Cationic Fiber Fines on the Mechanical Properties of Handsheets
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.C. Hemicellulose bioconversion. J. Ind. Microbiol. Biotechnol. 2003, 30, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Ebringerova, A.; Heinze, T. Xylan and xylan derivatives-Biopolymers with valuable properties, 1. Naturally occurring xylans structures, isolation procedures and properties. Macromol. Rapid Commun. 2000, 21, 542–556. [Google Scholar] [CrossRef]
- Ebringerová, A. Structural diversity and application potential of hemicelluloses. Macromol. Symp. 2005, 232, 1–12. [Google Scholar] [CrossRef]
- Spiridon, I.; Popa, V.I. Hemicelluloses: Major sources, properties and applications. In Monomers, Polymers and Composites from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2008; pp. 289–304. [Google Scholar]
- Wang, S.Y.; Ren, J.L.; Kong, W.Q.; Gao, C.D.; Liu, C.F.; Peng, F.; Sun, R.C. Influence of urea and glycerol on functional properties of biodegradable PVA/xylan composite films. Cellulose 2014, 21, 495–505. [Google Scholar] [CrossRef]
- Kong, W.Q.; Ren, J.L.; Wang, S.Y.; Li, M.F.; Sun, R.-C. A promising strategy for preparation of cationic xylan by environment-friendly semi-dry oven process. Fibers Polym. 2014, 15, 943–949. [Google Scholar] [CrossRef]
- Saxena, A.; Ragauskas, A.J. Water transmission barrier properties of biodegradable films based on cellulosic whiskers and xylan. Carbohydr. Polym. 2009, 78, 357–360. [Google Scholar] [CrossRef]
- Peng, X.W.; Zhong, L.X.; Ren, J.L.; Sun, R.C. Highly effective adsorption of heavy metal ions from aqueous solutions by macroporous xylan-rich hemicelluloses-based hydrogel. J. Agric. Food Chem. 2012, 60, 3909–3916. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.W.; Ren, J.L.; Zhong, L.X.; Peng, F.; Sun, R.C. Xylan-rich hemicelluloses-graft-acrylic acid ionic hydrogels with rapid responses to pH, salt, and organic solvents. J. Agric. Food Chem. 2011, 59, 8208–8215. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.D.; Ren, J.L.; Zhao, C.; Kong, W.Q.; Dai, Q.Q.; Chen, Q.F.; Liu, C.F.; Sun, R.C. Xylan-based temperature/pH sensitive hydrogels for drug controlled release. Carbohydr. Polym. 2016, 151, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Kataja-Aho, J.; Haavisto, S.; Asikainen, J.; Hyvärinen, S.; Vuoti, S. The influence of cationized birch xylan on wet and dry strength of fine paper. BioResources 2012, 7, 1713–1728. [Google Scholar] [CrossRef]
- Petzold-Welcke, K.; Schwikal, K.; Daus, S.; Heinze, T. Xylan derivatives and their application potential–Mini-review of own results. Carbohydr. Polym. 2014, 100, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Zhai, M.Z.; She, D.; Gao, Y.F. Synthesis and characterization of carboxymethyl xylan-g-poly (propylene oxide) and its application in films. Carbohydr. Polym. 2015, 133, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Q.; Wang, H.H.; Wen, X.X.; Zhang, A.P.; Wang, X.Y.; Zhong, L.X.; Liu, C.F.; Sun, R.C. Synthesis and characterization of xylan grafted with polyethylene glycol in ionic liquid and their use as moisture-absorption/retention biomaterials. Macromol. Mater. Eng. 2016, 301, 287–295. [Google Scholar] [CrossRef]
- Li, W.B.; Zhou, X.S. Modification of the water-insoluble hemicelluloses via free radical copolymerization in diluted alkali aqueous medium. J. Wood Chem. Technol. 2017, 37, 191–200. [Google Scholar] [CrossRef]
- Ünlü, C.H.; Öztekin, N.S.; Atıcı, O.G. Synthesis and thermal characterization of xylan-graft-polyacrylonitrile. Carbohydr. Polym. 2012, 90, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Mocchiutti, P.; Schnell, C.N.; Rossi, G.D.; Peresin, M.S.; Zanuttini, M.A.; Galván, M.V. Cationic and anionic polyelectrolyte complexes of xylan and chitosan. Interaction with lignocellulosic surfaces. Carbohydr. Polym. 2016, 150, 89–98. [Google Scholar]
- Silva, T.C.F.; Colodette, J.L.; Lucia, L.A.; Oliveira, R.C.; Oliveira, F.N.; Silva, L.H.M. Adsorption of chemically modified xylans on eucalyptus pulp and its effect on the pulp physical properties. Ind. Eng. Chem. Res. 2010, 50, 1138–1145. [Google Scholar] [CrossRef]
- Postma, D.; Chimphango, A.F.; Görgens, J.F. Cationization of Eucalyptus grandis 4-O-methyl glucuronoxylan for application as a wet-end additive in a papermaking process. Holzforschung 2014, 68, 519–527. [Google Scholar] [CrossRef]
- Ren, J.L.; Peng, F.; Sun, R.C.; Kennedy, J.F. Influence of hemicellulosic derivatives on the sulfate kraft pulp strength. Carbohydr. Polym. 2009, 75, 338–342. [Google Scholar] [CrossRef]
- Stationwala, M.I.; Mathieu, J.; Karnis, A. On the interaction of wood and mechanical pulping equipment. Part I: Fiber development and generation of fines. J. Pulp Paper Sci. 1996, 25, J155–J160. [Google Scholar]
- Luo, X.; Zhang, L. Immobilization of penicillin G acylase in epoxy-activated magnetic cellulose microspheres for improvement of biocatalytic stability and activities. Biomacromolecules 2010, 11, 2896–2903. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Panda, A.; Pal, S. Synthesis and characterization of a novel polymeric hydrogel based on hydroxypropyl methyl cellulose grafted with polyacrylamide. Cellulose 2012, 19, 933–945. [Google Scholar] [CrossRef]
- Singh, V.; Tiwari, A.; Tripathi, D.N.; Sanghi, R. Microwave assisted synthesis of guar-g-polyacrylamide. Carbohydr. Polym. 2004, 58, 1–6. [Google Scholar] [CrossRef]
- Wang, L.; Xu, Y. Preparation and characterization of graft copolymerization of ethyl acrylate onto hydroxypropyl methylcellulose in aqueous medium. Cellulose 2006, 13, 191–200. [Google Scholar] [CrossRef]
- Sun, R.C.; Sun, X.F.; Liu, G.Q.; Fowler, P.; Tomkinson, J. Structural and physicochemical characterization of hemicelluloses isolated by alkaline peroxide from barley straw. Polym. Int. 2002, 51, 117–124. [Google Scholar] [CrossRef]
- Biswa, D.; Singh, R. Characterisation of carboxymethyl cellulose and polyacrylamide graft copolymer. Carbohydr. Polym. 2004, 57, 379–387. [Google Scholar] [CrossRef]
- Reddy, K.O.; Maheswari, C.U.; Shukla, M.; Song, J.I.; Rajulu, A.V. Tensile and structural characterization of alkali treated Borassus fruit fine fibers. Compos. Part B Eng. 2013, 44, 433–438. [Google Scholar] [CrossRef]
- Kačuráková, M.; Ebringerova, A.; Hirsch, J.; Hromadkova, Z. Infrared study of arabinoxylans. J. Sci. Food Agric. 1994, 66, 423–427. [Google Scholar] [CrossRef]
- Chen, W.H.; Kuo, P.C. Isothermal torrefaction kinetics of hemicellulose, cellulose, lignin and xylan using thermogravimetric analysis. Energy 2011, 36, 6451–6460. [Google Scholar] [CrossRef]
- Peng, X.W.; Ren, J.L.; Sun, R.C. An efficient method for the synthesis of hemicellulosic derivatives with bifunctional groups in butanol/water medium and their rheological properties. Carbohydr. Polym. 2011, 83, 1922–1928. [Google Scholar] [CrossRef]
- Khodjab, M.; Jadac, A.; Eutamene, M.; Benbakhti, A. Preparation and aqueous properties of starch-grafted polyacrylamide copolymers. Starch/Stärke 2009, 61, 81–91. [Google Scholar]
- Söderqvist Lindblad, M.; Albertsson, A.C.; Ranucci, E.; Laus, M.; Giani, E. Biodegradable polymers from renewable sources: Rheological characterization of hemicellulose-based hydrogels. Biomacromolecules 2005, 6, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Hu, H. Preparation and characterization of crosslinked glyoxalated polyacrylamide paper-strengthening agent. J. Appl. Polym. Sci. 2012, 126, 459–469. [Google Scholar] [CrossRef]
- Grattoni, C.A.; Al-Sharji, H.H.; Yang, C.; Muggeridge, A.H.; Zimmerman, R.W. Rheology and permeability of crosslinked polyacrylamide gel. J. Colloid Interface Sci. 2001, 240, 601–607. [Google Scholar] [CrossRef] [PubMed]





| Number | Temperature (°C) | Monomer Concentration (mol/L) | Initiator Concentration (mol/L) | Time (h) | Graft Ratio (%) | Graft Efficiency (%) |
|---|---|---|---|---|---|---|
| 1 | 60 | 0.4 | 0.010 | 4 | 8.3 | 49 |
| 2 | 60 | 0.4 | 0.015 | 4 | 14.7 | 61 |
| 3 | 60 | 0.4 | 0.020 | 4 | 11.4 | 57 |
| 4 | 60 | 0.4 | 0.030 | 4 | 10.2 | 55 |
| 5 | 60 | 0.4 | 0.040 | 4 | 9.3 | 52 |
| 6 | 60 | 0.2 | 0.015 | 4 | 7.5 | 56 |
| 7 | 60 | 0.6 | 0.015 | 4 | 12.3 | 50 |
| 8 | 60 | 0.8 | 0.015 | 4 | 11.2 | 30 |
| 9 | 60 | 1.0 | 0.015 | 4 | 10.6 | 20 |
| 10 | 50 | 0.4 | 0.015 | 4 | 11 | 55 |
| 11 | 70 | 0.4 | 0.015 | 4 | 12.4 | 50 |
| 12 | 80 | 0.4 | 0.015 | 4 | 11.8 | 46 |
| 13 | 60 | 0.4 | 0.015 | 2 | 7 | 48 |
| 14 | 60 | 0.4 | 0.015 | 3 | 10 | 55 |
| 15 | 60 | 0.4 | 0.015 | 5 | 13 | 59 |
| 16 | 60 | 0.4 | 0.015 | 6 | 13 | 58.5 |
| The Amount of Xylan (wt %) | Tear Index (mN·m2/g) | Burst Index (kPa·m2/g) | Tensile Index (Nm/g) | Folding Strength (Time) |
|---|---|---|---|---|
| 0 | 6.02 ± 0.45 | 1.50 ± 0.11 | 22.00 ± 0.91 | 3 ± 0.4 |
| 0.3 | 6.30 ± 0.52 | 1.52 ± 0.13 | 20.13 ± 0.74 | 3 ± 0.4 |
| 0.5 | 6.53 ± 0.48 | 1.61 ± 0.17 | 22.57 ± 0.58 | 3 ± 0.5 |
| 1.0 | 6.54 ± 0.61 | 1.63 ± 0.15 | 23.45 ± 0.63 | 4 ± 0.5 |
| 1.5 | 6.49 ± 0.57 | 1.59 ± 0.17 | 23.12 ± 0.70 | 4 ± 0.5 |
| Grafting Ratio (%) | The Amount of Graft Copolymer (wt %) | Tear Index (mN·m2/g) | Burst Index (kPa·m2/g) | Tensile Index (Nm/g) | Folding Strength (Time) |
|---|---|---|---|---|---|
| 0.00 | 0 | 6.02 ± 0.45 | 1.50 ± 0.11 | 22.00 ± 0.91 | 3 ± 0.4 |
| 8.3 | 1.0 | 7.84 ± 0.32 | 2.15 ± 0.15 | 27.82 ± 0.77 | 5 ± 0.6 |
| 12.4 | 1.0 | 8.25 ± 0.61 | 2.10 ± 0.23 | 28.18 ± 0.83 | 5 ± 0.4 |
| 14.7 | 1.0 | 8.98 ± 0.55 | 2.20 ± 0.20 | 29.82 ± 0.76 | 6 ± 0.5 |
| 14.7 | 0.3 | 7.79 ± 0.51 | 1.57 ± 0.17 | 24.46 ± 0.98 | 3 ± 0.4 |
| 14.7 | 0.5 | 8.10 ± 0.33 | 1.97 ± 0.13 | 26.91 ± 0.87 | 4 ± 0.5 |
| 14.7 | 1.5 | 7.52 ± 0.38 | 1.85 ± 0.16 | 26.30 ± 0.83 | 4 ± 0.5 |
| Sample | Dosage of FF and PAMX (wt %) | Dosage of CF and PAMX (wt %) | Tear Index (mN·m2/g) | Burst Index (kPa·m2/g) | Tensile Index (Nm/g) | Folding Strength (Time) |
|---|---|---|---|---|---|---|
| The blank | 0 | - | 6.02 ± 0.45 | 1.50 ± 0.11 | 22.00 ± 0.91 | 3 ± 0.4 |
| 1 | 1.0 + 0 | - | 8.18 ± 0.57 | 1.70 ± 0.16 | 26.46 ± 0.83 | 4 ± 0.5 |
| 2 | 0.5 + 1.0 | - | 8.19 ± 0.36 | 1.96 ± 0.13 | 29.78 ± 0.73 | 5 ± 0.4 |
| 3 | 0 + 1.0 | 0 + 1.0 | 8.98 ± 0.55 | 2.20 ± 0.20 | 29.82 ± 0.76 | 6 ± 0.5 |
| 4 | - | 1.0 + 0 | 8.01 ± 0.38 | 1.87 ± 0.17 | 29.88 ± 0.87 | 6 ± 0.5 |
| 5 | - | 0.5 + 1.0 | 8.22 ± 0.51 | 1.81 ± 0.16 | 32.80 ± 0.90 | 6 ± 0.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, G.-B.; Kong, W.-Q.; Liu, C.-F.; Sun, R.-C.; Ren, J.-L. Synthesis and Characteristic of Xylan-grafted-polyacrylamide and Application for Improving Pulp Properties. Materials 2017, 10, 971. https://doi.org/10.3390/ma10080971
Xu G-B, Kong W-Q, Liu C-F, Sun R-C, Ren J-L. Synthesis and Characteristic of Xylan-grafted-polyacrylamide and Application for Improving Pulp Properties. Materials. 2017; 10(8):971. https://doi.org/10.3390/ma10080971
Chicago/Turabian StyleXu, Gui-Bin, Wei-Qing Kong, Chuan-Fu Liu, Run-Cang Sun, and Jun-Li Ren. 2017. "Synthesis and Characteristic of Xylan-grafted-polyacrylamide and Application for Improving Pulp Properties" Materials 10, no. 8: 971. https://doi.org/10.3390/ma10080971
APA StyleXu, G.-B., Kong, W.-Q., Liu, C.-F., Sun, R.-C., & Ren, J.-L. (2017). Synthesis and Characteristic of Xylan-grafted-polyacrylamide and Application for Improving Pulp Properties. Materials, 10(8), 971. https://doi.org/10.3390/ma10080971







