Thermotropic Liquid Crystal-Assisted Chemical and Biological Sensors
Abstract
:1. Introduction
2. Sensor Formats
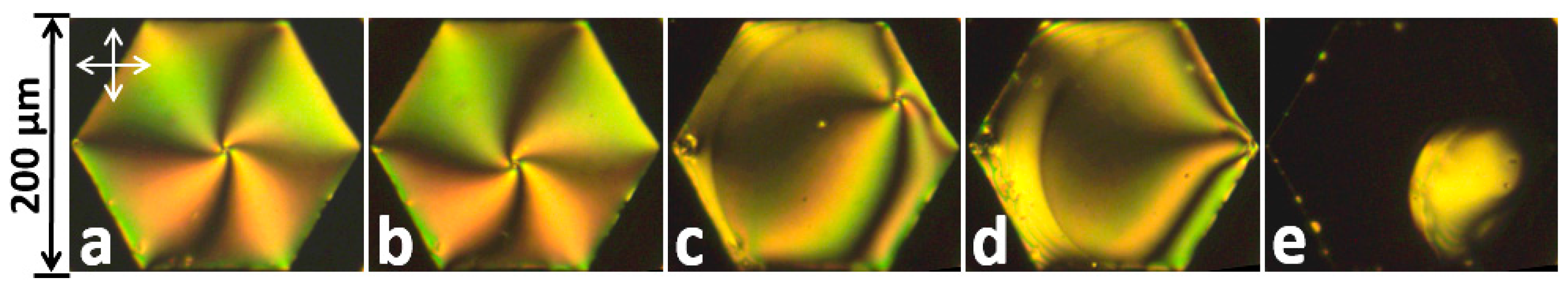
2.1. Freely-Suspended LC Films
2.2. LC Films with Solid Supports
2.3. Grids to Hold LC Films
2.4. LC shells and Droplets
2.5. LC in Capillaries and Fibers
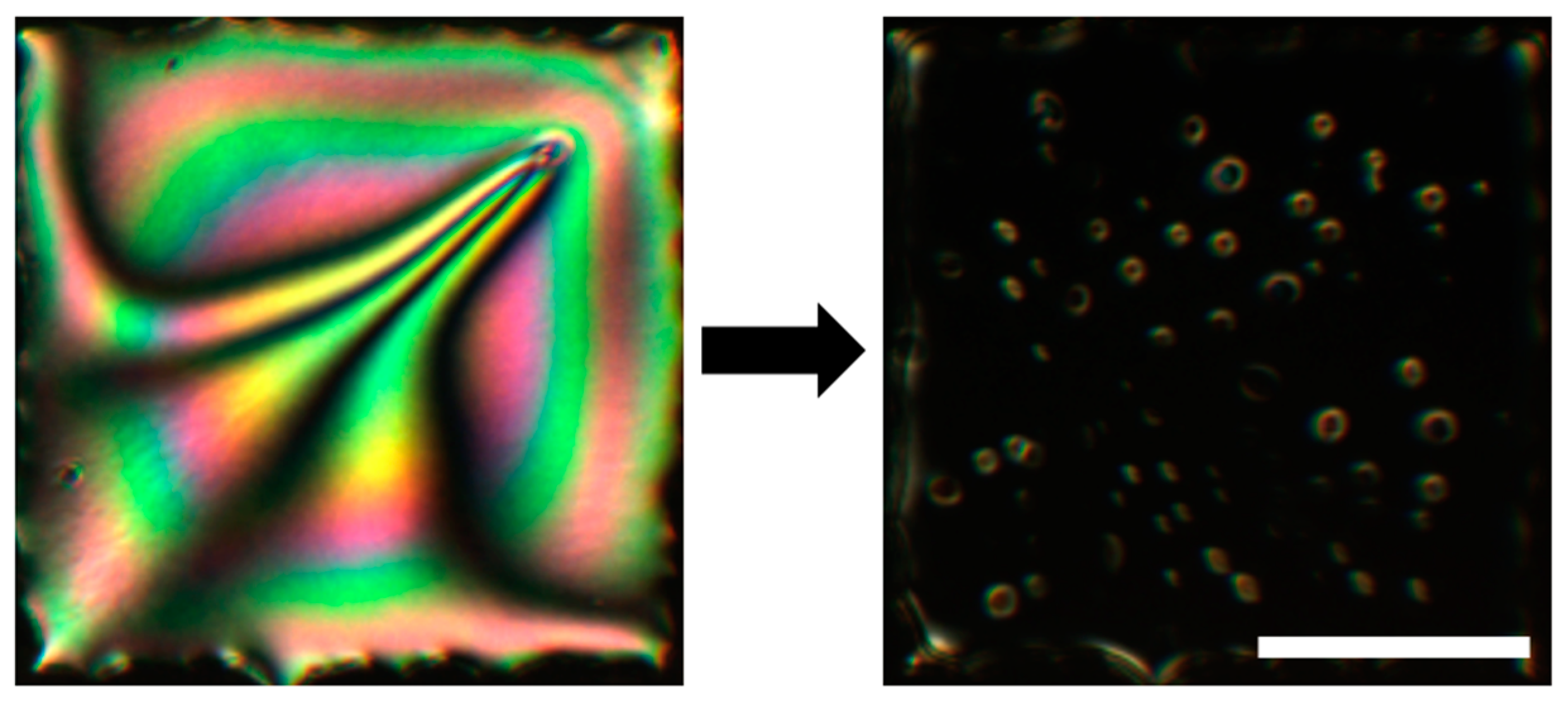
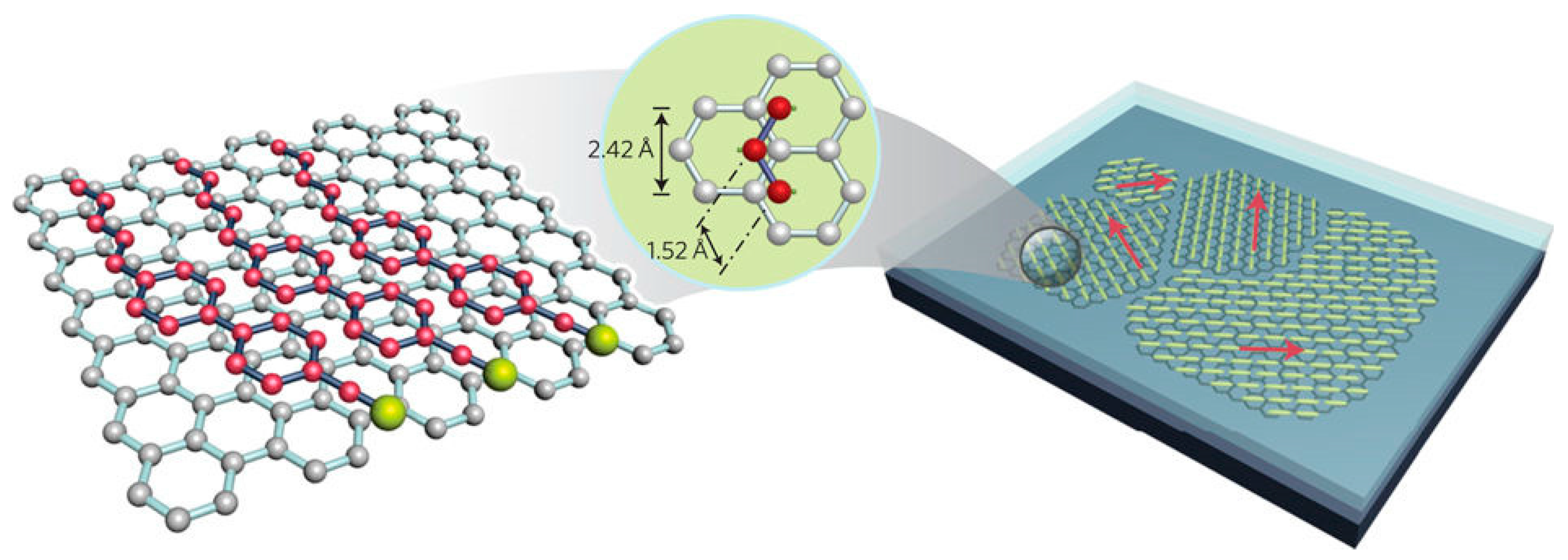
2.6. LC-Assisted Direct Visualization of Graphene Features
3. Increasing Detection Limits and Sensitivity
3.1. Flow Cell Design
3.2. Combining LC Films with Other Techniques
3.3. Choice of Sensing LC Materials
3.3.1. Tuning LC Elastic Constants
3.3.2. Dichroic LC and Polarizers
3.3.3. LC Temperature and Phase
3.3.4. Tailored LC Mesogens
3.3.5. Ionic Liquid Crystals
4. Analytes and Solvents
4.1. Hydrophilic-Lipophilic Balance
4.2. Influence of Aqueous Solution Composition
4.2.1. Effect of pH
4.2.2. Effect of Salt Type
4.2.3. Effect of co-Solute: Glycine
5. LC-Assisted Sensors for Specific Detection of Analytes
5.1. Specific Biosensing
5.2. Specific Sensing of Gases
5.3. Other Approaches
6. Computational Approaches to LC Sensor Designs
6.1. Computational Chemistry Methods
6.2. Molecular Dynamics
7. Summary and Future Directions
- Detection limits need to be as low as or lower than the sensitivities of existing non-LC sensors. Additionally, a linear sensor response over the interesting range is desirable [6].
- New types of detection modes that are not based on measurements performed at often slowly-achieved equilibrium states, may become very useful. Further, the final equilibrium states induced by different analytes may appear similar and even indistinguishable but the dynamics in achieving that final equilibrium state may reveal the presence of a specific analyte. Thus, non-equilibrium processes deserve serious attention in the future development of LC-assisted sensor platforms [5].
- One of the most important of customer requirements is that the sensors need to be extremely specific towards a single kind of analyte of interest. Research that demonstrates true and reliable specificity of LC-assisted sensors employing antibodies, aptamers and similar molecules remains scarce [14]. Reproducing and simplifying the biomimetic mechanisms of molecular recognition may serve as an efficient way of ensuring the required high specificity of biosensors [36].
- Another important market requirement is that the final sensor platform must be compact and especially easy and ready to use without much preparation. Thus, prefabricated devices with long shelf-lives are in high demand. At least two possible directions have been suggested: Ionic LCs may prevent fragile enzymes and antibodies from denaturing [99]. Aptamers, much more stable than antibodies, may provide the required level of specificity [121].
- Fundamental research on the behavior of LC-based sensors towards specific analytes in the presence of interfering species [55,108] is only at the beginning. This extremely important problem will need to be extensively addressed prior to any serious attempts at commercializing new types of sensors. It is likely that LC/analyte interactions, as well as chemical reactions, will need to be clearly understood and visualized at the molecular level for the successful development of superior future sensors [124,139].
Acknowledgments
Conflicts of Interest
References
- Palffy-Muhoray, P. The diverse world of liquid crystals. Phys. Today 2007, 60, 54–60. [Google Scholar] [CrossRef]
- Li, Q. Liquid Crystals Beyond Displays, 1st ed.; Wiley: Kent, OH, USA, 2012. [Google Scholar]
- Van der Asdonk, P.; Kouwer, P.H.J. Liquid crystal templating as an approach to spatially and temporally organise soft matter. Chem. Soc. Rev. 2017, 46, 5935–5949. [Google Scholar] [CrossRef] [PubMed]
- Schenning, A.; Crafword, G.P.; Broer, D.J. Liquid Crystal Sensors; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Popov, P.; Mann, E.K.; Jákli, A. Accurate Optical Detection of Amphiphiles at Liquid-Crystal-Water Interfaces. Phys. Rev. Appl. 2014, 1, 34003. [Google Scholar] [CrossRef]
- Iglesias, W.; Abbott, N.L.; Mann, E.K.; Jákli, A. Improving liquid-crystal-based biosensing in aqueous phases. ACS Appl. Mater. Interfaces 2012, 4, 6884–6890. [Google Scholar] [CrossRef] [PubMed]
- Carlton, R.J.; Hunter, J.T.; Miller, D.S.; Abbasi, R.; Mushenheim, P.C.; Tan, L.N.; Abbott, N.L. Chemical and biological sensing using liquid crystals. Liq. Cryst. Rev. 2013, 1, 29–51. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.N.; Carlton, R.J.; Cleaver, K.; Abbott, N.L. Liquid Crystal-Based Sensors for Rapid Analysis of Fatty Acid Contamination in Biodiesel. Mol. Cryst. Liq. Cryst. 2014, 1406, 37–41. [Google Scholar] [CrossRef]
- Popov, P.; Mann, E.K.; Jakli, A. Thermotropic liquid crystal films for biosensors and beyond. J. Mater. Chem. B 2017, 5, 5061–5078. [Google Scholar] [CrossRef]
- Hunter, J.T.; Abbott, N.L. Dynamics of the chemo-optical response of supported films of nematic liquid crystals. Sens. Actuators B Chem. 2013, 183, 71–80. [Google Scholar] [CrossRef]
- Reyes, C.G.; Sharma, A.; Lagerwall, J.P.F. Non-electronic gas sensors from electrospun mats of liquid crystal core fibres for detecting volatile organic compounds at room temperature. Liq. Cryst. 2016, 43, 1986–2001. [Google Scholar] [CrossRef]
- Shibaev, P.V.; Wenzlick, M.; Murray, J.; Tantillo, A.; Howard-Jennings, J. Rebirth of Liquid Crystals for Sensoric Applications: Environmental and Gas Sensors. Adv. Condens. Matter Phys. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Hussain, A.; Semeano, A.T.S.; Palma, S.I.C.J.; Pina, A.S.; Almeida, J.; Medrado, B.F.; Pádua, A.C.C.S.; Carvalho, A.L.; Dionísio, M.; Li, R.W.C.; et al. Tunable Gas Sensing Gels by Cooperative Assembly. Adv. Funct. Mater. 2017, 27, 1700803. [Google Scholar] [CrossRef] [PubMed]
- Popov, P.; Honaker, L.W.; Kooijman, E.E.; Mann, E.K.; Jákli, A.I. A liquid crystal biosensor for specific detection of antigens. Sens. Bio-Sens. Res. 2016, 8, 31–35. [Google Scholar] [CrossRef]
- Gupta, V.K.; Skaife, J.J.; Dubrovsky, T.B.; Abbott, N.L. Optical Amplification of Ligand-Receptor Binding Using Liquid Crystals. Science 1998, 279, 2077–2080. [Google Scholar] [CrossRef] [PubMed]
- Brake, J.M.; Abbott, N.L. Coupling of the orientations of thermotropic liquid crystals to protein binding events at lipid-decorated interfaces. Langmuir 2007, 23, 8497–8507. [Google Scholar] [CrossRef] [PubMed]
- Popov, P. Liquid Crystal Interfaces: Experiments, Simulations and Biosensors. Kent State University, 2015. Available online: http://rave.ohiolink.edu/etdc/view?acc_num=kent1434926908 (accessed on 21 December 2017).
- Walker, J.M. Biosensors and Biodetection; Springer: New York, NY, USA, 2017. [Google Scholar]
- Narayan, R.J. Medical Biosensors for Point of Care (POC) Applications, 1st ed.; Woodhead Publishing: Sawston, UK, 2016. [Google Scholar]
- Urban, G. Applications of Nanomaterials in Sensors and Diagnostics; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Ahmed, M.U.; Saaem, I.; Wu, P.C.; Brown, A.S. Personalized diagnostics and biosensors: A review of the biology and technology needed for personalized medicine. Crit. Rev. Biotechnol. 2014, 34, 180–196. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Nurunnabi, M.; Morshed, M.; Paul, A.; Polini, A.; Kuila, T.; Al Hariri, M.; Lee, Y.; Jaffa, A.A. Recent Advances in Application of Biosensors in Tissue Engineering. Biomed. Res. Int. 2014, 2014, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Shang, Y.; Zhang, J.; Cheang, L.H.; Zeng, X.; Tu, M. The effects of liquid crystal-based composite substrates on cell functional responses of human umbilical cord-derived mesenchymal stem cells by mechano-regulatory process. J. Biomater. Appl. 2017, 32, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Ünlü, N.L.; Kanik, F.E.; Seymour, E.; Connor, J.H.; Ünlü, M.S. Optical-Based Detectors. In Biosensors and Biodetection, 2nd ed.; Walker, J.M., Ed.; Springer: New York, NY, USA, 2017; Volume 1. [Google Scholar]
- Fang, Y. Label-Free Cell-Based Assays with Optical Biosensors in Drug Discovery. Assay Drug Dev. Technol. 2006, 4, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.A. Optical biosensors: Where next and how soon? Drug Discov. Today 2006, 11, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Gavela, A.F.; García, D.G.; Ramirez, J.; Lechuga, L. Last Advances in Silicon-Based Optical Biosensors. Sensors 2016, 16, 285. [Google Scholar] [CrossRef] [PubMed]
- Zanchetta, G.; Lanfranco, R.; Giavazzi, F.; Bellini, T.; Buscaglia, M. Emerging applications of label-free optical biosensors. Nanophotonics 2017, 6, 627–645. [Google Scholar] [CrossRef]
- Hussain, Z.; Qazi, F.; Ahmed, M.I.; Usman, A.; Riaz, A.; Abbasi, A.D. Liquid crystals based sensing platform-technological aspects. Biosens. Bioelectron. 2016, 85, 110–127. [Google Scholar] [CrossRef] [PubMed]
- Setia, S.; Sidiq, S.; De, J.; Pani, I.; Pal, S.K. Applications of liquid crystals in biosensing and organic light-emitting devices: Future aspects. Liq. Cryst. 2016, 43, 2009–2050. [Google Scholar] [CrossRef]
- Wang, D.; Park, S.-Y.; Kang, I.-K. Liquid crystals: Emerging materials for use in real-time detection applications. J. Mater. Chem. C. 2015, 3, 9038–9047. [Google Scholar] [CrossRef]
- Munir, S.; Kang, I.-K.; Park, S.-Y. Polyelectrolytes functionalized nematic liquid crystal-based biosensors: An overview. TrAC Trends Anal. Chem. 2016, 83, 80–94. [Google Scholar] [CrossRef]
- Van’t Hag, L.; Gras, S.L.; Conn, C.E.; Drummond, C.J. Lyotropic liquid crystal engineering moving beyond binary compositional space—Ordered nanostructured amphiphile self-assembly materials by design. Chem. Soc. Rev. 2017, 46, 2705–2731. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S. Lyotropic Chromonic Liquid Crystals; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Shiyanovskii, S.V.; Lavrentovich, O.D.; Schneider, T.; Ishikawa, T.; Smalyukh, I.I.; Woolverton, C.J.; Niehaus, G.D.; Doane, K.J. Lyotropic Chromonic Liquid Crystals for Biological Sensing Applications. Mol. Cryst. Liq. Cryst. 2005, 434. [Google Scholar] [CrossRef]
- De Souza, J.; Pontes, K.; Alves, T.; Amaral, V.; Rebelo, M.; Hausen, M.; Chaud, M. Spotlight on Biomimetic Systems Based on Lyotropic Liquid Crystal. Molecules 2017, 22, 419. [Google Scholar] [CrossRef] [PubMed]
- Crystal Diagnostics LTD, (n.d.). Available online: http://www.crystaldiagnostics.com/ (accessed on 23 November 2017).
- Van der Asdonk, P.; Collings, P.J.; Kouwer, P.H.J. Fully Stable and Homogeneous Lyotropic Liquid Crystal Alignment on Anisotropic Surfaces. Adv. Funct. Mater. 2017, 27, 1701209. [Google Scholar] [CrossRef]
- Berride, F.; Troche, E.; Feio, G.; Cabrita, E.; Sierra, T.; Azquez, A.N.V.; Cid, M. Chiral amplification of disodium cromoglycate chromonics induced by a codeine derivative. Soft Matter 2017, 13, 6810–6815. [Google Scholar] [CrossRef] [PubMed]
- Salamonczyk, M.; Zhang, J.; Portale, G.; Zhu, C.; Kentzinger, E.; Gleeson, J.T.; Jakli, A.; de Michele, C.; Dhont, J.K.G.; Sprunt, S.; et al. Smectic phase in suspensions of gapped DNA duplexes. Nat. Commun. 2016, 7, 13358. [Google Scholar] [CrossRef] [PubMed]
- Bellini, T.; Zanchetta, G.; Fraccia, T.P.; Cerbino, R.; Tsai, E.; Smith, G.P.; Moran, M.J.; Walba, D.M.; Clark, N.A. Liquid crystal self-assembly of random-sequence DNA oligomers. Proc. Natl. Acad. Sci. USA 2012, 109, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Barbero, G.; Evangelista, L.R. Adsorption phenomena and anchoring energy in nematic liquid crystals. Liq. Cryst. Rev. 2014, 2, 72. [Google Scholar] [CrossRef]
- Kim, H.J.; Jang, C. Micro-capillary sensor for imaging trypsin activity using confined nematic liquid crystals. J. Mol. Liq. 2016, 222, 596–600. [Google Scholar] [CrossRef]
- Kim, H.J.; Jang, C.-H. Liquid crystal-based capillary sensory platform for the detection of bile acids. Chem. Phys. Lipids 2017, 204, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Enz, E.; La Ferrara, V.; Scalia, G. Confinement-Sensitive Optical Response of Cholesteric Liquid Crystals in Electrospun Fibers. ACS Nano 2013, 7, 6627–6635. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.H.; Henx, B.; Lagerwall, J.P.F. Taming Liquid Crystal Self-Assembly: The Multifaceted Response of Nematic and Smectic Shells to Polymerization. Adv. Mater. 2016, 28, 10170–10174. [Google Scholar] [CrossRef] [PubMed]
- Jakli, A.; Mann, E.K.; Popov, P. System and Method Thereof for Accurate Optical Detection of Amphiphiles at a Liquid Crystal Interface. 2015. Available online: http://www.freepatentsonline.com/y2015/0233816.html (accessed on 21 December 2017).
- Lee, M.-J.; Sung, Y.-C.; Hsiao, Y.-C.; Lee, W. Chiral liquid crystals as biosensing platforms. Proc. SPIE 2016, 9940, 12–17. [Google Scholar] [CrossRef]
- Jang, J.H.; Park, S.Y. pH-responsive cholesteric liquid crystal double emulsion droplets prepared by microfluidics. Sens. Actuators B Chem. 2017, 241, 636–643. [Google Scholar] [CrossRef]
- Lee, H.-G.; Munir, S.; Park, S.-Y. Cholesteric Liquid Crystal Droplets for Biosensors. ACS Appl. Mater. Interfaces 2016, 8, 26407–26417. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-J.; Chang, C.-H.; Lee, W. Label-free protein sensing by employing blue phase liquid crystal. Biomed. Opt. Express 2017, 8, 1712. [Google Scholar] [CrossRef] [PubMed]
- Bukusoglu, E.; Martinez-Gonzalez, J.A.; Wang, X.; Zhou, Y.; de Pablo, J.J.; Abbott, N.L. Strain-induced alignment and phase behavior of blue phase liquid crystals confined to thin films. Soft Matter 2017. [Google Scholar] [CrossRef] [PubMed]
- Hartono, D.; Bi, X.; Yang, K.-L.; Yung, L.-Y.L. An Air-Supported Liquid Crystal System for Real-Time and Label-Free Characterization of Phospholipases and Their Inhibitors. Adv. Funct. Mater. 2008, 18, 2938–2945. [Google Scholar] [CrossRef]
- Popov, P.; Mann, E.K.; Jakli, A. Liquid-Crystal-Based Biosensor without Alignment Substrate. Biophys. J. 2014, 106, 415a. [Google Scholar] [CrossRef]
- Popov, N.; Smirnova, A.; Usol’tseva, N.; Popov, P. Determination of Concentrations of Surface-Active Materials in Aqueous Solutions at Different pH Values Using Liquid Crystals. Liq. Cryst. Their Appl. 2017, 17, 34–42. [Google Scholar] [CrossRef]
- Popov, P.; Honaker, L.W.; Mirheydari, M.; Mann, E.K.; Jákli, A. Chiral nematic liquid crystal microlenses. Sci. Rep. 2017, 7, 1603. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Zhong, Y.; Chen, R.; Wang, F.; Luo, D. Highly sensitive and selective liquid crystal optical sensor for detection of ammonia. Opt. Express 2017, 25, 13549. [Google Scholar] [CrossRef] [PubMed]
- Pantoja, M.A.B.; Abbott, N.L. Surface-Controlled Orientational Transitions in Elastically Strained Films of Liquid Crystal That Are Triggered by Vapors of Toluene. ACS Appl. Mater. Interfaces 2016, 8, 13114–13122. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhou, Y.; Kim, Y.K.; Miller, D.S.; Zhang, R.; Martinez-Gonzalez, J.A.; Bukusoglu, E.; Zhang, B.; Brown, T.M.; de Pablo, J.J.; et al. Patterned surface anchoring of nematic droplets at miscible liquid–liquid interfaces. Soft Matter 2017, 13, 5714–5723. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.; Lavrentovich, M.O.; Durey, G.; Darmon, A.; Haase, M.F.; Li, N.; Lee, D.; Stebe, K.J.; Kamien, R.D.; Lopez-Leon, T. Change in Stripes for Cholesteric Shells via Anchoring in Moderation. Phys. Rev. X 2017, 7, 41029. [Google Scholar] [CrossRef]
- Noh, J.; de Sousa, K.R.; Lagerwall, J.P.F. Influence of interface stabilisers and surrounding aqueous phases on nematic liquid crystal shells. Soft Matter 2015, 12, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.K.; Shum, H.C.; Rowat, A.C.; Lee, D.; Agresti, J.J.; Utada, A.S.; Chu, L.; Kim, J.; Fernandez-nieves, A.; Martinez, C.J.; et al. Designer emulsions using microfluidics. Mater. Today 2008, 11, 18–27. [Google Scholar] [CrossRef]
- Brosseau, Q.; Vrignon, J.; Baret, J.-C. Microfluidic Dynamic Interfacial Tensiometry (μDIT). Soft Matter 2014, 10, 3066–3076. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.Y.; Joshipura, I.D.; Lin, Y.; Kalantar-Zadeh, K.; Mitchell, A.; Khoshmanesh, K.; Dickey, M.D. Liquid-Metal Microdroplets Formed Dynamically with Electrical Control of Size and Rate. Adv. Mater. 2016, 28, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Humar, M.; Muševič, I. Surfactant sensing based on whispering-gallery-mode lasing in liquid-crystal microdroplets. Opt. Express 2011, 19, 2243–2248. [Google Scholar] [CrossRef] [PubMed]
- Buyuktanir, E.A.; Frey, M.W.; West, J.L. Self-assembled, optically responsive nematic liquid crystal/polymer core-shell fibers: Formation and characterization. Polymer 2010, 51, 4823–4830. [Google Scholar] [CrossRef]
- West, J.L.; Wang, J.; Jákli, A. Airbrushed Liquid Crystal/Polymer Fibers for Responsive Textiles. Adv. Sci. Technol. 2017, 100, 43–49. [Google Scholar] [CrossRef]
- Wang, J.; West, J.L. Morphology Tuning of Electrospun Liquid Crystal/Polymer Fibers. Chemphyschem 2016, 17, 3080–3085. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kolacz, J.; Chen, Y.; Jákli, A.; Kawalec, J.; Benitez, M.; West, J.L. Smart Fabrics Functionalized by Liquid Crystals. J. Soc. Inf. Disp. 2017, 48, 147–149. [Google Scholar] [CrossRef]
- Kye, Y.; Kim, C.; Lagerwall, J. Multifunctional responsive fibers produced by dual liquid crystal core electrospinning. J. Mater. Chem. C 2015, 3, 8979–8985. [Google Scholar] [CrossRef]
- Kim, D.K.; Hwang, M.; Lagerwall, J.P.F. Liquid crystal functionalization of electrospun polymer fibers. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 855–867. [Google Scholar] [CrossRef]
- Urbanski, M.; Reyes, C.G.; Noh, J.; Sharma, A.; Geng, Y.; Jampani, V.S.R.; Lagerwall, J.P.F. Liquid crystals in micron-scale droplets, shells and fibers. J. Phys. Condens. Matter 2017, 29, 133003. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, V.; Tiwari, J.N.; Kemp, K.C.; Perman, J.A.; Bourlinos, A.B.; Kim, K.S.; Zboril, R. Noncovalent Functionalization of Graphene and Graphene Oxide for Energy Materials, Biosensing, Catalytic, and Biomedical Applications. Chem. Rev. 2016, 116, 5464–5519. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Jang, C.H. Liquid crystal as sensing platforms for determining the effect of graphene oxide-based materials on phospholipid membranes and monitoring antibacterial activity. Sens. Actuators B Chem. 2017, 254, 72–80. [Google Scholar] [CrossRef]
- Kim, D.W.; Kim, Y.H.; Jeong, H.S.; Jung, H.T. Direct visualization of large-area graphene domains and boundaries by optical birefringency. Nat. Nanotechnol. 2011, 7, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, M.A.; Tien, D.H.; Iqbal, M.W.; Eom, J.; Park, J.H.; Hwang, C.; Seo, Y. Nematic Liquid Crystal on a Two Dimensional Hexagonal Lattice and its Application. Sci. Rep. 2015, 5, 13331. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, M.A.; Hussain, S.; Lee, J.; Jung, J.; Lee, N.; Kim, G.; Seo, Y. Study of Grains and Boundaries of Molybdenum Diselenide and Tungsten Diselenide Using Liquid Crystal. Nano Lett. 2017, 17, 1474–1481. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.J.; Lee, B.H.; Kwon, Y.R.; Choi, Y.E.; Murali, G.; Lee, J.H.; Nguyen, V.L.; Lee, Y.H.; Lee, S.H. Monitoring defects on monolayer graphene using nematic liquid crystals. Opt. Express 2015, 23, 14162. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; Kinnamon, D.; Garvey, A. Detection of graphene chirality using achiral liquid crystalline platforms. J. Appl. Phys. 2015, 118, 114302. [Google Scholar] [CrossRef]
- Basu, R.; Kinnamon, D.; Garvey, A. Graphene and liquid crystal mediated interactions. Liq. Cryst. 2016, 43, 2375–2390. [Google Scholar] [CrossRef]
- Zhang, X.L.; Liu, Y.; Fan, T.; Hu, N.; Yang, Z.; Chen, X.; Wang, Z.-Y.; Yang, J. Design and Performance of a Portable and Multichannel SPR Device. Sensors 2017, 17, 1435. [Google Scholar] [CrossRef] [PubMed]
- Lynn, N.S.; Sipova, H.; Adam, P.; Homola, J. Enhancement of affinity-based biosensors: Effect of sensing chamber geometry on sensitivity. Lab Chip 2013, 13, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Tomassetti, M.; Merola, G.; Martini, E.; Campanella, L.; Sanzò, G.; Favero, G.; Mazzei, F. Comparison between a Direct-Flow SPR Immunosensor for Ampicillin and a Competitive Conventional Amperometric Device: Analytical Features and Possible Applications to Real Samples. Sensors 2017, 17, 819. [Google Scholar] [CrossRef] [PubMed]
- Larson, R.G. The Structure and Rheology of Complex Fluids, OUP USA. 1999. Available online: https://books.google.com/books?id=Vt9fw_pf1LUC (accessed on 21 December 2017).
- Abuabed, A. Study of the Effect of Nematic Order Degradation in Liquid Crystal-Based Surface Plasmon Resonance Sensors. Photonics 2017, 4, 24. [Google Scholar] [CrossRef]
- Wu, P.-C.; Karn, A.; Lee, M.-J.; Lee, W.; Chen, C.-Y. Dye-liquid-crystal-based biosensing for quantitative protein assay. Dyes Pigment 2018, 150, 73–78. [Google Scholar] [CrossRef]
- Popova, M.; Bretz, S.L.; Hartley, C.S. Visualizing Molecular Chirality in the Organic Chemistry Laboratory Using Cholesteric Liquid Crystals. J. Chem. Educ. 2016, 93, 1096–1099. [Google Scholar] [CrossRef]
- Mulder, D.J.; Schenning, A.P.H.J.; Bastiaansen, C.W.M. Chiral-nematic liquid crystals as one dimensional photonic materials in optical sensors. J. Mater. Chem. C 2014, 2, 6695–6705. [Google Scholar] [CrossRef]
- Matsumura, K.; Opiekun, M.; Oka, H.; Vachani, A.; Albelda, S.M.; Yamazaki, K.; Beauchamp, G.K. Urinary volatile compounds as biomarkers for lung cancer: A proof of principle study using odor signatures in mouse models of lung cancer. PLoS ONE 2009, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sponring, A.; Filipiak, W.; Mikoviny, T.; Ager, C.; Schubert, J.; Miekisch, W.; Amann, A.; Troppmair, J. Release of volatile organic compounds from the lung cancer cell line NCI-H2087 in vitro. Anticancer Res. 2009, 29, 419–426. [Google Scholar] [PubMed]
- Filipiak, W.; Sponring, A.; Mikoviny, T.; Ager, C.; Schubert, J.; Miekisch, W.; Amann, A.; Troppmair, J. Release of volatile organic compounds (VOCs) from the lung cancer cell line CALU-1 in vitro. Cancer Cell Int. 2008, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Minh, T.D.C.; Blake, D.R.; Galassetti, P.R. The clinical potential of exhaled breath analysis for diabetes mellitus. Diabetes Res. Clin. Pract. 2012, 97, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Van Delden, R.A.; Feringa, B.L. Colour indicator for enantiomeric excess and assignment of the configuration of the major enantiomer of an amino acid ester. Chem. Commun. 2002, 2, 174–175. [Google Scholar] [CrossRef]
- Eelkema, R.; Feringa, B.L. Amplification of chirality in liquid crystals. Org. Biomol. Chem. 2006, 4, 3729–3745. [Google Scholar] [CrossRef] [PubMed]
- Kek, K.J.; Lee, J.J.Z.; Otono, Y.; Ishihara, S. Chemical gas sensors using chiral nematic liquid crystals and its application. J. Soc. Inf. Disp. 2017. [Google Scholar] [CrossRef]
- Otono, Y.; Kek, K.J.; Jia, J.; Lee, Z.; Ishihara, S.; Nakano, Y.; Hashimotodani, K.; Oka, H. Chemical gas sensors using chiral nematic liquid crystals. J. Soc. Inf. Disp. 2016, 75, 1021–1024. [Google Scholar] [CrossRef]
- Eimura, H.; Miller, D.S.; Wang, X.; Abbott, N.L.; Kato, T. Self-Assembly of Bioconjugated Amphiphilic Mesogens Having Specific Binding Moieties at Aqueous-Liquid Crystal Interfaces. Chem. Mater. 2016, 28, 1170–1178. [Google Scholar] [CrossRef]
- Munir, S.; Park, S. Liquid-crystal droplets functionalized with a non-enzymatic moiety for glucose sensing. Sens. Actuators B Chem. 2018, 257, 579–585. [Google Scholar] [CrossRef]
- Fernandez, A.A.; Kouwer, P. Key Developments in Ionic Liquid Crystals. Int. J. Mol. Sci. 2016, 17, 731. [Google Scholar] [CrossRef] [PubMed]
- Atta, N.; BinSabt, M.; Hassan, S.; Galal, A. Ionic Liquid Crystals Modifier for Selective Determination of Terazosin Antihypertensive Drug in Presence of Common Interference Compounds. Crystals 2017, 7, 27. [Google Scholar] [CrossRef]
- Toledo Hijo, A.A.; Maximo, G.J.; Costa, M.C.; Cunha, R.L.; Pereira, J.F.; Kurnia, K.A.; Batista, E.A.; Meirelles, A.J. Phase Behavior and Physical Properties of New Biobased Ionic Liquid Crystals. J. Phys. Chem. B 2017, 121, 3177–3189. [Google Scholar] [CrossRef] [PubMed]
- Munje, R.D.; Muthukumar, S.; Jagannath, B.; Prasad, S. A new paradigm in sweat based wearable diagnostics biosensors using Room Temperature Ionic Liquids (RTILs). Sci. Rep. 2017, 7, 1950. [Google Scholar] [CrossRef] [PubMed]
- Griffin, W.C. Classification of surface-active agents by“ HLB”. J. Soc. Cosmet. Chem. 1949, 1, 311–326. [Google Scholar]
- Kale, S.N.; Deore, S.L. Emulsion Micro Emulsion and Nano Emulsion: A Review. Syst. Rev. Pharm. 2016, 8, 39–47. [Google Scholar] [CrossRef]
- Ansel, H.C.; Popovich, N.G.; Allen, L.V. Disperse Systems. In Ansel’s Pharmaceutical Dosage Forms and Drug Delivery Systems, 9th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; p. 794. [Google Scholar]
- Sigma-Aldrich. Surfactants Classified by HLB Numbers, (n.d.). Available online: http://www.sigmaaldrich.com/materials-science/material-science-products.html?TablePage=22686648%0D (accessed on 3 August 2017).
- Senske, M.; Constantinescu-Aruxandei, D.; Havenith, M.; Herrmann, C.; Rtner, H.W.; Ebbinghaus, S. The temperature dependence of the Hofmeister series: Thermodynamic fingerprints of cosolute-protein interactions. Phys. Chem. Chem. Phys. 2016, 18, 29647–30206. [Google Scholar] [CrossRef] [PubMed]
- Carlton, R.J.; Ma, C.D.; Gupta, J.K.; Abbott, N.L. Influence of specific anions on the orientational ordering of thermotropic liquid crystals at aqueous interfaces. Langmuir 2012, 28, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Hallett, J.E.; Hayward, D.W.; Arnold, T.; Bartlett, P.; Richardson, R.M. X-ray reflectivity reveals ionic structure at liquid crystal–aqueous interfaces. Soft Matter 2017, 13, 5535. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Zhou, Q. Enhancing NMDA Receptor Function: Recent Progress on Allosteric Modulators. Neural Plast. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Usol’tseva, N.V.; Smirnova, А.I.; Zharnikova, N.V.; Kurbatova, M.S.; Giricheva, N.I.; Badelin, V.G. Effect of Glycine on Lyomesophase Formation by Sodium Dodecylsulfate—Water Systems. Liq. Cryst. Their Appl. 2016, 16, 70–79. [Google Scholar] [CrossRef]
- Shen, J.; He, F.; Chen, L.; Ding, L.; Liu, H.; Wang, Y.; Xiong, X. Liquid crystal-based detection of DNA hybridization using surface immobilized single-stranded DNA. Microchim. Acta 2017, 184, 3137–3144. [Google Scholar] [CrossRef]
- Kim, H.J.; Rim, J.; Jang, C.-H. Liquid-Crystal-Based Immunosensor for Diagnosis of Tuberculosis in Clinical Specimens. ACS Appl. Mater. Interfaces 2017, 9, 21209–21215. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.P.; Arulanandam, B.P.; Matta, L.L.; Weis, A.; Valdes, J.J. Biosensor recognition elements. Curr. Issues Mol. Biol. 2008, 10, 1–12. [Google Scholar] [PubMed]
- Eni-olorunda, I.; Sadana, A.; Hopkins, N.A.E.; Meyer, S.C.; Ghosh, I.; Baldrich, E.; Herold, K.E.; Rasooly, A.; Laurenceau, E.; Lim, S.; et al. Recognition Receptors in Biosensors; Springer: New York, NY, USA, 2010. [Google Scholar]
- Lim, S.A.; Ahmed, M.U. Chapter 1. Introduction to Food Biosensors. In Food Biosensors; Royal Society of Chemistry: Cambridge, UK, 2017; pp. 1–21. [Google Scholar]
- Deshpande, P.S.; Kashyap, R.S.; Ramteke, S.S.; Nagdev, K.J.; Purohit, H.J.; Taori, G.M.; Daginawala, H.F. Evaluation of the IS6110 PCR assay for the rapid diagnosis of tuberculous meningitis. Cerebrospinal Fluid Res. 2007, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Peng, Y.; Xu, L.; Zhou, W.; Wang, Q.; Guo, L. Liquid-Crystal Biosensor Based on Nickel-Nanosphere-Induced Homeotropic Alignment for the Amplified Detection of Thrombin. ACS Appl. Mater. Interfaces 2015, 7, 23418–23422. [Google Scholar] [CrossRef] [PubMed]
- Platypus Technologies, LLC, (n.d.). Available online: http://www.platypustech.com/dosimeters (accessed on 23 November 2017).
- Jeddi, I.; Saiz, L. Three-dimensional modeling of single stranded DNA hairpins for aptamer-based biosensors. Sci. Rep. 2017, 7, 1178. [Google Scholar] [CrossRef] [PubMed]
- Ruan, M.; Seydou, M.; Noel, V.; Piro, B.; Maurel, F.; Barbault, F. Molecular Dynamics Simulation of a RNA Aptasensor. J. Phys. Chem. B 2017, 121, 4071–4080. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Pal, S.K. Liquid Crystal Unveiled Interactions between Melittin and Phospholipids at Aqueous-Liquid Crystal Interface. ChemistrySelect 2017, 2, 4779–4786. [Google Scholar] [CrossRef]
- Munir, S.; Khan, M.; Park, S.-Y. Bienzyme liquid-crystal-based cholesterol biosensor. Sens. Actuators B Chem. 2015, 220, 508–515. [Google Scholar] [CrossRef]
- Szilvási, T.; Roling, L.T.; Yu, H.; Rai, P.; Choi, S.; Twieg, R.J.; Mavrikakis, M.; Abbott, N.L. Design of Chemoresponsive Liquid Crystals through Integration of Computational Chemistry and Experimental Studies. Chem. Mater. 2017, 29, 3563–3571. [Google Scholar] [CrossRef]
- Hunter, J.T.; Abbott, N.L. Adsorbate-Induced Anchoring Transitions of Liquid Crystals on Surfaces Presenting Metal Salts with Mixed Anions. ACS Appl. Mater. Interfaces 2014, 6, 2362–2369. [Google Scholar] [CrossRef] [PubMed]
- Orellana, L.; Yoluk, O.; Carrillo, O.; Orozco, M.; Lindahl, E. Prediction and validation of protein intermediate states from structurally rich ensembles and coarse-grained simulations. Nat. Commun. 2016, 7, 12575. [Google Scholar] [CrossRef] [PubMed]
- Poma, A.B.; Cieplak, M.; Theodorakis, P.E. Combining the MARTINI and Structure-Based Coarse-Grained Approaches for the Molecular Dynamics Studies of Conformational Transitions in Proteins. J. Chem. Theory Comput. 2017, 13, 1366–1374. [Google Scholar] [CrossRef] [PubMed]
- Niesen, M.J.M.; Wang, C.Y.; van Lehn, R.C.; Miller, T.F. Structurally detailed coarse-grained model for Sec-facilitated co-translational protein translocation and membrane integration. PLoS Comput. Biol. 2017, 13, e1005427. [Google Scholar] [CrossRef] [PubMed]
- Ohadi, D.; Uline, M.J. Molecular Modeling of Liquid Crystal/Phospholipid Interface as a Label-Free Biosensor. Biophys. J. 2017, 112, 592a. [Google Scholar] [CrossRef]
- Zhang, W.; Du, Y.; Cranford, S.W.; Wang, M.L. Biosensor Design through Molecular Dynamics Simulation. Int. J. Biol. Biomol. Agric. Food Biotechnol. Eng. 2016, 10, 10–14. [Google Scholar]
- Wang, Y.; Cai, W.; Chen, L.; Wang, G. Molecular dynamics simulation reveals how phosphorylation of tyrosine 26 of phosphoglycerate mutase 1 upregulates glycolysis and promotes tumor growth. Oncotarget 2017, 8, 12093–12107. [Google Scholar] [CrossRef] [PubMed]
- Okimoto, N.; Suenaga, A.; Taiji, M. Evaluation of protein–ligand affinity prediction using steered molecular dynamics simulations. J. Biomol. Struct. Dyn. 2016, 1102, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Izrailev, S.; Stepaniants, S.; Balsera, M.; Oono, Y.; Schulten, K. Molecular dynamics study of unbinding of the avidin-biotin complex. Biophys. J. 1997, 72, 1568–1581. [Google Scholar] [CrossRef]
- Perilla, J.R.; Schulten, K. Physical properties of the HIV-1 capsid from all-atom molecular dynamics simulations. Nat. Commun. 2017, 8, 15959. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Perilla, J.R.; Yufenyuy, E.L.; Meng, X.; Chen, B.; Ning, J.; Ahn, J.; Gronenborn, A.M.; Schulten, K.; Aiken, C.; et al. Mature HIV-1 capsid structure by cryo-electron microscopy and all-atom molecular dynamics. Nature 2013, 497, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, G.; Sato, S.; Iwadate, M.; Umeyama, H.; Hayakawa, M.; Murakami, Y.; Yoneda, S. Molecular Dynamics Simulations to Determine the Structure and Dynamics of Hepatitis B Virus Capsid Bound to a Novel Anti-viral Drug. Chem. Pharm. Bull. 2016, 64, 1393–1396. [Google Scholar] [CrossRef] [PubMed]
- Sadati, M.; Ramezani-Dakhel, H.; Bu, W.; Sevgen, E.; Liang, Z.; Erol, C.; Rahimi, M.; Qazvini, N.T.; Lin, B.; Abbott, N.L.; et al. Molecular Structure of Canonical Liquid Crystal Interfaces. J. Am. Chem. Soc. 2017, 139, 3841–3850. [Google Scholar] [CrossRef] [PubMed]
- Popov, P.; Lacks, D.J.; Jákli, A.; Mann, E.K. Insertion of liquid crystal molecules into hydrocarbon monolayers. J. Chem. Phys. 2014, 141, 54901. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.Y.; Zuo, F.; Zhao, Z.-G.; Chen, J.; Xu, D. Molecular Dynamics Investigations of An Indicator Displacement Assay Mechanism in Liquid Crystal Sensor. Phys. Chem. Chem. Phys. 2017, 19, 23924–23933. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.D.; Carrillo, J.-M.Y.; Matheson, M.A.; Brown, W.M. Rupture mechanism of liquid crystal thin films realized by large-scale molecular simulations. Nanoscale 2014, 6, 3083–3096. [Google Scholar] [CrossRef] [PubMed]
- Pollack, L. Bionanotechnology and the Computational Microscope. 2013. Available online: http://www.ks.uiuc.edu/History/BioNano/ (accessed on 25 July 2017).
- Popov, P.; Steinkerchner, L.; Mann, E.K. Molecular dynamics study of rhodamine 6G diffusion at n-decane-water interfaces. Phys. Rev. E 2015, 91, 53308. [Google Scholar] [CrossRef] [PubMed]
- Harder, E.; Damm, W.; Maple, J.; Wu, C.; Reboul, M.; Xiang, J.Y.; Wang, L.; Lupyan, D.; Dahlgren, M.K.; Knight, J.L.; et al. OPLS3: A Force Field Providing Broad Coverage of Drug-like Small Molecules and Proteins. J. Chem. Theory Comput. 2016, 12, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Cipcigan, F.S.; Sokhan, V.P.; Crain, J.; Martyna, G.J. Electronic coarse graining enhances the predictive power of molecular simulation allowing challenges in water physics to be addressed. J. Comput. Phys. 2016, 326, 222–233. [Google Scholar] [CrossRef]
- Bi, X.; Hartono, D.; Yang, K.-L. Real-Time Liquid Crystal pH Sensor for Monitoring Enzymatic Activities of Penicillinase. Adv. Funct. Mater. 2009, 19, 3760–3765. [Google Scholar] [CrossRef]
- Hussain, Z.; Zafiu, C.; Küpcü, S.; Pivetta, L.; Hollfelder, N.; Masutani, A.; Kilickiran, P.; Sinner, E.-K. Liquid crystal based sensors monitoring lipase activity: A new rapid and sensitive method for cytotoxicity assays. Biosens. Bioelectron. 2014, 56, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Van Duin, A.C.T.; Dasgupta, S.; Lorant, F.; Goddard, W.A. ReaxFF: A Reactive Force Field for Hydrocarbons. J. Phys. Chem. A 2001, 105, 9396–9409. [Google Scholar] [CrossRef]
- Hu, S.; Sun, W.; Fu, J.; Zhang, L.; Fan, Q.; Zhang, Z.; Wu, W.; Tang, Y. Reactive molecular dynamics simulations on the thermal decomposition of poly alpha-methyl styrene. J. Mol. Model. 2017, 23, 179. [Google Scholar] [CrossRef] [PubMed]
- Delhommelle, J. Recent advances in the molecular simulation of chemical reactions. Mol. Simul. 2015, 41, 1–2. [Google Scholar] [CrossRef]
- Ribeiro, J.V.; Bernardi, R.C.; Rudack, T.; Stone, J.E.; Phillips, J.C.; Freddolino, P.L.; Schulten, K. QwikMD—Integrative Molecular Dynamics Toolkit for Novices and Experts. Sci. Rep. 2016, 6, 26536. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, N.J.; Sheikh, O. Forecasting of biosensor technologies for emerging point of care and medical IoT applications using bibliometrics and patent analysis. In Proceedings of the IEEE 2016 Portland International Conference on Management of Engineering and Technology, Honolulu, HI, USA, 4–8 September 2016; pp. 3082–3093. [Google Scholar]
- ProXentia Srl. Available online: http://www.proxentia.com/ (accessed on 23 November 2017).
- Dynamic Biosensors Inc. Available online: http://www.dynamic-biosensors.com/ (accessed on 23 November 2017).
- Top Biosensor Companies. VentureRadar. Available online: https://www.ventureradar.com/keyword/Biosensors (accessed on 23 November 2017).
- De Gennes, P.G.; Prost, J. The Physics of Liquid Crystals, 2nd ed.; Claredon Press: Oxford, UK, 1995. [Google Scholar]
- Xu, M.; Jones, O.D.; Wang, L.; Zhou, X.; Davis, H.G.; Bryant, J.L.; Ma, J.; Isaacs, W.B.; Xu, X. Characterization of tubular liquid crystal structure in embryonic stem cell derived embryoid bodies. Cell Biosci. 2017, 7, 3. [Google Scholar] [CrossRef] [PubMed]





















© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popov, N.; Honaker, L.W.; Popova, M.; Usol’tseva, N.; Mann, E.K.; Jákli, A.; Popov, P. Thermotropic Liquid Crystal-Assisted Chemical and Biological Sensors. Materials 2018, 11, 20. https://doi.org/10.3390/ma11010020
Popov N, Honaker LW, Popova M, Usol’tseva N, Mann EK, Jákli A, Popov P. Thermotropic Liquid Crystal-Assisted Chemical and Biological Sensors. Materials. 2018; 11(1):20. https://doi.org/10.3390/ma11010020
Chicago/Turabian StylePopov, Nicolai, Lawrence W. Honaker, Maia Popova, Nadezhda Usol’tseva, Elizabeth K. Mann, Antal Jákli, and Piotr Popov. 2018. "Thermotropic Liquid Crystal-Assisted Chemical and Biological Sensors" Materials 11, no. 1: 20. https://doi.org/10.3390/ma11010020
APA StylePopov, N., Honaker, L. W., Popova, M., Usol’tseva, N., Mann, E. K., Jákli, A., & Popov, P. (2018). Thermotropic Liquid Crystal-Assisted Chemical and Biological Sensors. Materials, 11(1), 20. https://doi.org/10.3390/ma11010020





