Mechanical Properties of a Newly Additive Manufactured Implant Material Based on Ti-42Nb
Abstract
:1. Introduction
2. Results
2.1. SLM Powder Synthesis and Characterization
2.2. Additive Manufacturing
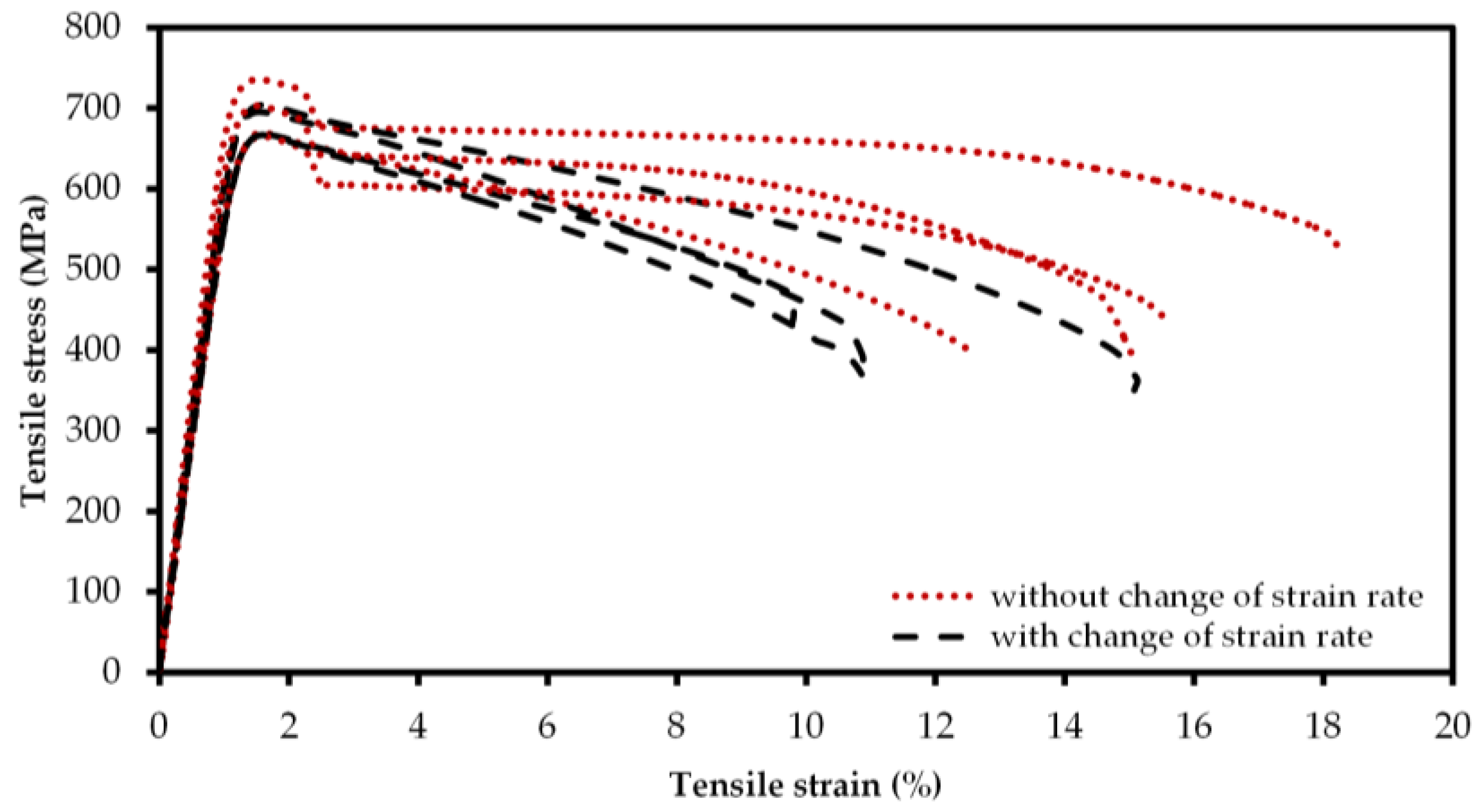
2.3. Mechanical Characterization
3. Discussion
4. Materials and Methods
4.1. Powder Synthesis
4.2. Specimen Manufacturing
4.3. Mechanical Test Setup
4.4. Chemical and Microstructural Characterization
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chandrasekar, C.R.; Grimer, R.J.; Carter, S.R.; Tillman, R.M.; Abudu, A.T. Modular endoprosthetic replacement for metastatic tumours of the proximal femur. J. Orthop. Surg. Res. 2008, 3, 50. [Google Scholar] [CrossRef] [PubMed]
- Lazerges, C.; Dagneaux, L.; Degeorge, B.; Tardy, N.; Coulet, B.; Chammas, M. Composite reverse shoulder arthroplasty can provide good function and quality of life in cases of malignant tumour of the proximal humerus. Int. Orthop. 2017, 41, 2619–2625. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, Y.; Wu, S.; Lin, H.; Yang, Y.; Fan, S.; Gu, C.; Wang, J.; Song, C. Customized a Ti6Al4V Bone Plate for Complex Pelvic Fracture by Selective Laser Melting. Materials 2017, 10, 35. [Google Scholar] [CrossRef] [PubMed]
- Dattani, R. Femoral osteolysis following total hip replacement. Postgrad. Med. J. 2007, 83, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Ries, M.D.; Link, T.M. Monitoring and risk of progression of osteolysis after total hip arthroplasty. Instr. Course Lect. 2013, 62, 207–214. [Google Scholar] [PubMed]
- Niinomi, M.; Nakai, M.; Hieda, J. Development of new metallic alloys for biomedical applications. Acta Biomater. 2012, 8, 3888–3903. [Google Scholar] [CrossRef] [PubMed]
- Sidambe, A. Biocompatibility of Advanced Manufactured Titanium Implants—A Review. Materials 2014, 7, 8168–8188. [Google Scholar] [CrossRef] [PubMed]
- Niinomi, M. Mechanical properties of biomedical titanium alloys. Mater. Sci. Eng. A 1998, 243, 231–236. [Google Scholar] [CrossRef]
- Markhoff, J.; Krogull, M.; Schulze, C.; Rotsch, C.; Hunger, S.; Bader, R. Biocompatibility and Inflammatory Potential of Titanium Alloys Cultivated with Human Osteoblasts, Fibroblasts and Macrophages. Materials 2017, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Quinn, R.K. Electrochemical and Surface Analytical Characterization of Titanium and Titanium Hydride Thin Film Electrode Oxidation. J. Electrochem. Soc. 1978, 125, 1790–1796. [Google Scholar] [CrossRef]
- Hussein, M.; Mohammed, A.; Al-Aqeeli, N. Wear Characteristics of Metallic Biomaterials: A Review. Materials 2015, 8, 2749–2768. [Google Scholar] [CrossRef]
- Jardini, A.L.; Larosa, M.A.; Maciel, F.R.; Zavaglia, C.A.; Bernardes, L.F.; Lambert, C.S.; Calderoni, D.R.; Kharmandayan, P. Cranial reconstruction: 3D biomodel and custom-built implant created using additive manufacturing. J. Cranio-Maxillo-Fac. Surg. 2014, 42, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
- Pilliar, R.M.; Deporter, D.A.; Watson, P.A.; Valiquette, N. Dental implant design—Effect on bone remodeling. J. Biomed. Mater. Res. 1991, 25, 467–483. [Google Scholar] [CrossRef] [PubMed]
- Elias, C.N.; Rocha, F.A.; Nascimento, A.L.; Coelho, P.G. Influence of implant shape, surface morphology, surgical technique and bone quality on the primary stability of dental implants. J. Mech. Behav. Biomed. Mater. 2012, 16, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, C.; Zhao, H.; Qu, S.; Li, X.; Li, Y. New Developments of Ti-Based Alloys for Biomedical Applications. Materials 2014, 7, 1709–1800. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-W.; Lee, C.-C.; Fu, P.-Y.; Lin, S.-C. The effects of flute shape and thread profile on the insertion torque and primary stability of dental implants. Med. Eng. Phys. 2012, 34, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Murr, L.E.; Quinones, S.A.; Gaytan, S.M.; Lopez, M.I.; Rodela, A.; Martinez, E.Y.; Hernandez, D.H.; Martinez, E.; Medina, F.; Wicker, R.B. Microstructure and mechanical behavior of Ti-6Al-4V produced by rapid-layer manufacturing, for biomedical applications. J. Mech. Behav. Biomed. Mater. 2009, 2, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Additive manufacturing techniques and their biomedical applications. Fam. Med. Community Health 2017, 5, 286–298. [Google Scholar] [CrossRef]
- Han, M.-K.; Kim, J.-Y.; Hwang, M.-J.; Song, H.-J.; Park, Y.-J. Effect of Nb on the Microstructure, Mechanical Properties, Corrosion Behavior, and Cytotoxicity of Ti-Nb Alloys. Materials 2015, 8, 5986–6003. [Google Scholar] [CrossRef] [PubMed]
- Markhoff, J.; Weinmann, M.; Schulze, C.; Bader, R. Influence of different grained powders and pellets made of Niobium and Ti-42Nb on human cell viability. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 73, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Zweymueller, K.A.; Lintner, F.K.; Semltich, M.F. Biologic Fixation of a Press-Fit Titanium Hip Joint Endoprosthesis. Clin. Orthop. Relat. Res. 1988, 235, 195–206. [Google Scholar]
- McKellop, H.; Ebramzadeh, E.; Niederer, P.G.; Sarmiento, A. Comparison of the stability of press-fit hip prosthesis femoral stems using a synthetic model femur. J. Orthop. Res. 1991, 9, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Adler, E.; Stuchin, S.A.; Kummer, F.J. Stability of press-fit acetabular cups. J. Arthroplast. 1992, 7, 295–301. [Google Scholar] [CrossRef]
- Morscher, E.W. Current status of acetabular fixation in primary total hip arthroplasty. Clin. Orthop. Relat. Res. 1992, 274, 172–193. [Google Scholar] [CrossRef]
- Prashanth, K.; Zhuravleva, K.; Okulov, I.; Calin, M.; Eckert, J.; Gebert, A. Mechanical and Corrosion Behavior of New Generation Ti-45Nb Porous Alloys Implant Devices. Technologies 2016, 4, 33. [Google Scholar] [CrossRef]
- Lindahl, O.; Lindgren, A.G.H. Cortical Bone in Man II. Variation in Tensile Strength with Age and Sex. Acta Orthop. Scand. 1967, 38, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Evans, F.G. Mechanical properties and histology of cortical bone from younger and older men. Anat. Rec. 1976, 185, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.; Kuhn, J.L.; Ciarelli, M.J.; Goldstein, S.A. The elastic moduli of human subchondral, trabecular, and cortical bone tissue and the size-dependency of cortical bone modulus. J. Biomech. 1990, 23, 1103–1113. [Google Scholar] [CrossRef]
- Rho, J.Y.; Ashman, R.B.; Turner, C.H. Young’s modulus of trabecular and cortical bone material: Ultrasonic and microtensile measurements. J. Biomech. 1993, 26, 111–119. [Google Scholar] [CrossRef]
- Melvin, J.W. Fracture Mechanics of Bone. J. Biomech. Eng. 1993, 115, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Rho, J.-Y.; Kuhn-Spearing, L.; Zioupos, P. Mechanical properties and the hierarchical structure of bone. Med. Eng. Phys. 1998, 20, 92–102. [Google Scholar] [CrossRef]
- Kuroda, D.; Niinomi, M.; Morinaga, M.; Kato, Y.; Yashiro, T. Design and mechanical properties of new β type titanium alloys for implant materials. Mater. Sci. Eng. A 1998, 243, 244–249. [Google Scholar] [CrossRef]
- Sharir, A.; Barak, M.M.; Shahar, R. Whole bone mechanics and mechanical testing. Vet. J. 2008, 177, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Facchini, L.; Magalini, E.; Robotti, P.; Molinari, A.; Höges, S.; Wissenbach, K. Ductility of a Ti-6Al-4V alloy produced by selective laser melting of prealloyed powders. Rapid Prototyp. J. 2010, 16, 450–459. [Google Scholar] [CrossRef]
- Engh, C.A.; Bobyn, J.D.; Glassman, A.H. Porous-coated hip replacement. The factors governing bone ingrowth, stress shielding, and clinical results. J. Bone Jt. Surg. Br. Vol. 1987, 69, 45–55. [Google Scholar]
- Niinomi, M.; Nakai, M. Titanium-Based Biomaterials for Preventing Stress Shielding between Implant Devices and Bone. Int. J. Biomater. 2011, 2011, 836587. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.; Tanner, K.E. Fatigue failure of cancellous bone: A possible cause of implant migration and loosening. J. Bone Jt. Surg. Br. Vol. 1997, 79, 181–182. [Google Scholar] [CrossRef]
- Krakhmalev, P.; Fredriksson, G.; Yadroitsava, I.; Kazantseva, N.; Du Plessis, A.; Yadroitsev, I. Deformation Behavior and Microstructure of Ti6Al4V Manufactured by SLM. Phys. Procedia 2016, 83, 778–788. [Google Scholar] [CrossRef]
- Rengier, F.; Mehndiratta, A.; von Tengg-Kobligk, H.; Zechmann, C.M.; Unterhinninghofen, R.; Kauczor, H.-U.; Giesel, F.L. 3D printing based on imaging data: Review of medical applications. Int. J. Comput. Assist. Radiol. Surg. 2010, 5, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Gross, B.C.; Erkal, J.L.; Lockwood, S.Y.; Chen, C.; Spence, D.M. Evaluation of 3D printing and its potential impact on biotechnology and the chemical sciences. Anal. Chem. 2014, 86, 3240–3253. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Possel, J.K.; Wacongne, C.; van Ham, A.F.; Klink, P.C.; Roelfsema, P.R. 3D printing and modelling of customized implants and surgical guides for non-human primates. J. Neurosci. Methods 2017, 286, 38–55. [Google Scholar] [CrossRef] [PubMed]
- Badrossamay, M.; Childs, T.H.C. Further studies in selective laser melting of stainless and tool steel powders. Int. J. Mach. Tools Manuf. 2007, 47, 779–784. [Google Scholar] [CrossRef]
- Mumtaz, K.A.; Erasenthiran, P.; Hopkinson, N. High density selective laser melting of Waspaloy®. J. Mater. Process. Technol. 2008, 195, 77–87. [Google Scholar] [CrossRef]
- Jinhui, L.; Ruidi, L.; Wenxian, Z.; Liding, F.; Huashan, Y. Study on formation of surface and microstructure of stainless steel part produced by selective laser melting. Mater. Sci. Technol. 2013, 26, 1259–1264. [Google Scholar] [CrossRef]
- Li, R.; Shi, Y.; Wang, Z.; Wang, L.; Liu, J.; Jiang, W. Densification behavior of gas and water atomized 316L stainless steel powder during selective laser melting. Appl. Surf. Sci. 2010, 256, 4350–4356. [Google Scholar] [CrossRef]
- Louvis, E.; Fox, P.; Sutcliffe, C.J. Selective laser melting of aluminium components. J. Mater. Process. Technol. 2011, 211, 275–284. [Google Scholar] [CrossRef]
- Santos, E.C.; Osakada, K.; Shiomi, M.; Kitamura, Y.; Abe, F. Microstructure and mechanical properties of pure titanium models fabricated by selective laser melting. Proc. Inst. Mech. Eng. Part C 2004, 218, 711–719. [Google Scholar] [CrossRef]
- Thijs, L.; Verhaeghe, F.; Craeghs, T.; van Humbeeck, J.; Kruth, J.-P. A study of the microstructural evolution during selective laser melting of Ti–6Al–4V. Acta Mater. 2010, 58, 3303–3312. [Google Scholar] [CrossRef]
- Leuders, S.; Thöne, M.; Riemer, A.; Niendorf, T.; Tröster, T.; Richard, H.A.; Maier, H.J. On the mechanical behaviour of titanium alloy TiAl6V4 manufactured by selective laser melting: Fatigue resistance and crack growth performance. Int. J. Fatigue 2013, 48, 300–307. [Google Scholar] [CrossRef]
- Lyons, B. Additive Manufacturing in Aerospace: Examples and Research Outlook. Bridge 2012, 44, 13–19. [Google Scholar]
- Zadpoor, A.A.; Malda, J. Additive Manufacturing of Biomaterials, Tissues, and Organs. Ann. Biomed. Eng. 2017, 45, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, B.; Kruth, J.-P. Selective laser melting of biocompatible metals for rapid manufacturing of medical parts. Rapid Prototyp. J. 2007, 13, 196–203. [Google Scholar] [CrossRef]
- Long, M.; Rack, H.J. Titanium alloys in total joint replacement—A materials science perspective. Biomaterials 1998, 19, 1621–1639. [Google Scholar] [CrossRef]
- Ankem, S.; Greene, C.A. Recent developments in microstructure/property relationships of beta titanium alloys. Mater. Sci. Eng. A 1999, 263, 127–131. [Google Scholar] [CrossRef]
- Niinomi, M. Recent research and development in titanium alloys for biomedical applications and healthcare goods. Sci. Technol. Adv. Mater. 2003, 4, 445–454. [Google Scholar] [CrossRef]
- Rack, H.J.; Qazi, J.I. Titanium alloys for biomedical applications. Mater. Sci. Eng. C 2006, 26, 1269–1277. [Google Scholar] [CrossRef]
- Fischer, M.; Joguet, D.; Robin, G.; Peltier, L.; Laheurte, P. In situ elaboration of a binary Ti-26Nb alloy by selective laser melting of elemental titanium and niobium mixed powders. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 62, 852–859. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Laheurte, P.; Acquier, P.; Joguet, D.; Peltier, L.; Petithory, T.; Anselme, K.; Mille, P. Synthesis and characterization of Ti-27.5Nb alloy made by CLAD® additive manufacturing process for biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 75, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Han, C.; Choma, T.; Wei, Q.; Yan, C.; Song, B.; Shi, Y. Effect of Nb content on microstructure, property and in vitro apatite-forming capability of Ti-Nb alloys fabricated via selective laser melting. Mater. Des. 2017, 126, 268–277. [Google Scholar] [CrossRef]
- Murray, J.L. The Nb−Ti (Niobium-Titanium) system. Bull. Alloy Phase Diagr. 1981, 2, 55–61. [Google Scholar] [CrossRef]
- Ozaki, T.; Matsumoto, H.; Watanabe, S.; Hanada, S. Beta Ti Alloys with Low Young’s Modulus. Mater. Trans. 2004, 45, 2776–2779. [Google Scholar] [CrossRef]
- Ahmed, T.; Rack, H.J. Martensitic transformations in Ti-(16–26 at %) Nb alloys. J. Mater. Sci. 1996, 31, 4267–4276. [Google Scholar] [CrossRef]
- Herzog, D.; Seyda, V.; Wycisk, E.; Emmelmann, C. Additive manufacturing of metals. Acta Mater. 2016, 117, 371–392. [Google Scholar] [CrossRef]
- Afonso, C.R.M.; Aleixo, G.T.; Ramirez, A.J.; Caram, R. Influence of cooling rate on microstructure of Ti–Nb alloy for orthopedic implants. Mater. Sci. Eng. C 2007, 27, 908–913. [Google Scholar] [CrossRef]
- Al-Zain, Y.; Kim, H.Y.; Koyano, T.; Hosoda, H.; Miyazaki, S. A comparative study on the effects of the ω and α phases on the temperature dependence of shape memory behavior of a Ti–27Nb alloy. Scr. Mater. 2015, 103, 37–40. [Google Scholar] [CrossRef]
- Bönisch, M.; Calin, M.; Waitz, T.; Panigrahi, A.; Zehetbauer, M.; Gebert, A.; Skrotzki, W.; Eckert, J. Thermal stability and phase transformations of martensitic Ti-Nb alloys. Sci. Technol. Adv. Mater. 2013, 14, 5004. [Google Scholar] [CrossRef] [PubMed]
- Lopes, E.S.N.; Cremasco, A.; Afonso, C.R.M.; Caram, R. Effects of double aging heat treatment on the microstructure, Vickers hardness and elastic modulus of Ti–Nb alloys. Mater. Charact. 2011, 62, 673–680. [Google Scholar] [CrossRef]
- Rafi, H.K.; Karthik, N.V.; Gong, H.; Starr, T.L.; Stucker, B.E. Microstructures and Mechanical Properties of Ti6Al4V Parts Fabricated by Selective Laser Melting and Electron Beam Melting. J. Mater. Eng. Perform. 2013, 22, 3872–3883. [Google Scholar] [CrossRef]
- Mertens, A.; Reginster, S.; Paydas, H.; Contrepois, Q.; Dormal, T.; Lemaire, O.; Lecomte-Beckers, J. Mechanical properties of alloy Ti–6Al–4V and of stainless steel 316L processed by selective laser melting: Influence of out-of-equilibrium microstructures. Powder Metall. 2014, 57, 184–189. [Google Scholar] [CrossRef]
- Burstein, A.H.; Reilly, D.T.; Martens, M. Aging of bone tissue: Mechanical properties. J. Bone Jt. Surg. Am. Vol. 1976, 58, 82–86. [Google Scholar] [CrossRef]
- Rho, J.-Y.; Tsui, T.Y.; Pharr, G.M. Elastic properties of human cortical and trabecular lamellar bone measured by nanoindentation. Biomaterials 1997, 18, 1325–1330. [Google Scholar] [CrossRef]
- Mansour, H.A.; Ray, J.D.; Mukherjee, D.P. Stress shielding of femoral component with and without collar. In Proceedings of the IEEE Fourteenth Southern Biomedical Engineering Conference, Shreveport, LA, USA, 7–9 April 1995; pp. 53–54. [Google Scholar]
- Oshida, Y. Bioscience and Bioengineering of Titanium Materials; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Abdel-Hady Gepreel, M.; Niinomi, M. Biocompatibility of Ti-alloys for long-term implantation. J. Mech. Behav. Biomed. Mater. 2013, 20, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Friák, M.; Counts, W.A.; Ma, D.; Sander, B.; Holec, D.; Raabe, D.; Neugebauer, J. Theory-Guided Materials Design of Multi-Phase Ti-Nb Alloys with Bone-Matching Elastic Properties. Materials 2012, 5, 1853–1872. [Google Scholar] [CrossRef]
- Matlakhova, L.A.; Matlakhov, A.N.; Monteiro, S.N.; Fedotov, S.G.; Goncharenko, B.A. Properties and structural characteristics of Ti–Nb–Al alloys. Mater. Sci. Eng. A 2005, 393, 320–326. [Google Scholar] [CrossRef]
- Sumner, D.R.; Galante, J.O. Determinants of stress shielding: Design versus materials versus interface. Clin. Orthop. Relat. Res. 1992, 274, 202–212. [Google Scholar] [CrossRef]
- Heller, M.O.; Bergmann, G.; Kassi, J.-P.; Claes, L.; Haas, N.P.; Duda, G.N. Determination of muscle loading at the hip joint for use in pre-clinical testing. J. Biomech. 2005, 38, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Cheal, E.J.; Spector, M.; Hayes, W.C. Role of loads and prosthesis material properties on the mechanics of the proximal femur after total hip arthroplasty. J. Orthop. Res. 1992, 10, 405–422. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization. Metallic Materials—Tensile Testing—Part 1: Method of Test at Room Temperature (ISO 6892-1:2016); German Version EN ISO 6892-1:2016; ISO: Geneva, Switzerland, 2016. [Google Scholar]
- Yasa, E.; Deckers, J.; Kruth, J.-P. The investigation of the influence of laser re-melting on density, surface quality and microstructure of selective laser melting parts. Rapid Prototyp. J. 2011, 17, 312–327. [Google Scholar] [CrossRef]
- Deutsches Institut fuer Normung e.V. Testing of Metallic Materials—Compression Test at Room Temperature (DIN 50106:2016-11); DIN Deutsches Institut für Normung e. V.—Beuth Verlag: Berlin, Germany, 2016. [Google Scholar]
- Deutsches Institut fuer Normung e.V. Testing of Metallic Materials—Tensile Test Pieces (DIN 50125:2016-12); DIN Deutsches Institut für Normung e. V.—Beuth Verlag: Berlin, Germany, 2016. [Google Scholar]
- Wysocki, B.; Maj, P.; Krawczyńska, A.; Rożniatowski, K.; Zdunek, J.; Kurzydłowski, K.J.; Święszkowski, W. Microstructure and mechanical properties investigation of CP titanium processed by selective laser melting (SLM). J. Mater. Process. Technol. 2017, 241, 13–23. [Google Scholar] [CrossRef]
- Zhang, B.; Yongato, L.; Bai, Q. Defect Formation Mechanisms in Selective Laser Melting: A Review. Chin. J. Mech. Eng. 2017, 30, 515–527. [Google Scholar]
- Chlebus, E.; Kuźnicka, B.; Kurzynowski, T.; Dybała, B. Microstructure and mechanical behaviour of Ti–6Al–7Nb alloy produced by selective laser melting. Mater. Charact. 2011, 62, 488–495. [Google Scholar] [CrossRef]
- Sercombe, T.; Jones, N.; Day, R.; Kop, A. Heat treatment of Ti-6Al-7Nb components produced by selective laser melting. Rapid Prototyp. J. 2008, 14, 300–304. [Google Scholar] [CrossRef]
- Ptochos, E.; Labeas, G. Elastic modulus and Poisson’s ratio determination of micro-lattice cellular structures by analytical, numerical and homogenisation methods. J. Sandw. Struct. Mater. 2012, 14, 597–626. [Google Scholar] [CrossRef]
- Babaee, S.; Jahromi, B.H.; Ajdari, A.; Nayeb-Hashemi, H.; Vaziri, A. Mechanical properties of open-cell rhombic dodecahedron cellular structures. Acta Mater. 2012, 60, 2873–2885. [Google Scholar] [CrossRef]
- Hedayati, R.; Sadighi, M.; Mohammadi-Aghdam, M.; Zadpoor, A.A. Mechanical behavior of additively manufactured porous biomaterials made from truncated cuboctahedron unit cells. Int. J. Mech. Sci. 2016, 106, 19–38. [Google Scholar] [CrossRef]
- Frosch, K.-H.; Barvencik, F.; Viereck, V.; Lohmann, C.H.; Dresing, K.; Breme, J.; Brunner, E.; Stürmer, K.M. Growth behavior, matrix production, and gene expression of human osteoblasts in defined cylindrical titanium channels. J. Biomed. Mater. Res. Part A 2004, 68, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, C.; Li, C.; Qin, Y.; Zhong, L.; Chen, B.; Li, Z.; Liu, H.; Chang, F.; Wang, J. Analysis of factors influencing bone ingrowth into three-dimensional printed porous metal scaffolds: A review. J. Alloy. Compd. 2017, 717, 271–285. [Google Scholar] [CrossRef]











| Material | Manufacturing | Condition | E (GPa) | σ0.2 (MPa) | UTS/UCS (MPa) | εb (%) |
|---|---|---|---|---|---|---|
| CP-Ti [8] | - | Grade 1–4 | 102.7–104.1 | 170–485 | 240–550 | 15–24 |
| Ti-6Al-4V [8] | annealed | - | 110–114 | 825–869 | 895–930 | 6–10 |
| Ti-6Al-4V ELI [8] | mill annealed | - | 101–110 | 795–875 | 860–965 | 10–15 |
| Ti-6Al-7Nb [8] | - | - | 114 | 880–950 | 900–1050 | 8.1–15 |
| Ti-6Al-4V [34] | EBM | as built | 118 ± 5 | 830 ± 5 | 915 ± 10 | 13.2 ± 0.6 |
| hipped | 117 ± 4 | 795 ± 10 | 870 ± 10 | 13.7 ± 1.0 | ||
| wrought/annealed | 104 ± 2 | 790 ± 20 | 870 ± 10 | 18.1 ± 0.8 | ||
| Ti-6Al-4V [17,68,69] | SLM | - | 112 | 990–1330 | 1095–1407 | 2–5 |
| Ti-45Nb [25] (compression test) | green compacted + sintered | - | 18.5 ± 2.5 | - | 282 ± 26 | - |
| hot-pressed | - | 64.4 ± 9.4 | - | 667 ± 56 | - | |
| Hot-pressed + sintered | - | 60.3 ± 8.9 | - | 686 ± 64 | - | |
| Ti-26Nb * [57] (* at %; compression test) | Ingot | - | 69.9 ± 0.2 | - | - | - |
| SLM (in situ alloyed) | - | 77 ± 1.4 | - | - | - | |
| Ti-27.5Nb * [58] (* at %) | CLAD®-Process | as built | 70 ± 3 | - | - | - |
| Solution treated | 70 ± 4 | - | - | - | ||
| Ti-42Nb (present study) | SLM | CNC-machined | 60.51 ± 3.92 | 674.08 ± 24.77 | 683.17 ± 16.67 | 11.65 ± 2.03 |
| Material | Test Method | Anatomic Region | E (GPa) | σ0.2 (MPa) | UTS/UCS (σc 35) (MPa) | εf (%) |
|---|---|---|---|---|---|---|
| Cortical Bone | Tensile | Femur [26,27,70] | 13.6–16.8 | - | 68–141 | 1.07–2.83 |
| Tibia [70] | 16.2–23.83 | - | 84–157 | 1.56–3.09 | ||
| Compression | Tibia osteons [71] | 22.5–25.8 | - | - | - | |
| Femur [70] | 17.6 | - | 194 | - | ||
| Tibia [70] | 28 | - | 195 | - | ||
| Ti-42Nb (present study) | Tensile | - | 60.51 ± 3.92 | 674.08 ± 24.77 | 683.17 ± 16.67 | 11.65 ± 2.03 |
| Compression | - | - | 831.58 ± 30.11 | 1330.74 ±53.45 | - |
| EDX Measuring Spot | Specimen 10 | Specimen 11 | ||
|---|---|---|---|---|
| Ti (wt %) | Nb (wt %) | Ti (wt %) | Nb (wt %) | |
| 1 | 55.7 | 44.3 | 56.3 | 43.7 |
| 2 | 59.1 | 39.9 | 58.1 | 41.9 |
| 3 | 57.7 | 42.3 | 57.5 | 42.5 |
| Specimen | As Printed | As Machined | As Measured | |||
|---|---|---|---|---|---|---|
| d (mm) | h (mm) | d0 (mm) | h0 (mm) | d0 (mm) | h0 (mm) | |
| Tensile (Specimen 1–8) | 15.0 | 70.0 | 6.0 | 60.00 | 5.98 ± 0.01 | 60.06 ± 0.05 |
| Compression (Specimen 9–26) | 10.7 | 16.0 | 6.9 | 10.35 | 6.89 ± 0.02 | 10.33 ± 0.03 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schulze, C.; Weinmann, M.; Schweigel, C.; Keßler, O.; Bader, R. Mechanical Properties of a Newly Additive Manufactured Implant Material Based on Ti-42Nb. Materials 2018, 11, 124. https://doi.org/10.3390/ma11010124
Schulze C, Weinmann M, Schweigel C, Keßler O, Bader R. Mechanical Properties of a Newly Additive Manufactured Implant Material Based on Ti-42Nb. Materials. 2018; 11(1):124. https://doi.org/10.3390/ma11010124
Chicago/Turabian StyleSchulze, Christian, Markus Weinmann, Christoph Schweigel, Olaf Keßler, and Rainer Bader. 2018. "Mechanical Properties of a Newly Additive Manufactured Implant Material Based on Ti-42Nb" Materials 11, no. 1: 124. https://doi.org/10.3390/ma11010124
APA StyleSchulze, C., Weinmann, M., Schweigel, C., Keßler, O., & Bader, R. (2018). Mechanical Properties of a Newly Additive Manufactured Implant Material Based on Ti-42Nb. Materials, 11(1), 124. https://doi.org/10.3390/ma11010124





