Insights into Galvanic Corrosion Behavior of Ti-Cu Dissimilar Joint: Effect of Microstructure and Volta Potential
Abstract
:1. Introduction
2. Materials and Methods
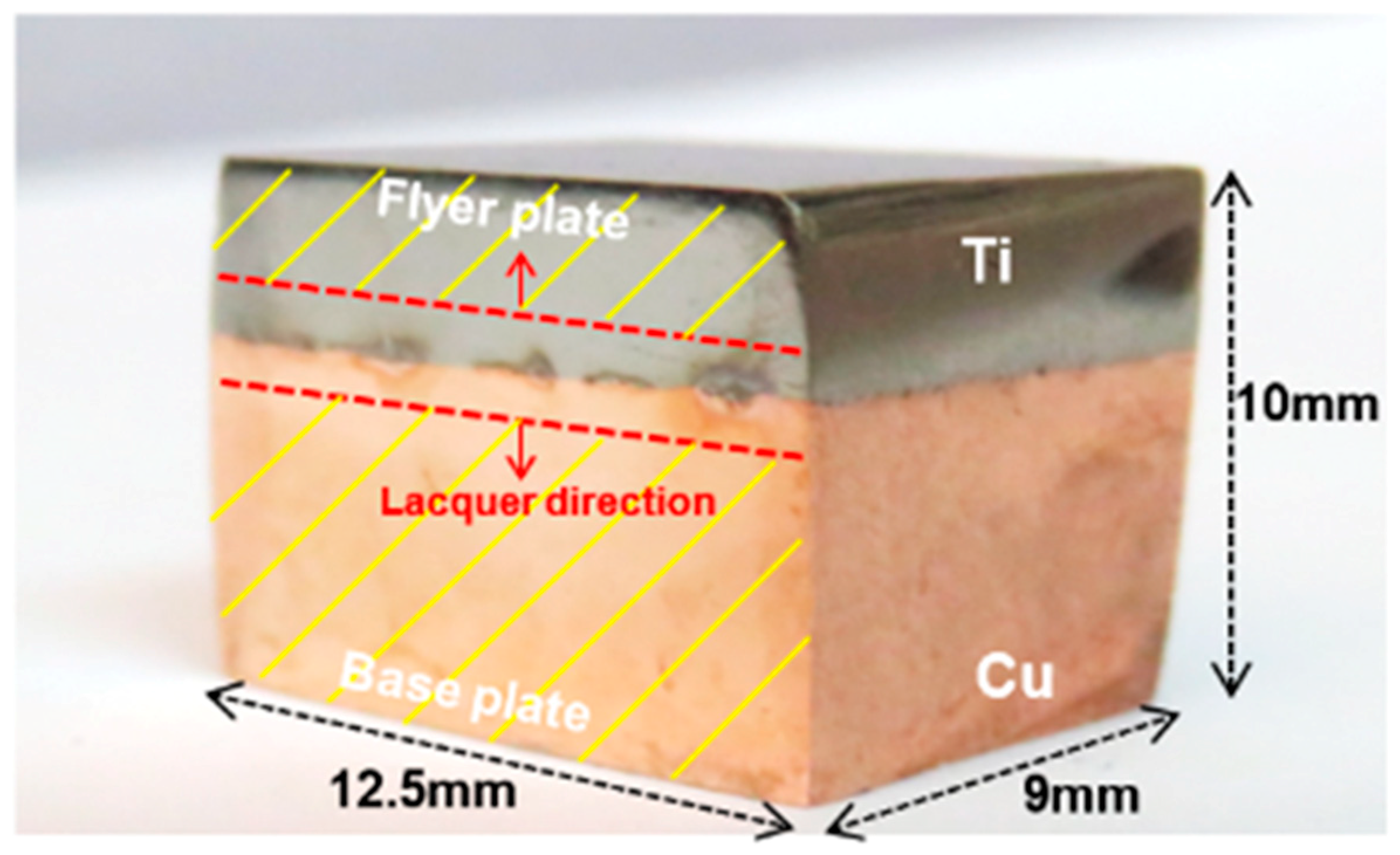
2.1. Materials and Preparation
2.2. Metallographic Studies and Hardness Measurement
2.3. Electrochemical Measurements
2.4. Volta Potential Measurement by SKPFM
3. Results
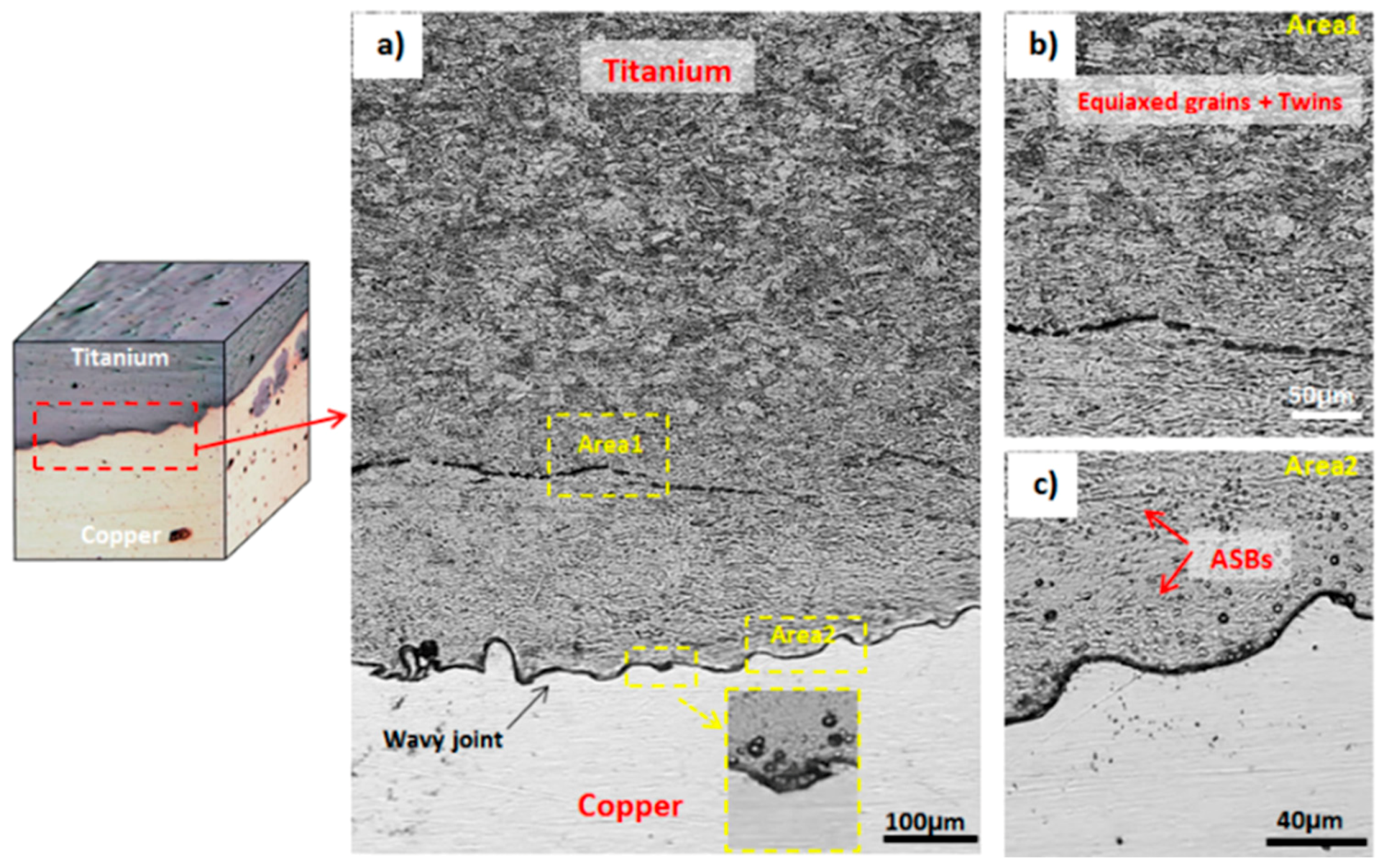
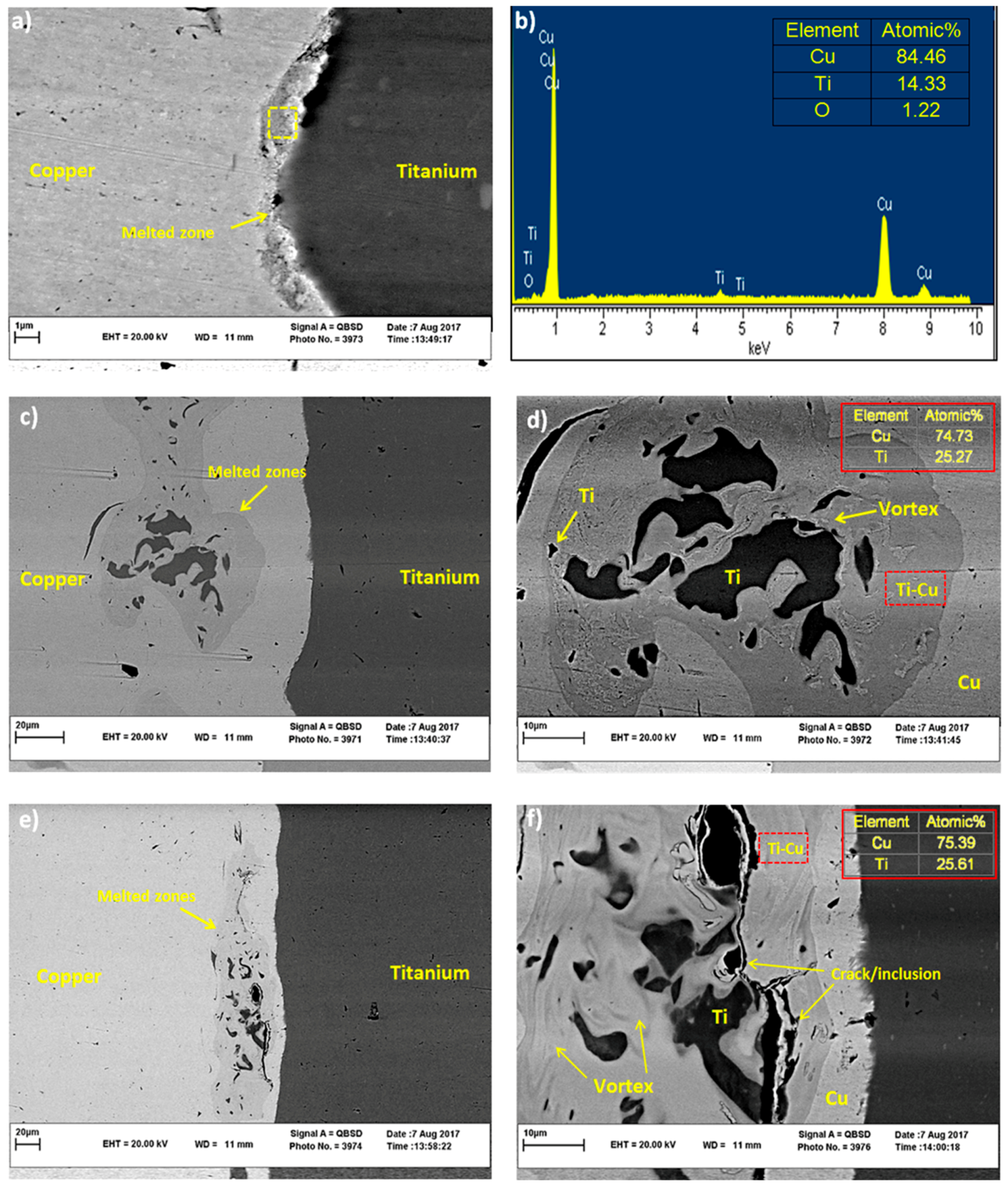
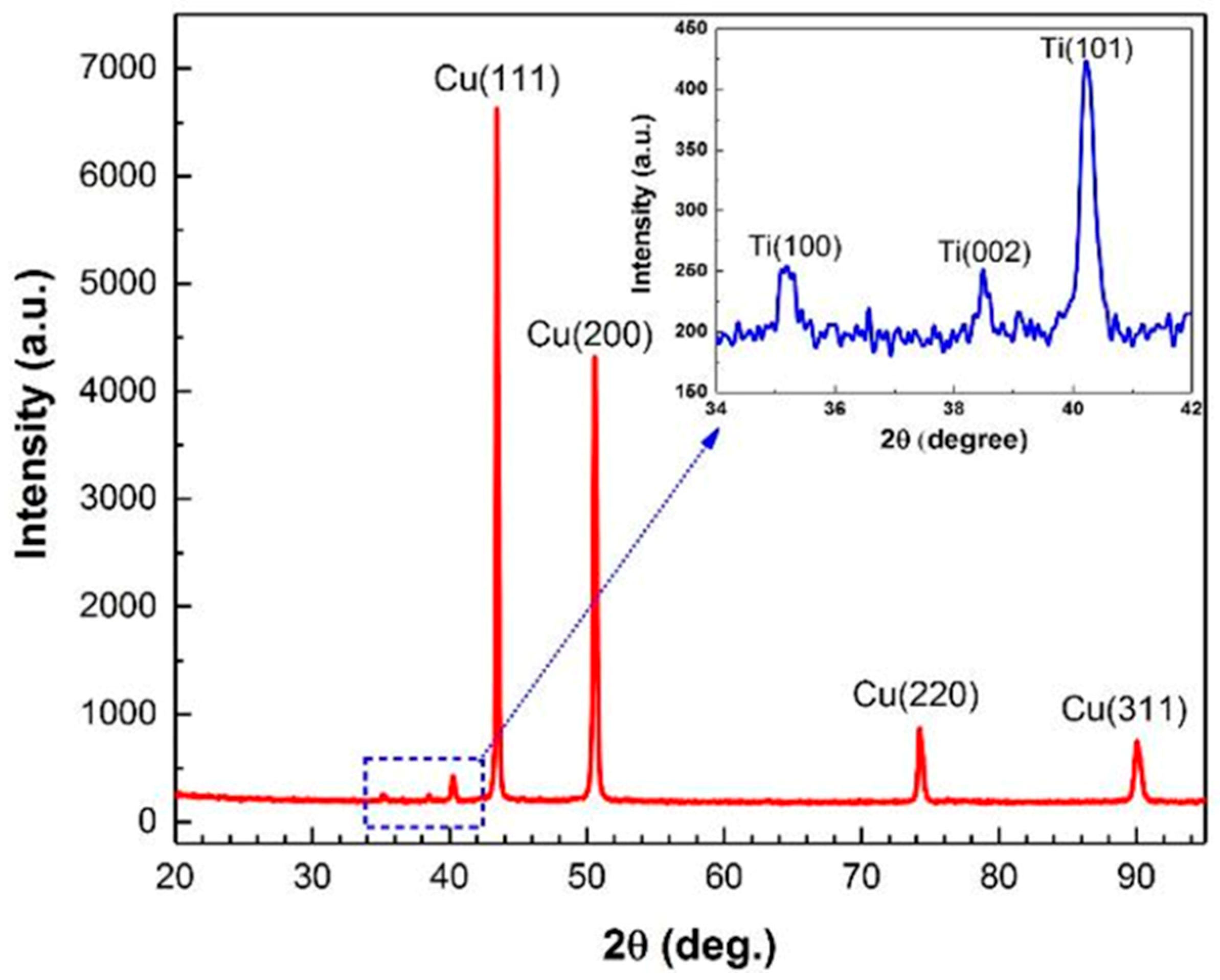
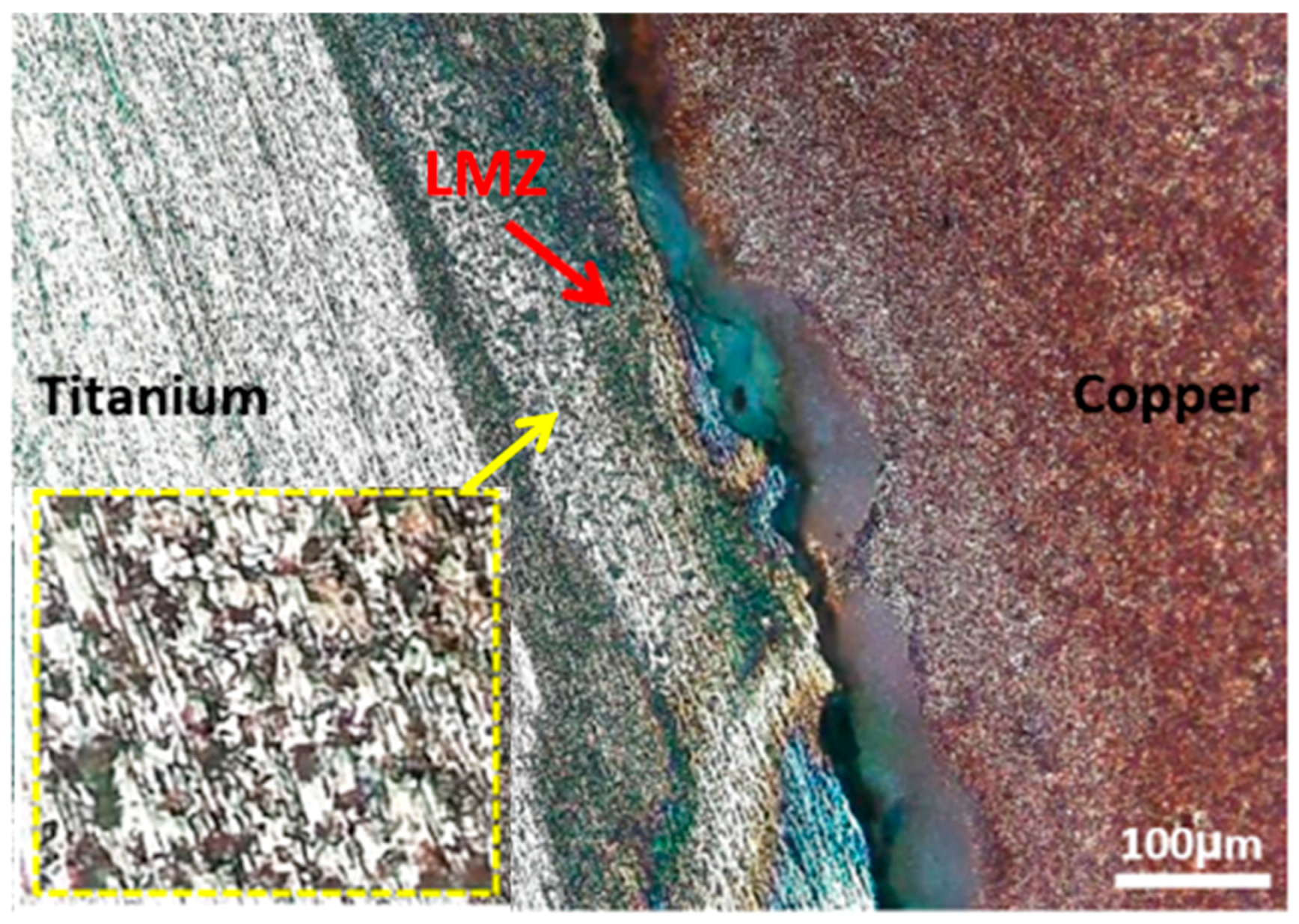
3.1. Microstructural Investigation
3.2. Micro-Hardness Test
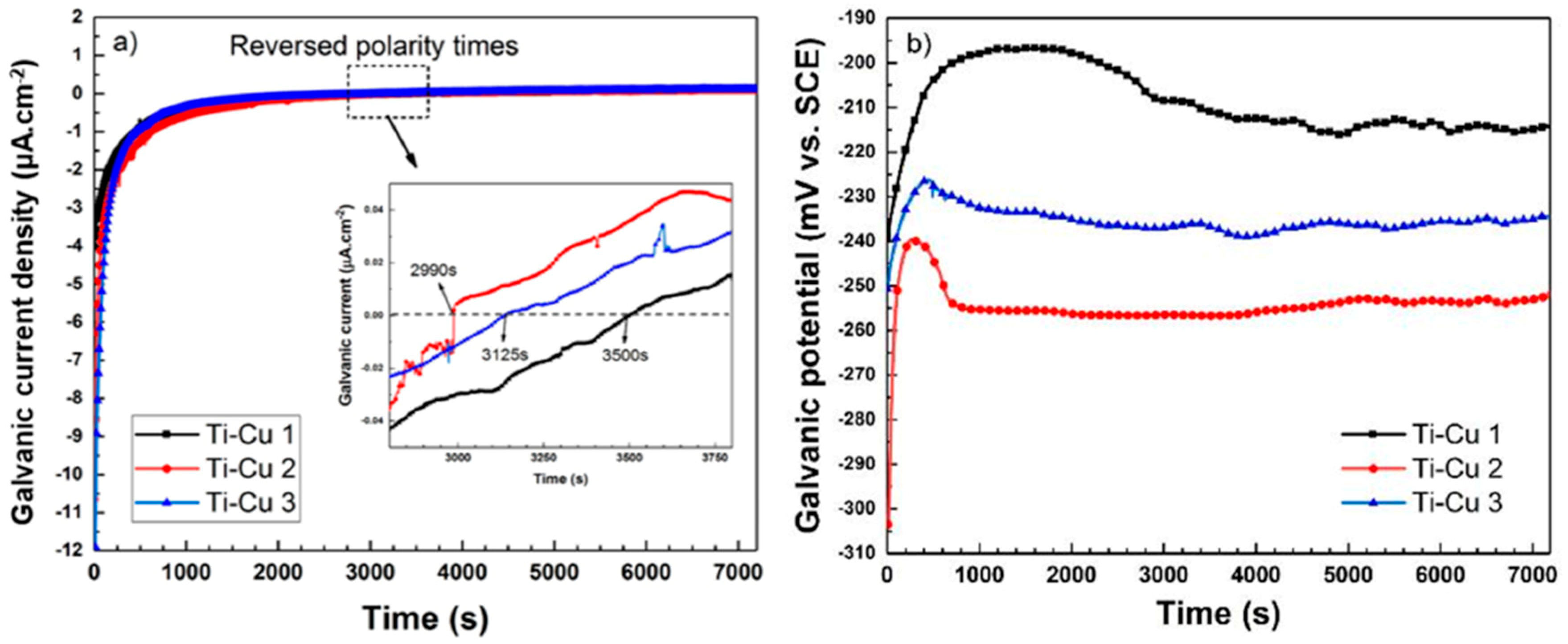
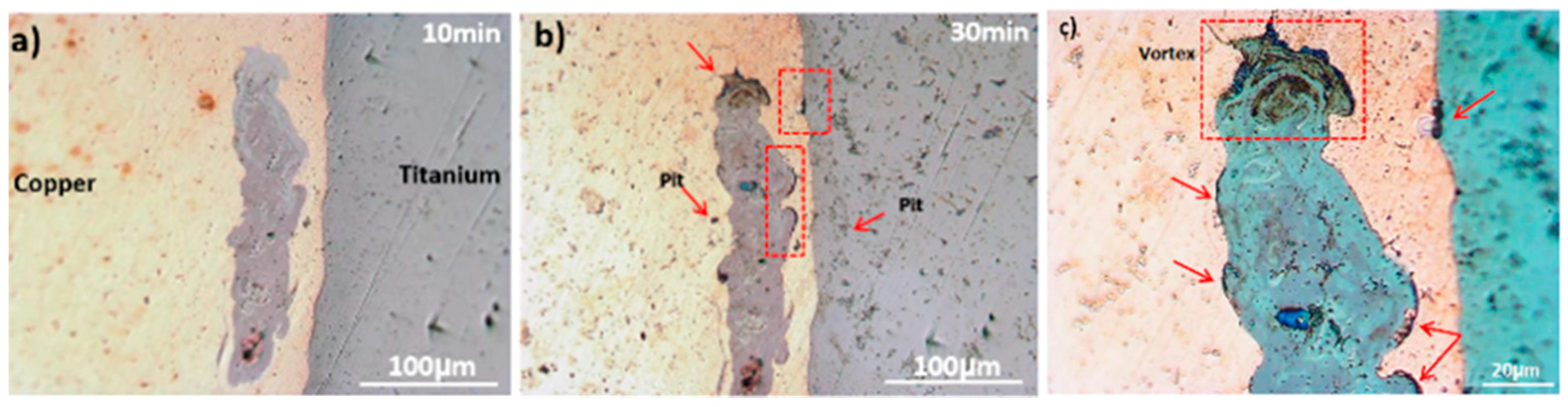
3.3. Corrosion Behavior
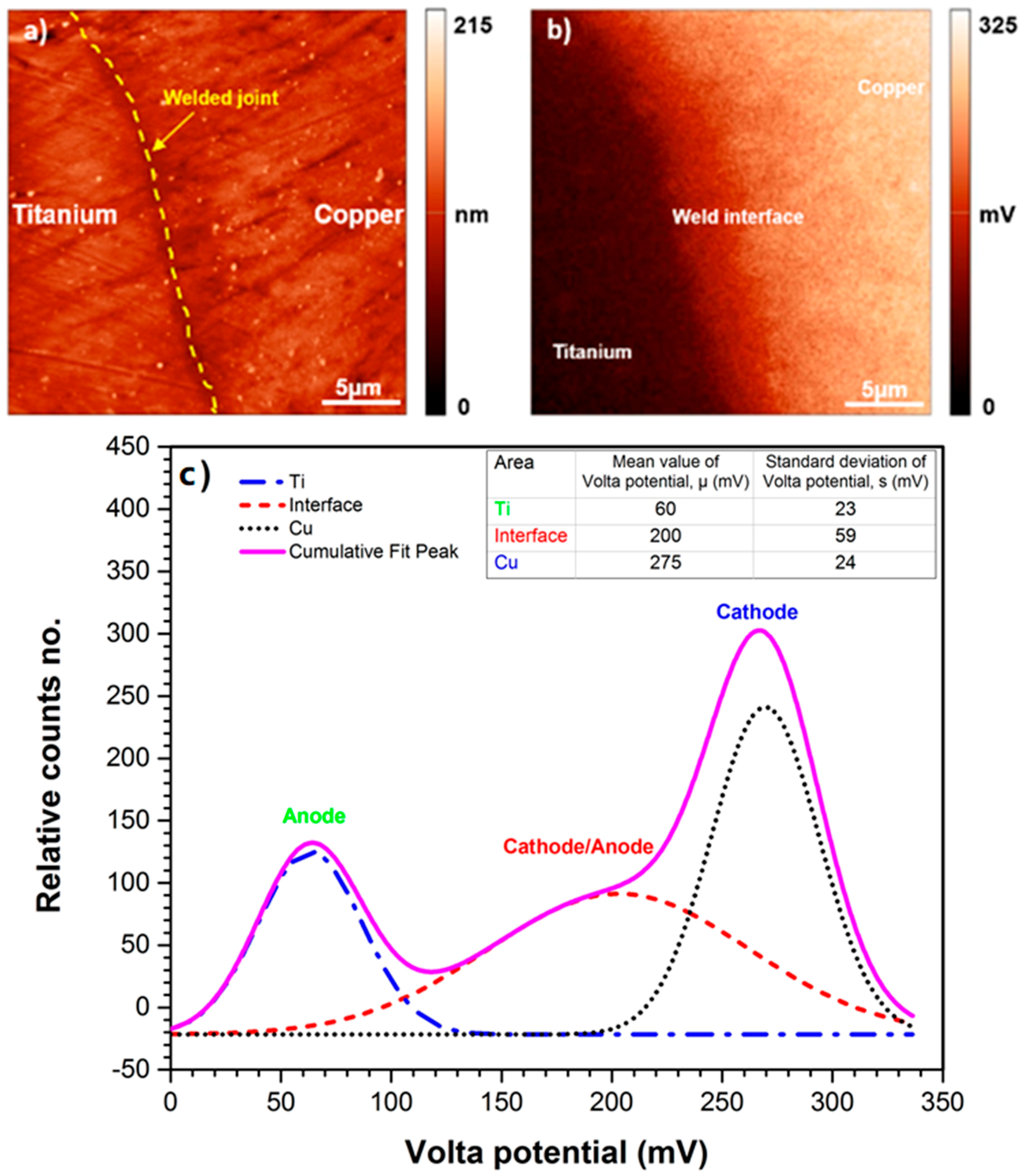
3.4. Predication of Volta Potential Measurement and Immersion Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bataev, I.A.; Lazurenko, D.V.; Tanaka, S.; Hokamoto, K.; Bataev, A.A.; Guo, Y.; Jorge, A.M. High cooling rates and metastable phases at the interfaces of explosively welded materials. Acta Mater. 2017, 135, 277–289. [Google Scholar] [CrossRef]
- Acarer, M. Electrical, Corrosion, and Mechanical Properties of Aluminum-Copper Joints Produced by Explosive Welding. J. Mater. Eng. Perform. 2012, 21, 2375–2379. [Google Scholar] [CrossRef]
- Choi, C.; Tan, P.; Ruan, D.; Dixon, B. A new concept of universal substitutive explosive welding. Mater. Des. 2017, 115, 393–403. [Google Scholar] [CrossRef]
- Liu, W.; Xu, Q.; Han, J.; Chen, X.; Min, Y. A novel combination approach for the preparation of superhydrophobic surface on copper and the consequent corrosion resistance. Corros. Sci. 2016, 110, 105–113. [Google Scholar] [CrossRef]
- Zhang, X.; Odnevall Wallinder, I.; Leygraf, C. Mechanistic studies of corrosion product flaking on copper and copper-based alloys in marine environments. Corros. Sci. 2014, 85, 15–25. [Google Scholar] [CrossRef]
- Petrović Mihajlović, M.B.; Radovanović, M.B.; Tasić, Ž.Z.; Antonijević, M.M. Imidazole based compounds as copper corrosion inhibitors in seawater. J. Mol. Liq. 2017, 225, 127–136. [Google Scholar] [CrossRef]
- Welbourn, R.J.; Truscott, C.L.; Skoda, M.A.; Zarbakhsh, A.; Clarke, S.M. Corrosion and inhibition of copper in hydrocarbon solution on a molecular level investigated using neutron reflectometry and XPS. Corros. Sci. 2017, 115, 68–77. [Google Scholar] [CrossRef]
- Tian, H.; Cheng, Y.F.; Li, W.; Hou, B. Triazolyl-acylhydrazone derivatives as novel inhibitors for copper corrosion in chloride solutions. Corros. Sci. 2015, 100, 341–352. [Google Scholar] [CrossRef]
- Mansfeld, F.; Liu, G.; Xiao, H.; Tsai, C.H.; Little, B.J. The corrosion behavior of copper alloys, stainless steels and titanium in seawater. Corros. Sci. 1994, 36, 2063–2095. [Google Scholar] [CrossRef]
- Liu, J.; Alfantazi, A.; Asselin, E. Effects of Temperature and Sulfate on the Pitting Corrosion of Titanium in High-Temperature Chloride Solutions. J. Electrochem. Soc. 2015, 162, C189–C196. [Google Scholar] [CrossRef]
- Casillas, N. Scanning Electrochemical Microscopy of Precursor Sites for Pitting Corrosion on Titanium. J. Electrochem. Soc. 1993, 140, L142. [Google Scholar] [CrossRef]
- Zu, G.; Li, X.; Zhang, J.; Zhang, H. Interfacial characterization and mechanical property of Ti/Cu clad sheet produced by explosive welding and annealing. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2015, 30, 1198–1203. [Google Scholar] [CrossRef]
- Vivek, A.; Liu, B.C.; Hansen, S.R.; Daehn, G.S. Accessing collision welding process window for titanium/copper welds with vaporizing foil actuators and grooved targets. J. Mater. Process. Technol. 2014, 214, 1583–1589. [Google Scholar] [CrossRef]
- Durgutlu, A.; Gülenç, B.; Findik, F. Examination of copper/stainless steel joints formed by explosive welding. Mater. Des. 2005, 26, 497–507. [Google Scholar] [CrossRef]
- Findik, F. Recent developments in explosive welding. Mater. Des. 2011, 32, 1081–1093. [Google Scholar] [CrossRef]
- Mudali, U.; Ananda Rao, B.M.; Shanmugam, K.; Natarajan, R.; Raj, B. Corrosion and microstructural aspects of dissimilar joints of titanium and type 304L stainless steel. J. Nucl. Mater. 2003, 321, 40–48. [Google Scholar] [CrossRef]
- Kahraman, N.; Gülenç, B. Microstructural and mechanical properties of Cu–Ti plates bonded through explosive welding process. J. Mater. Process. Technol. 2005, 169, 67–71. [Google Scholar] [CrossRef]
- Mendes, R.; Ribeiro, J.B.; Loureiro, A. Effect of explosive characteristics on the explosive welding of stainless steel to carbon steel in cylindrical configuration. Mater. Des. 2013, 51, 182–192. [Google Scholar] [CrossRef] [Green Version]
- Loureiro, A.; Mendes, R.; Ribeiro, J.B.; Leal, R.M.; Galvão, I. Effect of explosive mixture on quality of explosive welds of copper to aluminium. Mater. Des. 2016, 95, 256–267. [Google Scholar] [CrossRef]
- Zareie Rajani, H.R.; Akbari Mousavi, S.A.A. The effect of explosive welding parameters on metallurgical and mechanical interfacial features of Inconel 625/plain carbon steel bimetal plate. Mater. Sci. Eng. A 2012, 556, 454–464. [Google Scholar] [CrossRef]
- Hoseini Athar, M.M.; Tolaminejad, B. Weldability window and the effect of interface morphology on the properties of Al/Cu/Al laminated composites fabricated by explosive welding. Mater. Des. 2015, 86, 516–525. [Google Scholar] [CrossRef]
- Ning, J.; Zhang, L.-J.; Xie, M.-X.; Yang, H.-X.; Yin, X.-Q.; Zhang, J.-X. Microstructure and property inhomogeneity investigations of bonded Zr/Ti/steel trimetallic sheet fabricated by explosive welding. J. Alloys Compd. 2017, 698, 835–851. [Google Scholar] [CrossRef]
- Gloc, M.; Wachowski, M.; Plocinski, T.; Kurzydlowski, K.J. Microstructural and microanalysis investigations of bond titanium grade1/low alloy steel st52-3N obtained by explosive welding. J. Alloys Compd. 2016, 671, 446–451. [Google Scholar] [CrossRef]
- Song, J.; Kostka, A.; Veehmayer, M.; Raabe, D. Hierarchical microstructure of explosive joints: Example of titanium to steel cladding. Mater. Sci. Eng. A 2011, 528, 2641–2647. [Google Scholar] [CrossRef]
- Rahimi, E.; Rafsanjani-Abbasi, A.; Imani, A.; Hosseinpour, S.; Davoodi, A. Correlation of surface Volta potential with galvanic corrosion initiation sites in solid-state welded Ti-Cu bimetal using AFM-SKPFM. Corros. Sci. 2018, 140, 30–39. [Google Scholar] [CrossRef]
- American Society for Testing Materials. Standard Guide for Preparation of Metallographic Specimens; ASTM E3-11(2017); ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- Sarvghad-Moghaddam, M.; Parvizi, R.; Davoodi, A.; Haddad-Sabzevar, M.; Imani, A. Establishing a correlation between interfacial microstructures and corrosion initiation sites in Al/Cu joints by SEM–EDS and AFM–SKPFM. Corros. Sci. 2014, 79, 148–158. [Google Scholar] [CrossRef]
- Bettini, E.; Eriksson, T.; Boström, M.; Leygraf, C.; Pan, J. Influence of metal carbides on dissolution behavior of biomedical CoCrMo alloy: SEM, TEM and AFM studies. Electrochim. Acta 2011, 56, 9413–9419. [Google Scholar] [CrossRef]
- Rohwerder, M.; Turcu, F. High-resolution Kelvin probe microscopy in Corros. Sci.: Scanning Kelvin probe force microscopy (SKPFM) versus classical scanning Kelvin probe (SKP). Electrochim. Acta 2007, 53, 290–299. [Google Scholar] [CrossRef]
- Schmutz, P.; Frankel, G.S. Characterization of AA2024-T3 by scanning Kelvin probe force microscopy. J. Electrochem. Soc. 1998, 145, 2285–2295. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, W.; Cao, X.; Wu, J. The effect of annealing on the interface microstructure and mechanical characteristics of AZ31B/AA6061 composite plates fabricated by explosive welding. Mater. Des. 2015, 65, 1100–1109. [Google Scholar] [CrossRef]
- Prażmowski, M.; Rozumek, D.; Paul, H. Static and fatigue tests of bimetal Zr-steel made by explosive welding. Eng. Fail. Anal. 2017, 75, 71–81. [Google Scholar] [CrossRef]
- Fronczek, D.M.; Wojewoda-Budka, J.; Chulist, R.; Sypien, A.; Korneva, A.; Szulc, Z.; Schell, N.; Zieba, P. Structural properties of Ti/Al clads manufactured by explosive welding and annealing. Mater. Des. 2016, 91, 80–89. [Google Scholar] [CrossRef]
- Manikandan, P.; Hokamoto, K.; Fujita, M.; Raghukandan, K.; Tomoshige, R. Control of energetic conditions by employing interlayer of different thickness for explosive welding of titanium/304 stainless steel. J. Mater. Process. Technol. 2008, 195, 232–240. [Google Scholar] [CrossRef]
- Prasanthi, T.N.; Sudha, C.; Saroja, S. Effect of alloying elements on interdiffusion phenomena in explosive clads of 304LSS/Ti–5Ta–2Nb alloy. J. Mater. Sci. 2016, 51, 5290–5304. [Google Scholar] [CrossRef]
- Murray, J.L. The Cu−Ti (Copper-Titanium) system. Bull. Alloy Phase Diagrams 1983, 4, 81–95. [Google Scholar] [CrossRef]
- Elrefaey, A.; Tillmann, W. Solid state diffusion bonding of titanium to steel using a copper base alloy as interlayer. J. Mater. Process. Technol. 2009, 209, 2746–2752. [Google Scholar] [CrossRef]
- Saboktakin, M.; Razavi, G.R.; Monajati, H. The Investigate Metallurgical Properties of Roll BondingTitanium Clad Steel. IJAPM 2011, 177–180. [Google Scholar] [CrossRef]
- Blasco-Tamarit, E.; Igual-Muñoz, A.; García Antón, J.; García-García, D. Corrosion behaviour and galvanic coupling of titanium and welded titanium in LiBr solutions. Corros. Sci. 2007, 49, 1000–1026. [Google Scholar] [CrossRef]
- Jiang, Z.; Dai, X.; Middleton, H. Investigation on passivity of titanium under steady-state conditions in acidic solutions. Mater. Chem. Phys. 2011, 126, 859–865. [Google Scholar] [CrossRef]
- Pouilleau, J.; Devilliers, D.; Garrido, F.; Durand-Vidal, S.; Mahé, E. Structure and composition of passive titanium oxide films. Mater. Sci. Eng. B 1997, 47, 235–243. [Google Scholar] [CrossRef]
- Du, X.-Q.; Yang, Q.-S.; Chen, Y.; Yang, Y.; Zhang, Z. Galvanic corrosion behavior of copper/titanium galvanic couple in artificial seawater. T. Nonferr. Metal. Soc. 2014, 24, 570–581. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Z.; Gong, Y.; Gao, F.; Luo, Z.; Zhang, S.; Li, H. Water soluble corrosion inhibitors for copper in 3.5 wt% sodium chloride solution. Corros. Sci. 2017, 123, 339–350. [Google Scholar] [CrossRef]
- Davoodi, A.; Esfahani, Z.; Sarvghad, M. Microstructure and corrosion characterization of the interfacial region in dissimilar friction stir welded AA5083 to AA7023. Corros. Sci. 2016, 107, 133–144. [Google Scholar] [CrossRef]
- Alves, A.C.; Wenger, F.; Ponthiaux, P.; Celis, J.-P.; Pinto, A.M.; Rocha, L.A.; Fernandes, J. Corrosion mechanisms in titanium oxide-based films produced by anodic treatment. Electrochim. Acta 2017, 234, 16–27. [Google Scholar] [CrossRef]
- McCafferty, E. Introduction to Corrosion Science, 1st ed.; Springer: New York, NY, USA, 2010; ISBN 978-1-4419-0455-3. [Google Scholar]
- Qu, Q.; Wang, L.; Chen, Y.; Li, L.; He, Y.; Ding, Z. Corrosion Behavior of Titanium in Artificial Saliva by Lactic Acid. Materials 2014, 7, 5528–5542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.; Zhang, D.; Qiu, R.; Wan, Y.; Wu, J. Green approach to fabrication of a super-hydrophobic film on copper and the consequent corrosion resistance. Corros. Sci. 2014, 80, 366–373. [Google Scholar] [CrossRef]
- Bi, H.; Burstein, G.T.; Rodriguez, B.B.; Kawaley, G. Some aspects of the role of inhibitors in the corrosion of copper in tap water as observed by cyclic voltammetry. Corros. Sci. 2016, 102, 510–516. [Google Scholar] [CrossRef]
- Jiang, Z.; Dai, X.; Norby, T.; Middleton, H. Investigation of pitting resistance of titanium based on a modified point defect model. Corros. Sci. 2011, 53, 815–821. [Google Scholar] [CrossRef]
- Mckay, P.; Mitton, D.B. An Electrochemical Investigation of Localized Corrosion on Titanium in Chloride Environments. Corrosion 1985, 41, 52–62. [Google Scholar] [CrossRef]
- Donatus, U.; Thompson, G.E.; Zhou, X. Effect of Near-Ambient Temperature Changes on the Galvanic Corrosion of an AA2024-T3 and Mild Steel Couple. J. Electrochem. Soc. 2015, 162, C42–C46. [Google Scholar] [CrossRef]
- Yin, Z.F.; Yan, M.L.; Bai, Z.Q.; Zhao, W.Z.; Zhou, W.J. Galvanic corrosion associated with SM 80SS steel and Ni-based alloy G3 couples in NaCl solution. Electrochim. Acta 2008, 53, 6285–6292. [Google Scholar] [CrossRef]
- Lacroix, L.; Ressier, L.; Blanc, C.; Mankowski, G. Combination of AFM, SKPFM, and SIMS to study the corrosion behavior of S-phase particles in AA2024-T351. J. Electrochem. Soc. 2008, 155, C131–C137. [Google Scholar] [CrossRef] [Green Version]
- Jönsson, M.; Thierry, D.; LeBozec, N. The influence of microstructure on the corrosion behaviour of AZ91D studied by scanning Kelvin probe force microscopy and scanning Kelvin probe. Corros. Sci. 2006, 48, 1193–1208. [Google Scholar] [CrossRef]
- Iannuzzi, M.; Vasanth, K.L.; Frankel, G.S. Unusual Correlation between SKPFM and Corrosion of Nickel Aluminum Bronzes. J. Electrochem. Soc. 2017, 164, C488–C497. [Google Scholar] [CrossRef] [Green Version]
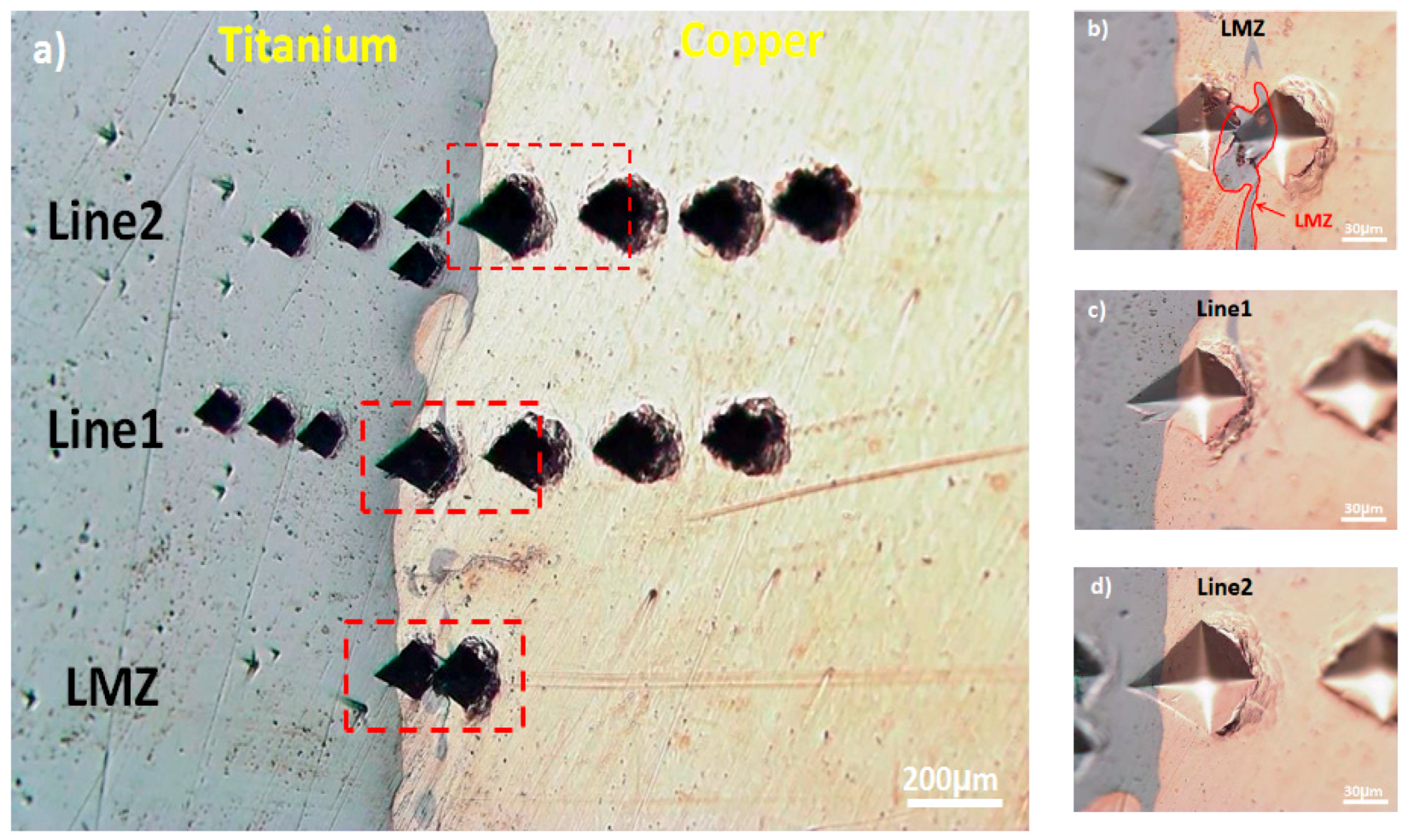


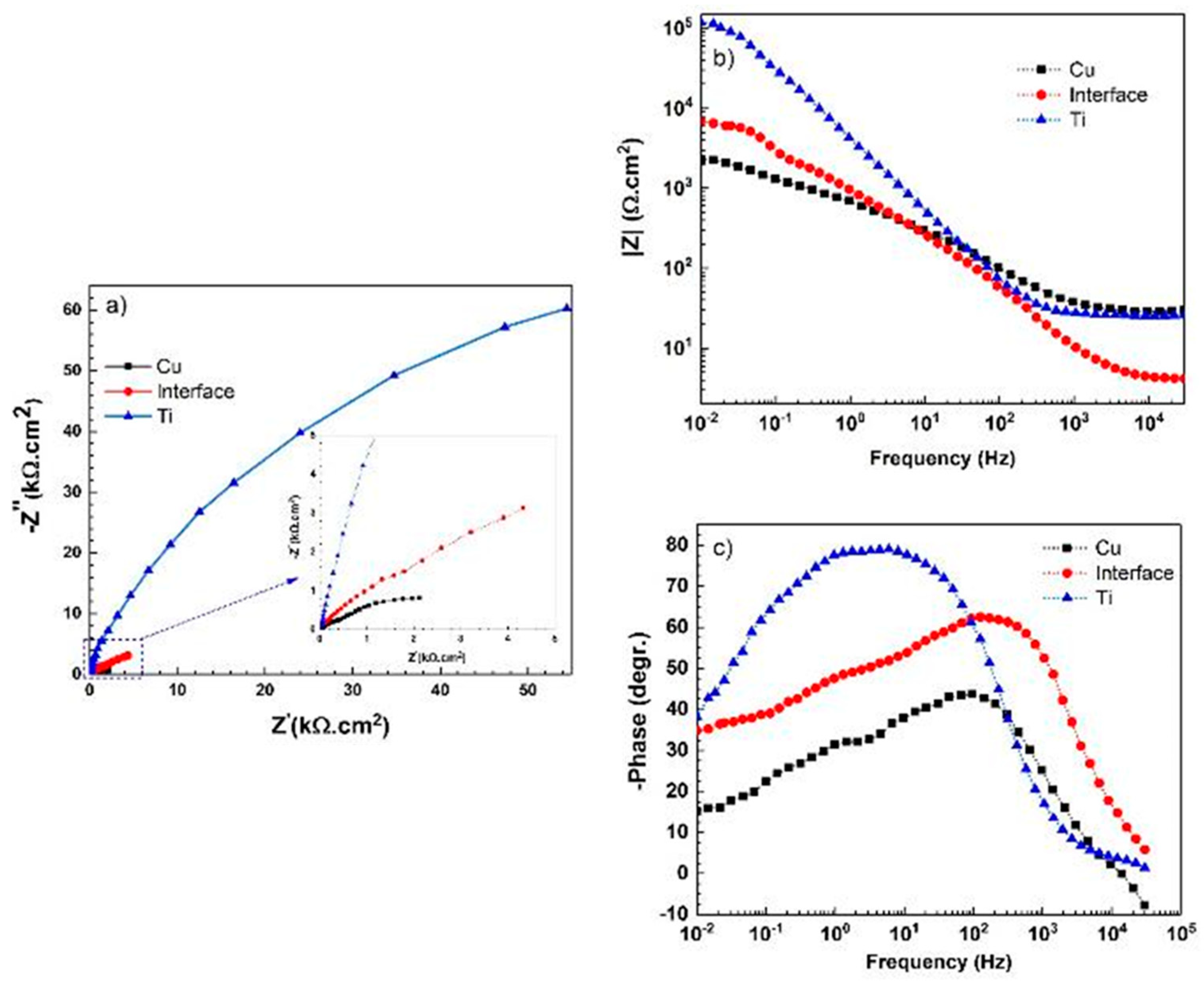
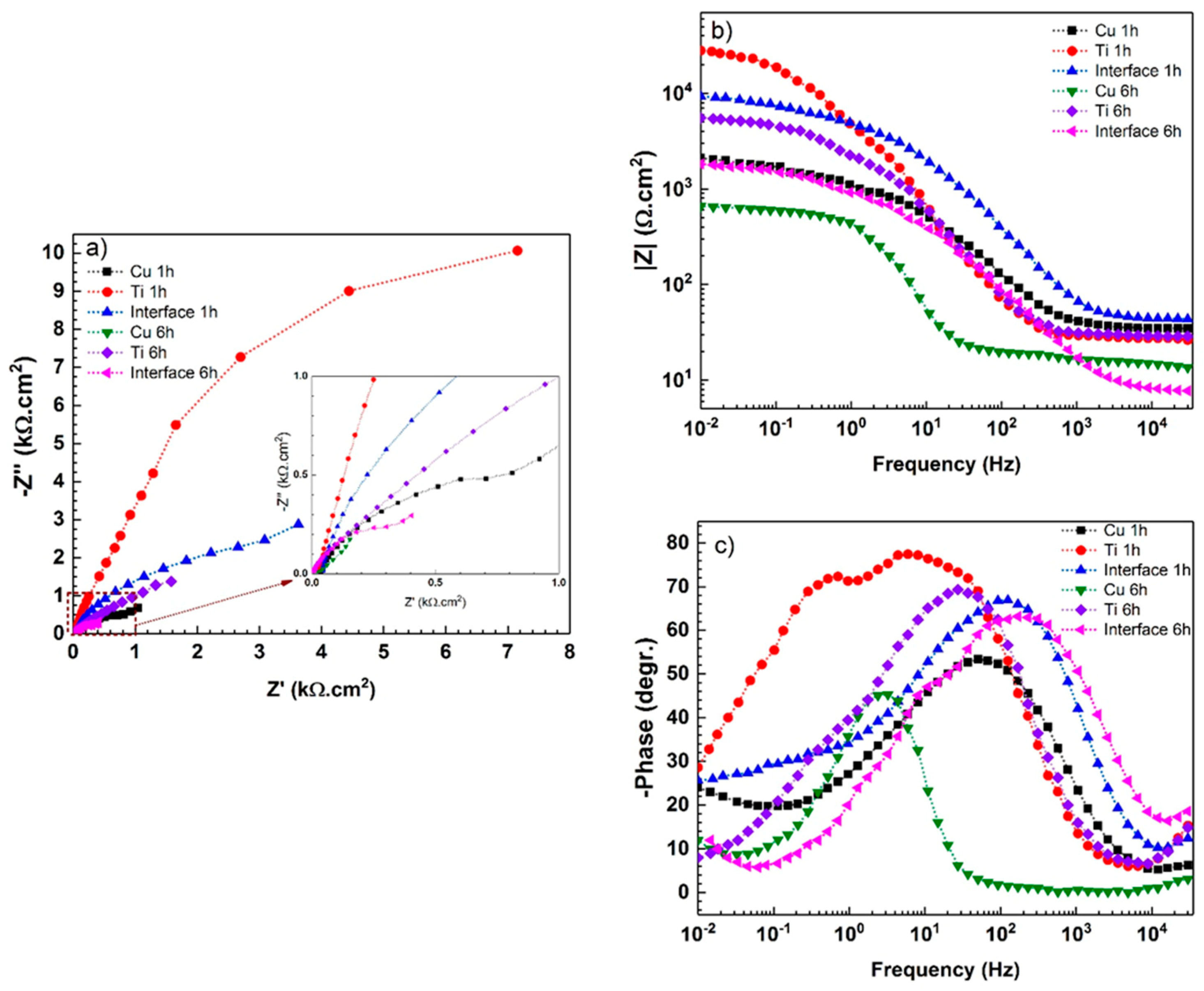

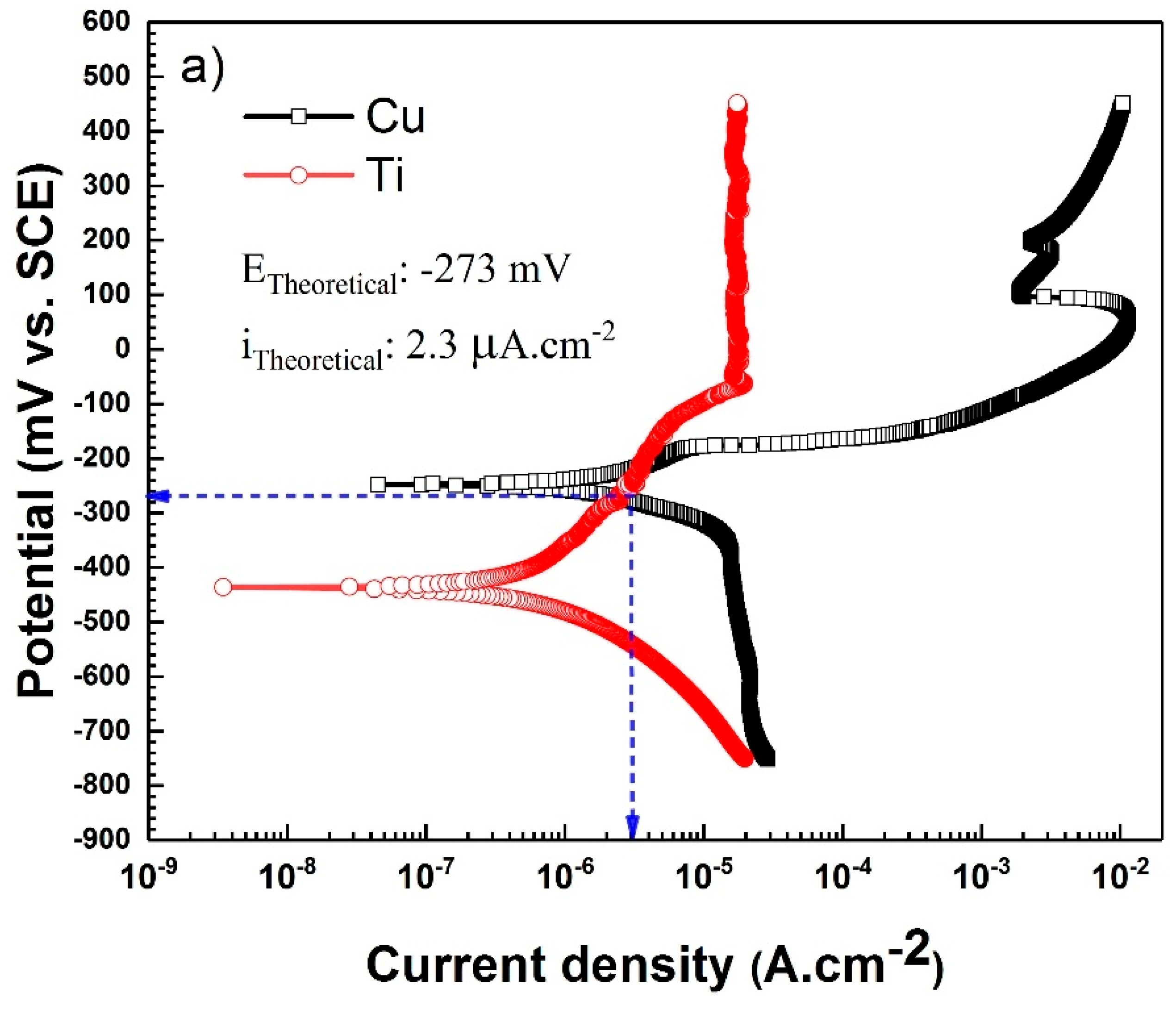


| Immersion Time | Surface | Rs (Ω·cm2) | Rf (kΩ·cm2) | CPEf (µF·S−1·cm−2) | n1 | Rct (kΩ·cm2) | CPEct (µF·S−1·cm−2) | W (Ω·s−0.5) | n2 |
|---|---|---|---|---|---|---|---|---|---|
| - | Titanium | 25.2 ± 1 | 123.1 ± 2 | 45.3 ± 0.2 | 0.89 ± 00.5 | - | - | - | - |
| Copper | 27.4 ± 2 | 0.51 ± 0.2 | 109.7 ± 1 | 0.72 ± 0.04 | 2.5 ± 0.6 | 240.8 ± 18 | - | 0.74 ± 0.01 | |
| Interface | 5.1 ± 0.5 | 0.55 ± 0.3 | 88.6 ± 0.3 | 0.78 ± 0.02 | 9.2 ± 0.4 | 291.6 ± 23 | - | 0.61 ± 0.04 | |
| 1 h | Titanium | 27.8 ± 3 | 4.7 ± 0.4 | 26.2 ± 3 | 0.97 ± 0.02 | 25.2 ± 2 | 12.9 ± 2 | - | 0.92 ± 0.03 |
| Copper | 34.1 ± 2 | 0.53 ± 0.6 | 2.8 ± 0.7 | 0.89 ± 0.03 | 2.2 ± 0.4 | 188.5 ± 35 | 352 ± 3 | 0.51 ± 0.02 | |
| Interface | 42.1 ± 4 | 2.4 ± 0.2 | 7.5 ± 0.6 | 0.92 ± 0.05 | 9.1 ± 0.2 | 64.2 ± 2 | 4029 ± 30 | 0.52 ± 0.02 | |
| 6 h | Titanium | 28.3 ± 2 | 0.3 ± 0.1 | 32.5 ± 3 | 0.84 ± 0.04 | 5.2 ± 1 | 30.6 ± 2 | - | 0.89 ± 0.02 |
| Copper | 13.4 ± 1 | 0.12 ± 0.1 | 75.8 ± 2 | 0.79 ± 0.03 | 0.7 ± 0.2 | 237.4 ± 50 | 150 ± 15 | 0.43 ± 0.01 | |
| Interface | 7.7 ± 2 | 1.2 ± 0.4 | 4.5 ± 2 | 0.92 ± 0.01 | 1.5 ± 0.3 | 8.4 ± 1 | 2626 ± 50 | 0.47 ± 0.05 |
| Immersion Time | Surface | icorr (µA·cm−2) | Ecorr (mV vs. SCE) | ba (mV/decade) | bc (mV/decade) |
|---|---|---|---|---|---|
| 0 | Titanium | 0.74 ± 0.3 | −435.8 ± 2 | 297.1 ± 1 | 174.4 ± 1 |
| Copper | 4.2 ± 0.2 | −250.4 ± 1 | 56.3 ± 2 | 185.5 ± 3 | |
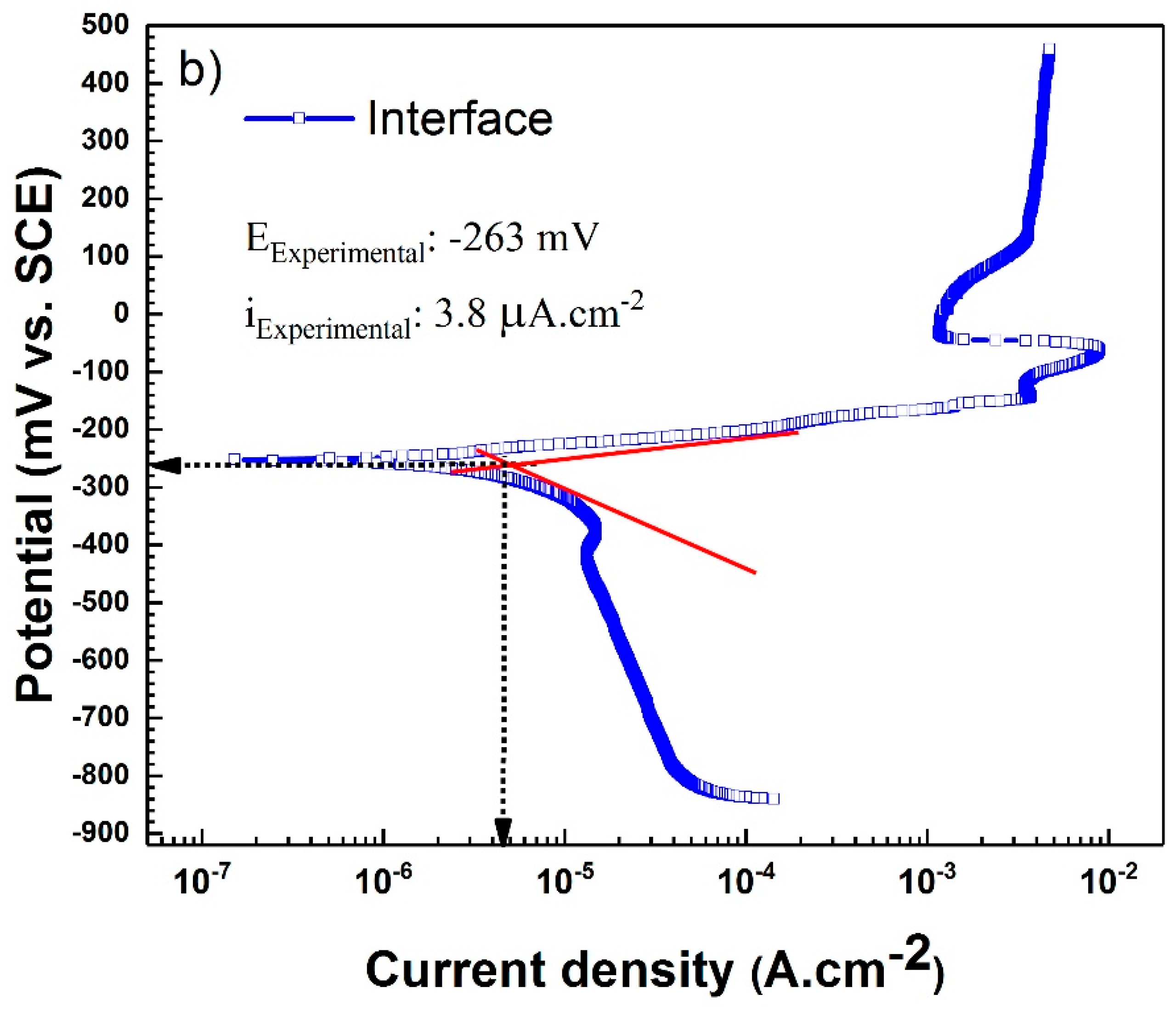
| Interface | 3.8 ± 0.4 | −263.5 ± 1 | 90.2 ± 1 | 162.4 ± 2 | |
| 1 h | Titanium | 0.87 ± 0.4 | −303.6 ± 2 | 301.8 ± 1 | 171.8 ± 2 |
| Copper | 5.9 ± 1 | −283.4 ± 2 | 214.2 ± 1 | 148.3 ± 1 | |
| Interface | 4.1 ± 1 | −273.3 ± 2 | 150.8 ± 2 | 274.5 ± 2 | |
| 6 h | Titanium | 1.4 ± 0.5 | −302.3 ± 2 | 197.2 ± 2 | 68.1 ± 2 |
| Copper | 8.5 ± 0.3 | −351.5 ± 2 | 104.8 ± 2 | 219.5 ± 1 | |
| Interface | 6.5 ± 0.5 | −275.3 ± 2 | 138.7 ± 2 | 201.7 ± 2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahimi, E.; Rafsanjani-Abbasi, A.; Imani, A.; Hosseinpour, S.; Davoodi, A. Insights into Galvanic Corrosion Behavior of Ti-Cu Dissimilar Joint: Effect of Microstructure and Volta Potential. Materials 2018, 11, 1820. https://doi.org/10.3390/ma11101820
Rahimi E, Rafsanjani-Abbasi A, Imani A, Hosseinpour S, Davoodi A. Insights into Galvanic Corrosion Behavior of Ti-Cu Dissimilar Joint: Effect of Microstructure and Volta Potential. Materials. 2018; 11(10):1820. https://doi.org/10.3390/ma11101820
Chicago/Turabian StyleRahimi, Ehsan, Ali Rafsanjani-Abbasi, Amin Imani, Saman Hosseinpour, and Ali Davoodi. 2018. "Insights into Galvanic Corrosion Behavior of Ti-Cu Dissimilar Joint: Effect of Microstructure and Volta Potential" Materials 11, no. 10: 1820. https://doi.org/10.3390/ma11101820
APA StyleRahimi, E., Rafsanjani-Abbasi, A., Imani, A., Hosseinpour, S., & Davoodi, A. (2018). Insights into Galvanic Corrosion Behavior of Ti-Cu Dissimilar Joint: Effect of Microstructure and Volta Potential. Materials, 11(10), 1820. https://doi.org/10.3390/ma11101820







