Detection of Singlet Oxygen Formation inside Photoactive Biohybrid Composite Material
Abstract
:1. Introduction
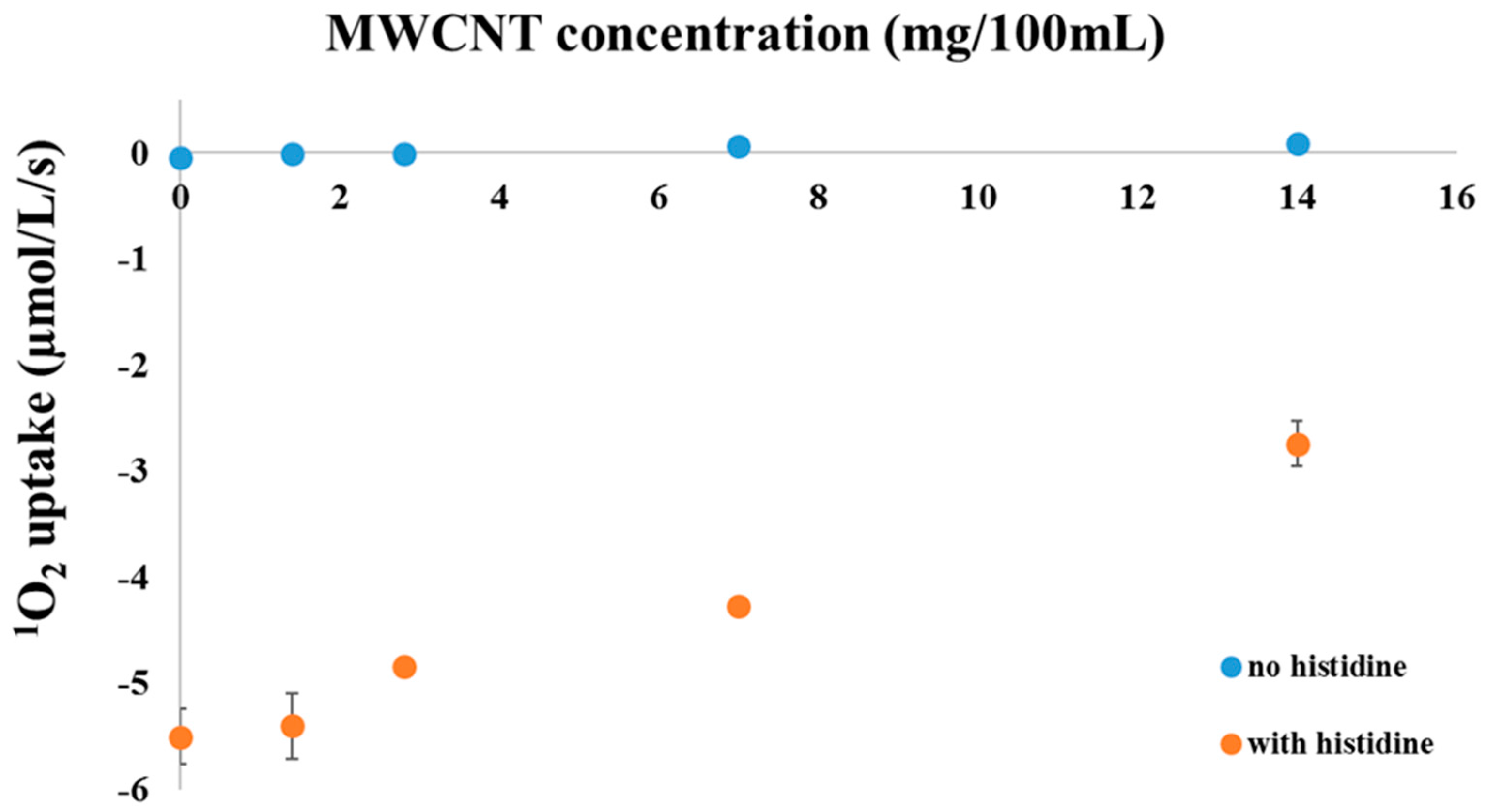
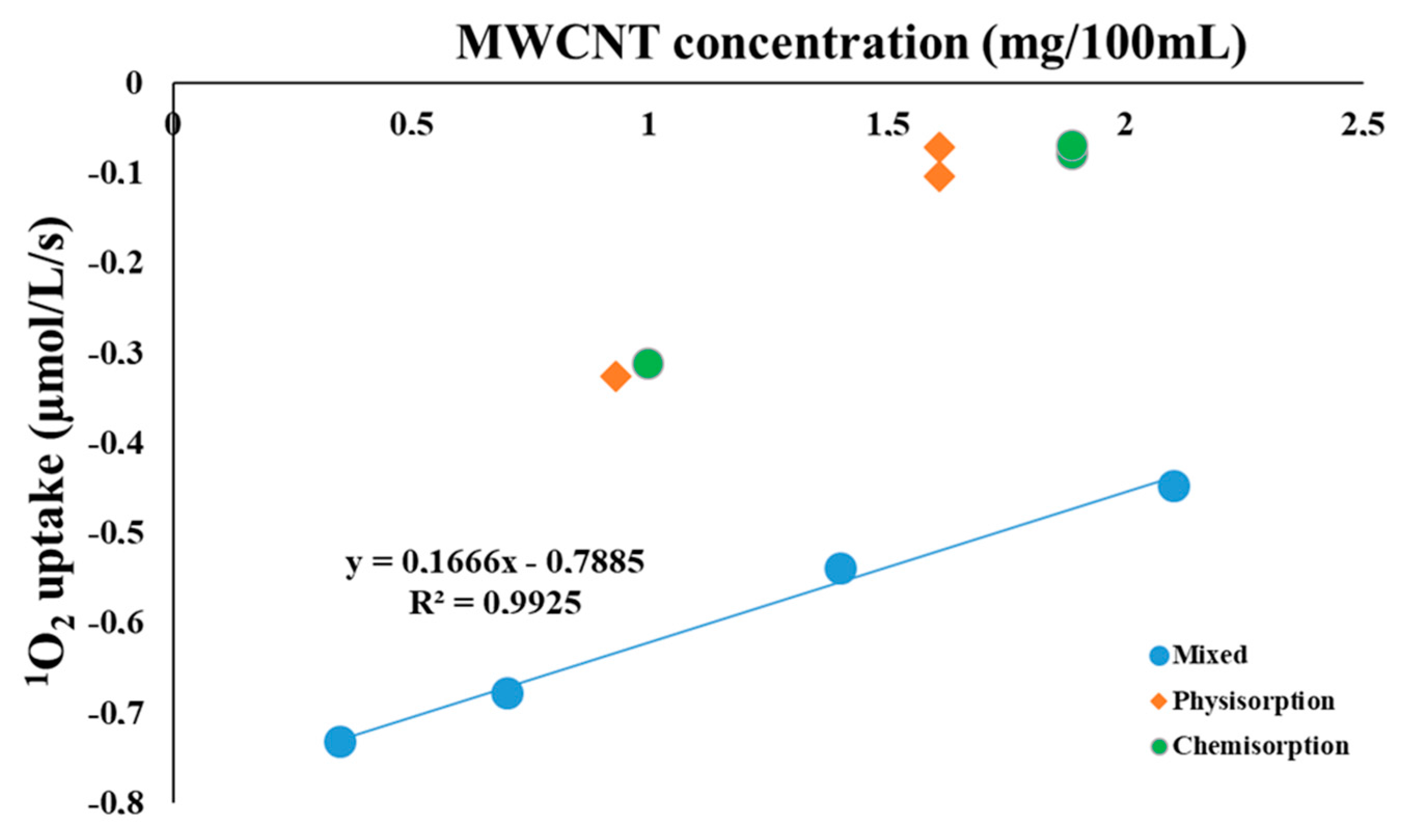
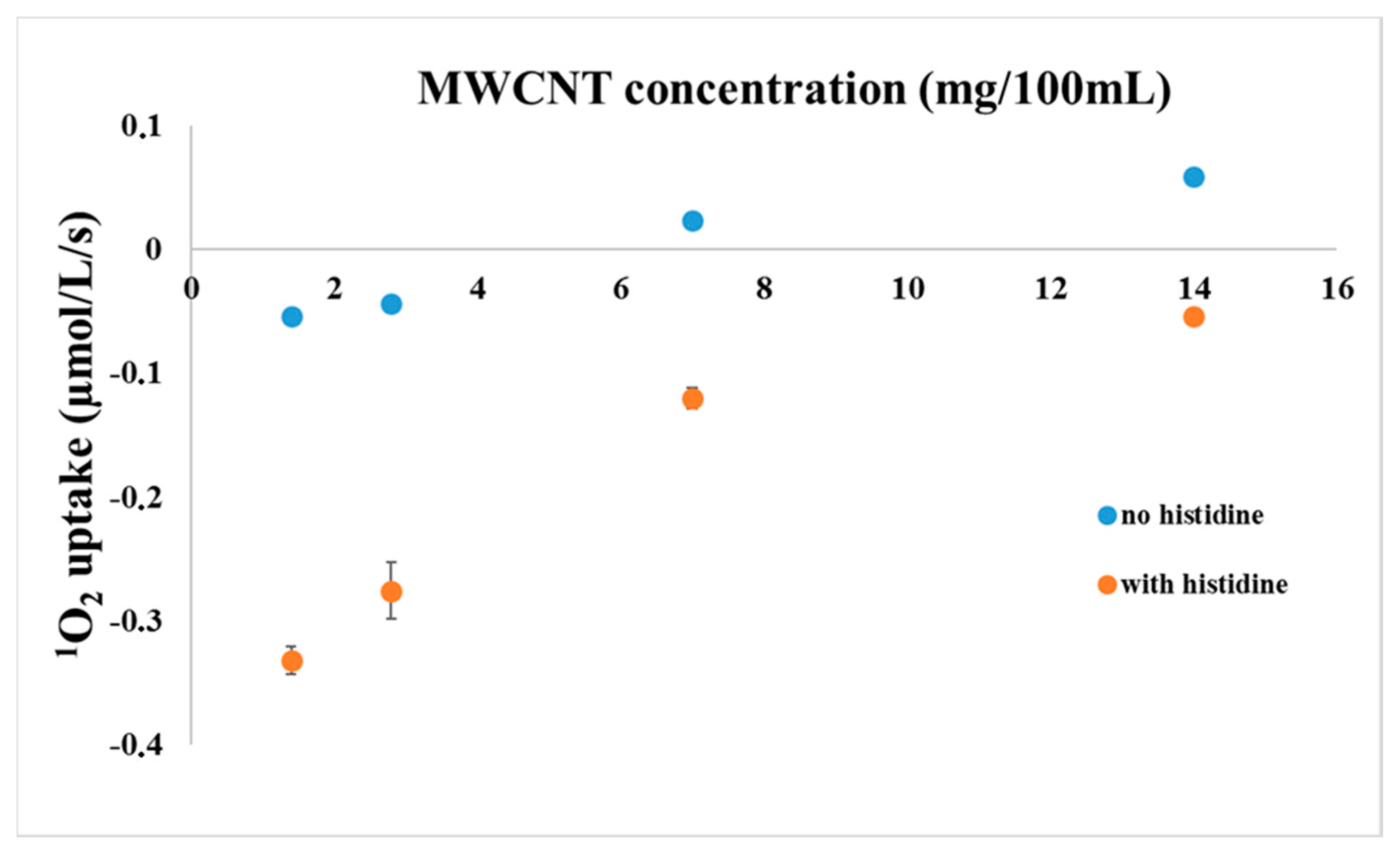
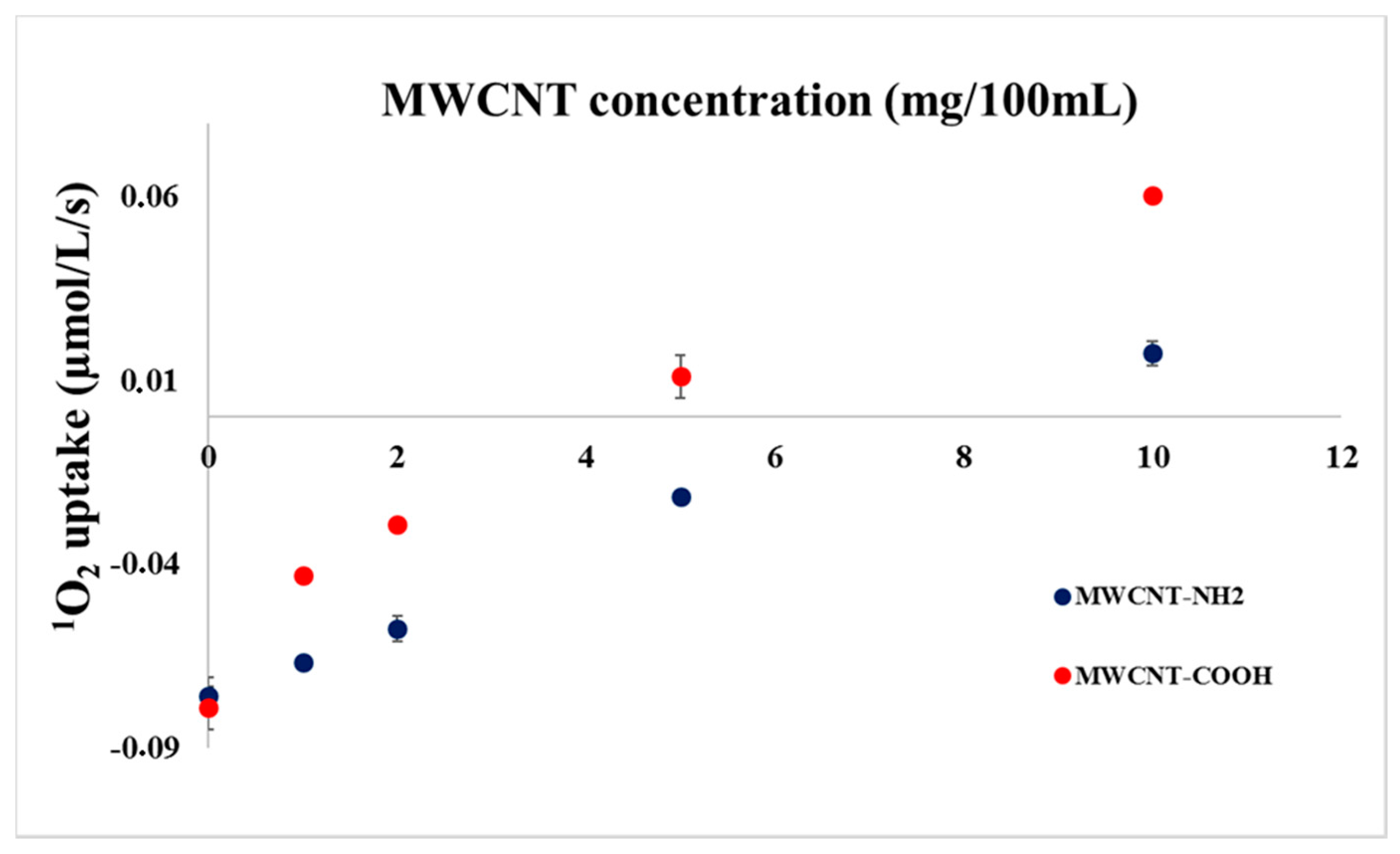
2. Results and Discussion
3. Materials and Methods
3.1. Sample Preparation
3.2. Singlet Oxygen Production Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Xua, J.; Bhattacharya, P.; Váró, G. Monolithically integrated bacteriorhodopsin/semiconductor opto-electronic integrated circuit for a bio-photoreceiver. Biosens. Bioelectron. 2004, 19, 885–892. [Google Scholar] [CrossRef]
- Meunier, C.F.; Rooke, J.C.; Hajdu, K.; Cutsem, P.V.; Cambier, P.; Leonard, A.; Su, B.L. Insight into cellular response of plant cells confined within silica-based matrices. Langmuir 2010, 26, 6568–6575. [Google Scholar] [CrossRef] [PubMed]
- Shoseyov, O.; Levy, I. Nanobiotechnology: Bioinspired Devices and Materials of the Future; Humana Press Inc.: Totowa, NJ, USA, 2008; ISBN 978-1-59745-218-2. [Google Scholar]
- Ormos, P.; Fábián, L.; Oroszi, L.; Wolff, E.K.; Ramsden, J.J.; Dér, A. Protein-based integrated optical switching and modulation. Appl. Phys. Lett. 2002, 80, 4060–4062. [Google Scholar] [CrossRef]
- Hajdu, K.; Szabó, T.; Magyar, M.; Bencsik, G.; Németh, Z.; Nagy, K.; Forró, L.; Váró, G.; Hernádi, K.; Nagy, L. Photosynthetic reaction center protein in nanostructures. Phys. Status Solidi B 2011, 248, 2700–2703. [Google Scholar] [CrossRef]
- Darder, M.; Aranda, P.; Ruiz-Hitzky, E. Bionanocomposites: A new concept of ecological, bioinspired, and functional hybrid materials. Adv. Mater. 2007, 19, 1309–1319. [Google Scholar] [CrossRef]
- Hajdu, K.; Gergely, C.; Martin, M.; Zimányi, L.; Agarwal, V.; Palestino, G.; Hernádi, K.; Németh, Z.; Nagy, L. Light-harvesting bio-nanomaterial using porous silicon and photosynthetic reaction center. Nanoscale Res. Lett. 2012, 7, 400. [Google Scholar] [CrossRef] [PubMed]
- Friebe, V.M.; Delgado, J.D.; Swainsbury, D.J.K.; Gruber, J.M.; Chanaewa, A.; Grondelle, R.; Hauff, E.L.; Millo, D.; Joes, M.R.; Frese, R.N. Plasmon enhanced photocurrent of photosynthetic pigment-proteins on nanoporous silver. Adv. Funct. Mater. 2015, 26, 285–292. [Google Scholar] [CrossRef]
- Allen, J.P.; Williams, J.C. Photosynthetic Reaction Centers. FEBS Lett. 1998, 438, 5–9. [Google Scholar] [CrossRef]
- Paddock, M.L.; Feher, G.; Okamura, M.Y. Proton Transfer Pathways and Mechanism in Bacterial Reaction Centers. FEBS Lett. 2003, 555, 45–50. [Google Scholar] [CrossRef]
- Wraight, C.A. Proton and Electron in the Acceptor Quinone Complex of Photosynthetic Reaction Centers from Rhodobacter Sphaeroides. Front. Biosci. 2004, 9, 309–337. [Google Scholar] [CrossRef] [PubMed]
- Nagy, L.; Magyar, M.; Szabó, T.; Hajdu, K.; Giotta, L.; Dorogi, M.; Milano, F. Photosynthetic Machineries in Nano-Systems. Curr. Protein Pept. Sci. 2014, 15, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Szabó, T.; Magyar, M.; Hajdu, K.; Dorogi, M.; Nyerki, E.; Tóth, T.; Lingvay, M.; Garab, G.; Hernádi, K.; Nagy, L. Structural and Functional Hierarchy in Photosynthetic Energy Conversion—From Molecules to Nanostructures. Nanoscale Res. Lett. 2015, 10, 458. [Google Scholar] [CrossRef] [PubMed]
- Szabó, T.; Nyerki, E.; Tóth, T.; Csekő, R.; Magyar, M.; Horváth, E.; Hernádi, K.; Endrődi, B.; Visy, C.; Forró, L.; Nagy, L. Generating photocurrent by nanocomposites based on photosynthetic reaction centre protein. Phys. Status Solidi B 2015, 252, 2614–2619. [Google Scholar] [CrossRef]
- Takshi, A.; Yaghoubi, H.; Wang, J.; Jun, D.; Beatty, J.T. Electrochemical Field-Effect Transistor Utilization to Study the Coupling Success Rate of Photosynthetic Protein Complexes to Cytochrome c. Biosensors 2017, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Ciornii, D.; Feifel, S.C.; Hejazi, M.; Kölsch, A.; Lokstein, H.; Zouni, A.; Lisdat, F. Construction of photobiocathodes using multi-walled carbon nanotubesand photosystem I. Phys. Status Solidi A 2017, 214, 9. [Google Scholar] [CrossRef]
- Kaniber, S.M.; Brandstetter, M.; Simmel, F.C.; Carmeli, I.; Holleitner, A.W. On-chip functionalization of carbon nanotubes with photosystem I. J. Am. Chem. Soc. 2010, 132, 2872–2873. [Google Scholar] [CrossRef] [PubMed]
- Feifel, S.C.; Lokstein, H.; Hejazi, M.; Zouni, A.; Lisdat, F. Unidirectional photocurrent of photosystem I on p-System-modified graphene electrodes: Nanobionic approaches for the construction of photobiohybrid systems. Langmuir 2015, 31, 10590–10598. [Google Scholar] [CrossRef] [PubMed]
- Kothe, T.; Pöller, S.; Zhao, F.; Fortgang, P.; Rögner, M.; Schuhmann, W.; Plumere, N. Engineered electron-transfer chain in photosystem 1 based photocathodes outperforms electron-transfer rates in natural photosynthesis. Chem. Eur. J. 2014, 20, 11029–11034. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.M.; Magdaong, M.; Shen, H.; Frank, A.; Rusling, J.F. Efficient photoelectrochemical energy conversion using spinach photosystem II (PSII) in lipid multilayer films. ChemistryOpen 2015, 4, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubi, H.; Schaefer, M.; Yaghoubi, S.; Jun, D.; Schlaf, R.; Beatty, T.; Takshi, A. A ZnO nanowire bio-hybrid solar cell. Nanotechnology 2017, 28, 5. [Google Scholar] [CrossRef] [PubMed]
- Miyachi, M.; Ikehira, S.; Nishiori, D.; Yamanoi, Y.; Yamada, M.; Iwai, M.; Tomo, T.; Allakhverdiev, S.I.; Nishihara, H. Photocurrent Generation of Reconstituted Photosystem II on a Self-Assembled Gold Film. Langmuir 2017, 33, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Badura, A.; Esper, B.; Ataka, K.; Grunwald, C.; Wol, C.; Kuhlmann, J.; Heberle, J.; Rogner, M. Light-Driven Water Splitting for (Bio-)Hydrogen Production: Photosystem 2 as the Central Part of a Bioelectrochemical Device. Photochem. Photobiol. 2006, 82, 1385–1390. [Google Scholar] [CrossRef] [PubMed]
- Volushin, R.A.; Kreslavski, V.D.; Zharmukhamedov, S.K.; Bedbenov, V.S.; Ramakrishna, S.; Allakhverdiev, S. Photoelectrochemical cells based on photosynthetic systems: A review. Biofuel Res. J. 2015, 6, 227–235. [Google Scholar] [CrossRef]
- Boghossian, A.A.; Zhang, J.; Barone, P.W.; Reuel, N.F.; Kim, J.H.; Heller, D.A.; Ahn, J.H.; Hilmer, A.J.; Rwei, A.; Arkalgud, J.R.; et al. Near-Infrared Fluorescent Sensors Based on Single-Walled Carbon Nanotubes for Life Sciences Applications. ChemSusChem 2011, 7, 848–863. [Google Scholar] [CrossRef] [PubMed]
- Carmeli, I.; Mangold, M.; Frolov, L.; Zebli, B.; Carmeli, C.; Richter, S.; Holleitner, A.W. A Photosynthetic Reaction Center Covalently Bound to Carbon Nanotubes. Adv. Mater. 2007, 19, 3901–3905. [Google Scholar] [CrossRef]
- Frolov, L.; Rosenwaks, Y.; Carmeli, C.; Carmeli, I. Fabrication of a Photoelectronic Device by Direct Chemical Binding of the Photosynthetic Reaction Center Protein to Metal Surfaces. Adv. Mater. 2005, 17, 2434–2437. [Google Scholar] [CrossRef]
- Davis, J.J.; Coles, R.J.; Allen, H.; Hill, O. Protein Electrochemistry at Carbon Nanotube Electrodes. J. Electroanal. Chem. 1997, 440, 279–282. [Google Scholar] [CrossRef]
- Dorogi, M.; Balint, Z.; Miko, C.; Vileno, B.; Milas, M.; Hernadi, K.; Forro, L.; Varo, G.; Nagy, L. Stabilization Effect of Single-Walled Carbon Nanotubes on the Functioning of Photosynthetic Reaction Centers. J. Phys. Chem. B 2006, 110, 21473–21479. [Google Scholar] [CrossRef] [PubMed]
- Kruss, S.; Hilmer, A.J.; Zhang, J.; Reuel, N.F.; Mu, B.; Strano, M.S. Carbon Nanotubes as Optical Biomedical Sensors. Adv. Drug Deliv. Rev. 2013, 65, 1933–1950. [Google Scholar] [CrossRef] [PubMed]
- Magyar, M.; Hajdu, K.; Szabó, T.; Endrődi, B.; Hernádi, K.; Horváth, E.; Magrez, A.; Forró, L.; Visy, C.; Nagy, L. Sensing Hydrogen Peroxide by Carbon Nanotube/Horseradish Peroxidase Bio-Nanocomposite. Phys. Status Solidi B 2013, 250, 2559–2563. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Eklund, P.C. Science of Fullerenes and Carbon Nanotubes, 1st ed.; Academic Press: San Diego, CA, USA, 1996; ISBN 9780080540771. [Google Scholar]
- Banerjee, S.; Kahn, M.G.; Wong, S.S. Rational Chemical Strategies for Carbon Nanotube Functionalization. Chemistry 2003, 9, 1898–1908. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, A. Functionalization of Single-Walled Carbon Nanotubes. Angew. Chem. Int. Ed. 2002, 41, 1853–1859. [Google Scholar] [CrossRef]
- Hideg, E.; Kós, P.B.; Vass, I. Photosystem II damage induced by chemically generated singlet oxygen in tobacco leaves. Phys. Plant 2007, 131, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J. Free Radicals in Biology and Medicine; Oxford Science Publications: New York, NY, USA, 1999. [Google Scholar]
- Tyystjärvi, E.; Phototoxicity, L.D. Noodén: Plant Cell Death Processes; Academic Press: San Diego, CA, USA, 2004; pp. 271–283. ISBN 0-12-520915-0. [Google Scholar]
- Tyystjärvi, E. Photoinhibition of Photosystem II. Int. Rev. Cell Mol. Biol. 2013, 300, 243–303. [Google Scholar] [PubMed]
- Gallejo, S.M.; Pena, L.B.; Barcia, R.A.; Azpilicueta, C.E.; Iannone, M.F.; Rosales, E.P.; Zawoznik, M.S.; Groppa, M.D.; Benavides, M.P. Unravelling cadmium toxicity and tolerance in plants: Insight into regulatory mechanisms. Environ. Exp. Bot. 2012, 83, 33–46. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Allakhverdiev, S.I.; Murata, N. Protein synthesis is the primary target of reactive oxygen species in the photoinhibition of photosystem II. Physiol. Plant 2011, 142, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Moan, J.; Pettersen, E.O.; Christensen, T. The mechanism of photodynamic inactivation of human cells in vitro in the presence of haematoporphyrin. Br. J. Cancer 1979, 39, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Krasnovsky, A.A., Jr. Singlet molecular oxygen in photobiochemical systems: IR phosphorescence studies. Membr. Cell Biol. 1998, 12, 665–690. [Google Scholar] [PubMed]
- Li, H.; Melø, T.B.; Arellano, J.B.; Razi Naqvi, K. Temporal profile of the singlet oxygen emission endogenously produced by photosystem II reaction centre in an aqueous buffer. Photosynth. Res. 2012, 112, 75–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, P.C.; Rodgers, M.A.J. Singlet molecular oxygen in micellar systems. 1. Distribution equilibria between hydrophobic and hydrophilic compartments. J. Phys. Chem. 1983, 87, 4894–4898. [Google Scholar] [CrossRef]
- Ehrenberg, B.; Anderson, J.L.; Foote, C.S. Kinetics and yield of singlet oxygen photosensitized by hypericin in organic and biological media. Photochem. Photobiol. 1998, 68, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Boldog, P.; Hajdu, K.; Magyar, M.; Hideg, É.; Hernádi, K.; Horváth, E.; Magrez, A.; Nagy, K.; Váró, G.; Forró, L.; et al. Carbon Nanotubes Quench Singlet Oxygen Generated by Photosynthetic Reaction Centers. Phys. Status Solidi B 2013, 250, 2539–2543. [Google Scholar] [CrossRef] [Green Version]
- Mattila, H.; Khorobrykh, S.; Havurinne, V.; Tyystjarvi, E. Reactive oxygen species: Reactions and detection from photosynthetic tissues. J. Photochem. Photobiol. B Biol. 2015, 152, 176–214. [Google Scholar] [CrossRef] [PubMed]
- Karonen, M.; Mattila, H.; Huang, P.; Mamedov, F.; Styring, S.; Tyystjarvi, E. A Tandem Mass Spectrometric Method for Singlet Oxygen Measurement. Photochem. Photobiol. 2014, 90, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Hideg, E.; Deák, Z.; Hakala-Yatkin, M.; Karonen, M.; Rutherford, A.W.; Tyystjärvi, E.; Vass, I.; Krieger-Liszkay, A. Pure forms of the singlet oxygen sensors TEMP and TEMPD do not inhibit Photosystem II. Biochim. Biophys. Acta 2011, 1807, 1658–1661. [Google Scholar] [CrossRef] [PubMed]
- Hideg, E.; Barta, C.; Kálai, T.; Vass, I.; Hideg, K.; Asada, K. Detection of singlet oxygen and superoxide with fluorescent sensors in leaves under stress by photoinhibition or UV radiation. Plant Cell Physiol. 2002, 43, 1154–1164. [Google Scholar] [CrossRef] [PubMed]
- Telfer, A.; Bishop, S.M.; Phillips, D.; Barber, J. Isolated photosynthetic reaction center of photosystem two as a sensitizer for the formation of singlet oxygen. J. Biol. Chem. 1994, 269, 13244–13253. [Google Scholar] [PubMed]
- Zhu, Z.; Tang, Z.; Phillips, J.A.; Yang, R.; Wang, H.; Tan, W. Regulation of Singlet Oxygen Generation Using Single-Walled Carbon Nanotubes. J. Am. Chem. Soc. 2008, 130, 10856–10857. [Google Scholar] [CrossRef] [PubMed]
- Hamon, M.A.; Stensaas, K.L.; Sugar, M.A.; Tumminello, K.C.; Allred, A.K. Reacting Soluble Single-Walled Carbon Nanotubes with Singlet Oxygen. Chem. Phys. Lett. 2007, 447, 1–4. [Google Scholar] [CrossRef]
- Lebedkin, S.; Kareev, I.; Hennrich, F.; Kappes, M.M. Efficient Quenching of Singlet Oxygen via Energy Transfer to Semiconducting Single-Walled Carbon Nanotubes. J. Phys. Chem. C 2008, 112, 16236–16239. [Google Scholar] [CrossRef]
- Chen, C.; Jafvert, C.T. Photoreactivity of Carboxylated Single-Walled Carbon Nanotubes in Sunlight: Reactive Oxygen Species Production in Water. Environ. Sci. Technol. 2010, 44, 6674–6679. [Google Scholar] [CrossRef] [PubMed]
- Francisco-Marquez, M.; Galano, A.; Martínez, A. On the Free Radical Scavenging Capability of Carboxylated Single-Walled Carbon Nanotubes. J. Phys. Chem. C 2010, 114, 6363–6370. [Google Scholar] [CrossRef]
- Fenoglio, I.; Tomatis, M.; Lison, D.; Muller, J.; Fonseca, A.B.; Nagy, J.; Fubini, B. Reactivity of Carbon Nanotubes: Free Radical Generation or Scavenging Activity? Free Radic. Biol. Med. 2006, 40, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Jafvert, C.T. The Role of Surface Functionalization in the Solar Light-Induced Production of Reactive Oxygen Species by Single-Walled Carbon Nanotubes in Water. Carbon 2011, 49, 5099–5106. [Google Scholar] [CrossRef]
- Wasserman, H.H.; Stiller, K.; Floyd, M.B. The reactions of heterocyclic systems with singlet oxygen. Photosensitized oxygenation of imidazoles. Tetrahedron Lett. 1968, 9, 3277–3280. [Google Scholar] [CrossRef]
- Rehman, A.U.; Cser, K.; Sass, L.; Vass, I. Characterization of singlet oxygen production and its involvement in photodamage of Photosystem II in the cyanobacterium Synechocystis PCC 6803 by histidine-mediated chemical trapping. Biochim. Biophys. Acta 2013, 1827, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Kinka, A.; Hajdu, K.; Magyar, M.; Mucsi, L.; Szabó, T.; Hernádi, K.; Horváth, E.; Magrez, A.; Forró, L.; Nagy, L. Equilibrium Concentration of Singlet Oxygen in Photoreaction of Reaction Center/Carbon Nanotube Bionanocomposites. Phys. Status Solidi B 2015, 252, 2479–2484. [Google Scholar] [CrossRef]
- Bellus, D. Physical quenchers of singlet molecular oxygen. Adv. Photochem. 1979, 11, 105–205. [Google Scholar]
- Siström, W.R. A requirement for sodium in the growth of Rhodopseudomonas sphaeroides. J. Gen. Microbiol. 1960, 22, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Tandori, J.; Nagy, L.; Puskás, Á.; Droppa, M.; Horváth, G.; Maróti, P. The IleL229 → Met mutation impairs the quinone binding to the QB-pocket in reaction centers of Rhodobacter sphaeroides. Photosynth. Res. 1995, 45, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Straley, S.C.; Parson, W.W.; Mauzerall, D.C.; Clayton, R.K. Pigment content and molar extinction coefficients of photochemical reaction centers from Rhodopseudomonas sphaeroides. Biochim. Biophys. Acta 1973, 305, 597–609. [Google Scholar] [CrossRef]
- Rehman, A.U.; Szabó, M.; Deák, Z.; Sass, L.; Larkum, A.; Ralph, P.; Vass, I. Symbiodinium sp. cells produce light-induced intra- and extracellular singlet oxygen, which mediates photodamage of the photosynthetic apparatus and has the potential to interact with the animal host in coral symbiosis. New Phytol. 2016, 212, 472–484. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Hurtado, J.; López, R.; Suárez, D.; Menéndez, M.I. Theoretical study of the oxidation of histidine by singlet oxygen. Chem. Eur. J. 2012, 18, 8437–8447. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hajdu, K.; Ur Rehman, A.; Vass, I.; Nagy, L. Detection of Singlet Oxygen Formation inside Photoactive Biohybrid Composite Material. Materials 2018, 11, 28. https://doi.org/10.3390/ma11010028
Hajdu K, Ur Rehman A, Vass I, Nagy L. Detection of Singlet Oxygen Formation inside Photoactive Biohybrid Composite Material. Materials. 2018; 11(1):28. https://doi.org/10.3390/ma11010028
Chicago/Turabian StyleHajdu, Kata, Ateeq Ur Rehman, Imre Vass, and László Nagy. 2018. "Detection of Singlet Oxygen Formation inside Photoactive Biohybrid Composite Material" Materials 11, no. 1: 28. https://doi.org/10.3390/ma11010028
APA StyleHajdu, K., Ur Rehman, A., Vass, I., & Nagy, L. (2018). Detection of Singlet Oxygen Formation inside Photoactive Biohybrid Composite Material. Materials, 11(1), 28. https://doi.org/10.3390/ma11010028





