Effects of Different Melting Technologies on the Purity of Superalloy GH4738
Abstract
:1. Introduction
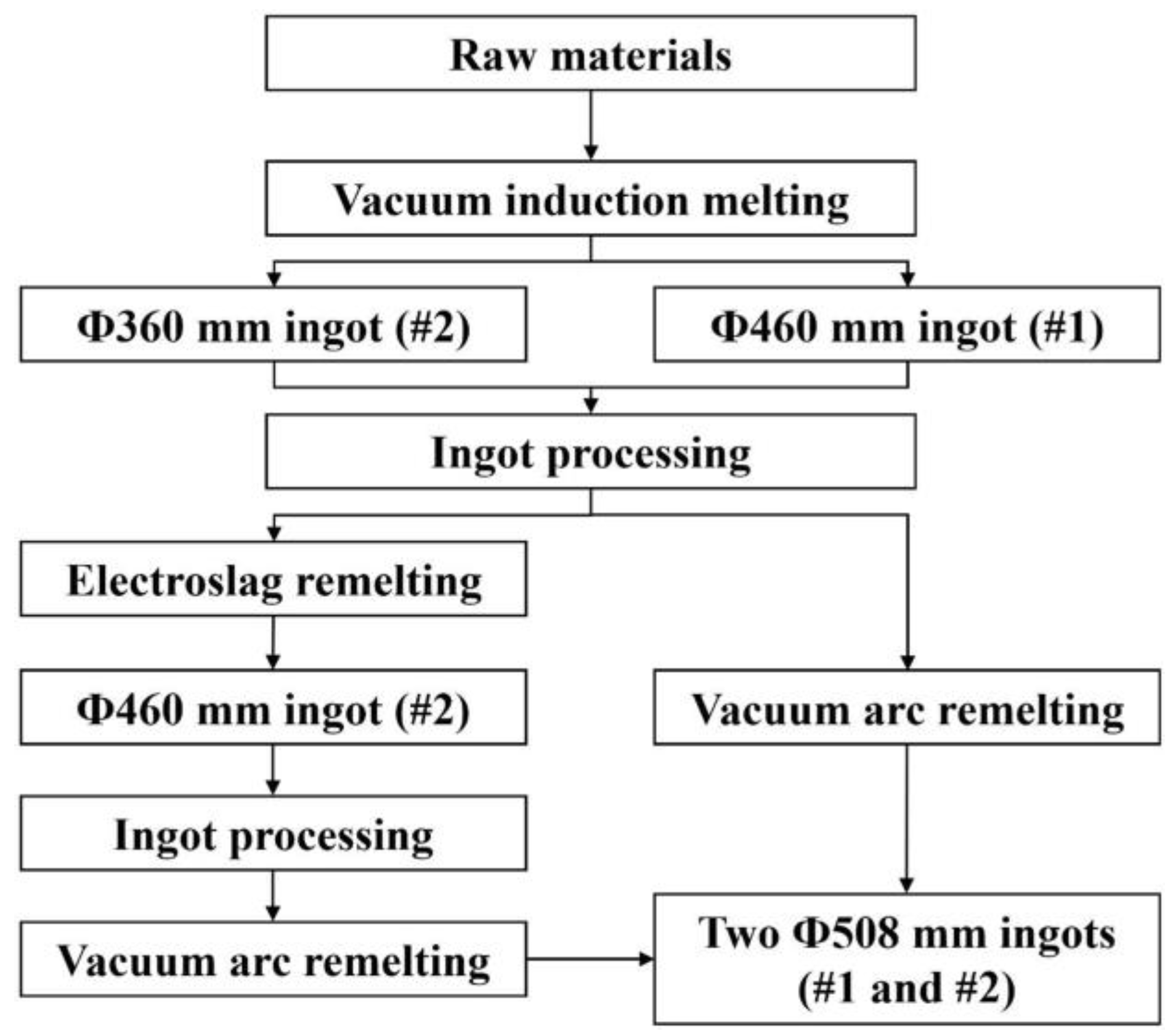
2. Experimental
2.1. Melting
2.2. Preparation and Testing
3. Results and Discussions
3.1. Chemical-Composition Analysis
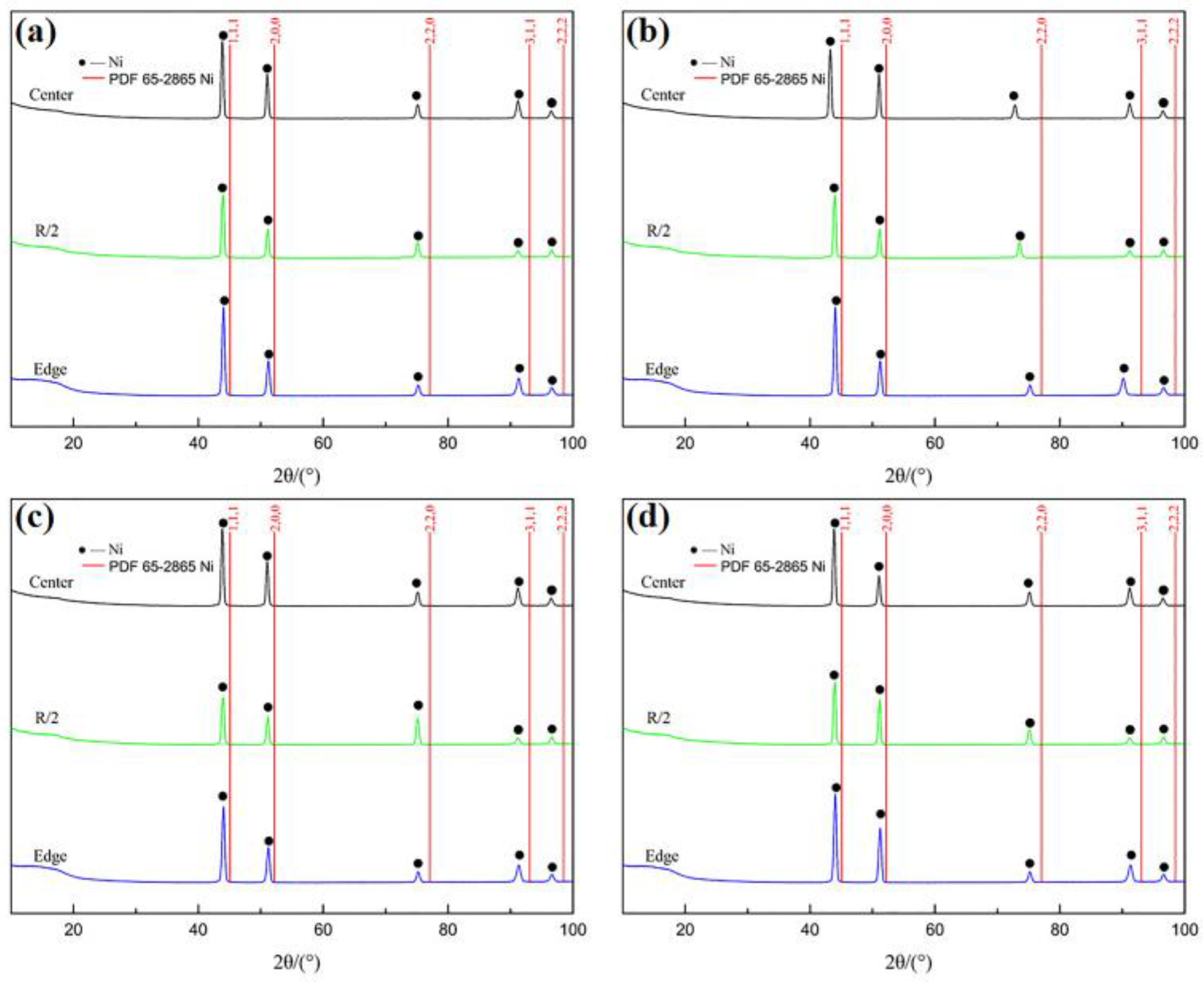
3.2. XRD Analyses
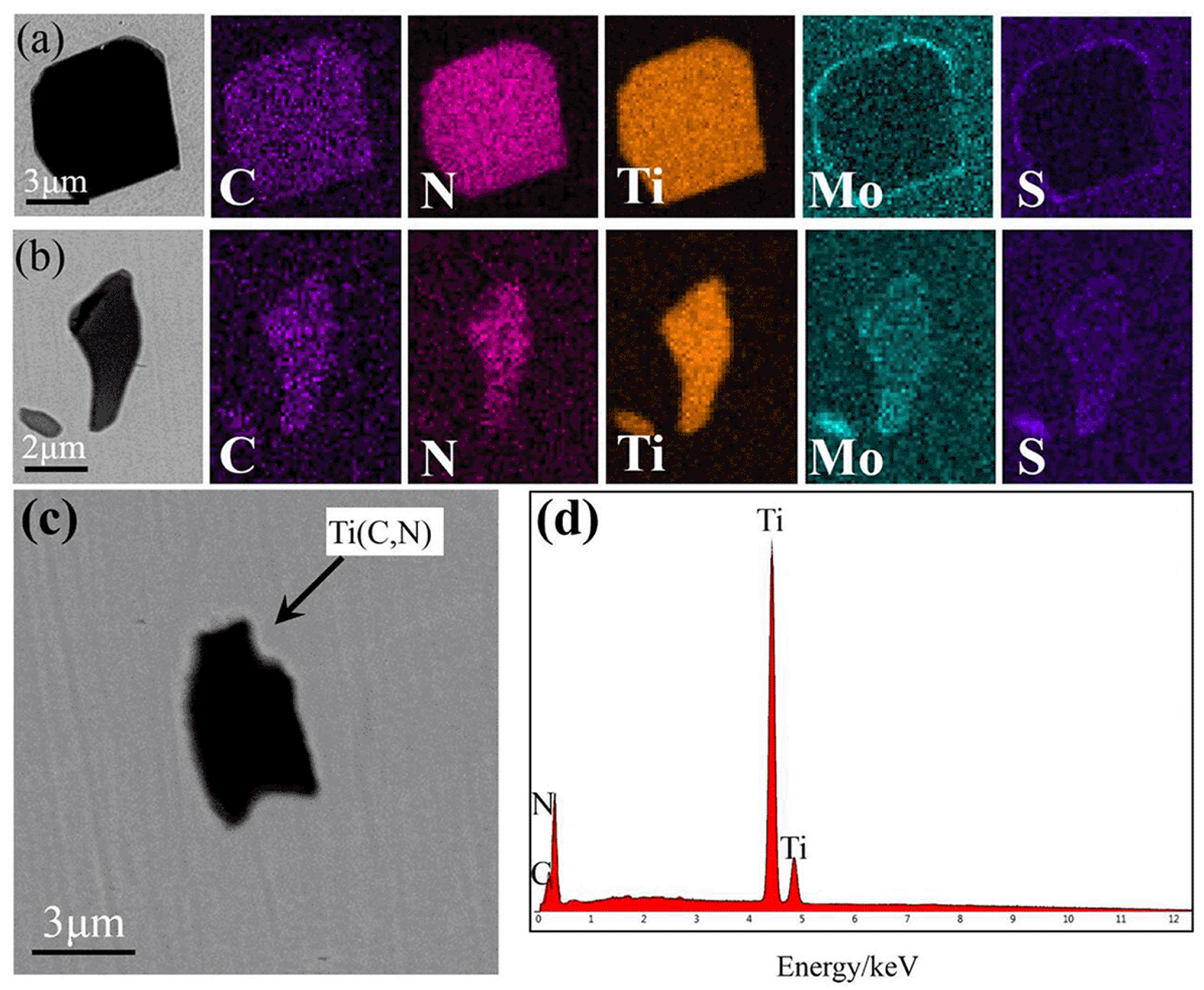
3.3. Inclusion Characteristics and Their Formation Mechanism
3.4. Analysis of Mechanical Properties
3.5. Analysis of White-Spot Formation Probability
4. Conclusions
- Double melting reduced the levels of N, S, and O in the GH4738 superalloy to 0.0057 wt.%, 0.002 wt.%, and 0.0012 wt.%, respectively, while triple melting reduced the contents of these elements to 0.0056 wt.%, 0.0007 wt.%, and 0.0008 wt.%, respectively. Hence, triple melting maintains lower levels of these harmful elements in the superalloy.
- The superalloy samples refined by both techniques exhibited only Ni diffraction peaks in their XRD spectra, which were shifted to lower values compared with the standard peak positions (PDF 65-2865 Ni).
- Four primary types of inclusion were present in the double-melted GH4738 superalloy samples, the number of inclusions was 5763 and the average inclusion size was 3.1 μm. Two dominant types of inclusion were present in the triple-melted superalloy samples; the number of inclusions was 3412 and the average inclusion size was 2.5 μm. Hence, triple melting generates fewer types and amounts of inclusion and the average inclusion size is also smaller.
- The average tensile strengths of the double-melted GH4738 superalloy samples were 1051 and 902 MPa at 25 °C and 535 °C, respectively, and its average cycle number was 1223. The average tensile strengths of the triple-melted GH4738 superalloy samples were 1164 and 1053 MPa at 25 °C and 535 °C, respectively, and its average cycle number was 1417. Hence, triple melting produces higher mechanical properties in the superalloy.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wen, Z.X.; Liang, J.W.; Liu, C.Y.; Pei, H.Q.; Wen, S.F.; Yue, Z.F. Prediction method for creep life of thin-wall specimen with film cooling holes in Ni-based single-crystal superalloy. Int. J. Mech. Sci. 2018, 141, 276–289. [Google Scholar] [CrossRef]
- Furuya, Y.; Matsuoka, S.; Kimura, T.; Hayaishi, M. Effects of inclusion and ODA sizes on gigacycle fatigue properties of high-strength steels. Tetsu Hagane 2005, 91, 630–638. [Google Scholar] [CrossRef]
- Kirka, M.M.; Brindley, K.A.; Neu, R.W.; Antolovich, S.D.; Shinde, S.R.; Gravett, P.W. Influence of coarsened and rafted microstructures on the thermomechanical fatigue of a Ni-base superalloy. Int. J. Fatigue 2015, 81, 191–201. [Google Scholar] [CrossRef] [Green Version]
- Mao, J.; Chang, K.M.; Yang, W.H.; Ray, K.; Vaze, S.P.; Ferrer, D.U. Cooling precipitation and strengthening study in powder metallurgy superalloy U720LI. Metall. Mater. Trans. A 2001, 32, 2441–2452. [Google Scholar]
- Carter, J.L.W.; Kuper, M.W.; Uchic, M.D.; Mills, M.J. Characterization of localized deformation near grain boundaries of superalloy René-104 at elevated temperature. Mater. Sci. Eng. A 2014, 605, 127–136. [Google Scholar] [CrossRef]
- Radis, R.; Schaffer, M.; Albu, M.; Kothleitner, G.; Pölt, P.; Kozeschnik, E. Multimodal size distributions of γ′ precipitates during continuous cooling of UDIMET 720 Li. Acta Mater. 2009, 57, 5739–5747. [Google Scholar] [CrossRef]
- Williams, J.C.; Starke, E.A., Jr. Progress in structural materials for aerospace systems. Acta Mater. 2003, 51, 5775–5799. [Google Scholar] [CrossRef]
- Zhang, B.J.; Zhao, G.P.; Zhang, W.Y.; Huang, S.; Chen, S.F. Investigation of high performance disc alloy GH4065 and associated advanced processing techniques. Acta Metall. Sin. 2015, 51, 1227–1234. [Google Scholar]
- Hegde, S.R.; Kearsey, R.M.; Beddoes, J.C. Designing homogenization-solution heat treatments for single crystal supperalloys. Mater. Sci. Eng. A 2010, 527, 5528–5538. [Google Scholar] [CrossRef]
- Schmiedt, A.B.; Dickert, H.H.; Bleck, W.; Kamps, U. Evaluation of maximum non-metallic inclusion sizes in engineering steels by fitting a generalized extreme value distribution based on vectors of largest observations. Acta Mater. 2015, 95, 1–9. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Zeng, L.; Hao, W.; Wang, J. Precipitation behavior of TiN in the GH4700 nickel-base superalloy. Rare Met. Mater. Eng. 2013, 42, 344–348. [Google Scholar]
- Bricknell, R.H.; Mulford, R.A.; Woodford, D.A. The role of sulfur in the air embrittlement of nickel and its alloys. Metall. Mater. Trans. A 1982, 13, 1223–1232. [Google Scholar] [CrossRef]
- Zhou, L.Z.; Guo, J.T. Effects of sulphur content on microstructure and mechanical properties of alloy K4169. Acta Metall. Sin. 1995, 31, 261–265. [Google Scholar]
- Song, H.W.; Guo, S.R.; Lu, D.Z.; Hu, Z. Influences of sulfur doping on property of superalloy IN718. Acta Metall. Sin. 1999, 35, 281–284. [Google Scholar]
- Wang, X.H.; Jiang, M.; Chen, B.; Li, H.B. Study on formation of non-metallic inclusions with lower melting temperatures in extra low oxygen special steels. Sci. China Technol. Sci. 2012, 55, 1863–1872. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, B.; Xu, D.K.; Han, E.H.; Ke, W. Effects of inclusion and loading direction on the fatigue behavior of hot rolled low carbon steel. Int. J. Fatigue 2010, 32, 1116–1125. [Google Scholar] [CrossRef]
- Furuya, Y.; Hirukawa, H.; Kimura, T.; Hayaishi, M. Gigacycle fatigue properties of high-strength steels according to inclusion and ODA sizes. Metall. Mater. Trans. A 2007, 38, 1722–1730. [Google Scholar] [CrossRef]
- Degawa, T.; Ototani, T. Refining of high purity Ni-base superalloy using calcia refractory. Tetsu Hagane 1987, 73, 1691–1697. [Google Scholar] [CrossRef]
- Wang, H.; Zhong, Y.B.; Li, Q.; Fang, Y.P.; Ren, W.L.; Lei, Z.S.; Ren, Z.M. Effect of current frequency on droplet evolution during magnetic-field-controlled electroslag remelting process via visualization method. Metall. Mater. Trans. B 2017, 48, 655–663. [Google Scholar] [CrossRef]
- Shevchenko, D.M.; Ward, R.M. Liquid metal pool behavior during the vacuum arc remelting of INCONEL 718. Metall. Mater. Trans. B 2009, 40, 263–270. [Google Scholar] [CrossRef]
- Hou, D.; Jiang, Z.H.; Dong, Y.W.; Li, Y.; Gong, W.; Liu, F.B. Mass transfer model of desulfurization in the electroslag remelting process. Metall. Mater. Trans. B 2017, 48, 1885–1897. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, L.F.; Wang, X.H.; Ren, Y.; Liu, X.F.; Shan, Q.L. Characteristics of inclusions in low carbon Al-killed steel during ladle furnace refining and calcium treatment. ISIJ Int. 2013, 53, 1401–1410. [Google Scholar] [CrossRef]
- Ahmadi, S.; Arabi, H.; Shokuhfar, A.; Rezaei, A. Evaluation of the electroslag remelting process in medical grade of 316LC stainless steel. J. Mater. Sci. Technol. 2009, 25, 592–596. [Google Scholar]
- Kanungo, S.B.; Parida, K.M.; Sant, B.R. Studies on MnO2—II. Relationship between physicochemical properties and electrochemical activity of some synthetic MnO2 of different crystallographic forms. Electrochim. Acta 1981, 26, 1147–1156. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Yang, S.F.; Li, J.S.; Guo, H.; Zheng, H.B. Effects of different hot working techniques on inclusions in GH4738 superalloy produced by VIM and VAR. Materials 2018, 11, 1024. [Google Scholar] [CrossRef] [PubMed]
- Toro, A.; Zhou, F.; Wu, M.H.; Geertruyden, W.V.; Misiolek, W.Z. Characterization of non-metallic inclusions in superelastic NiTi tubes. J. Mater. Eng. Perform. 2009, 18, 448–458. [Google Scholar] [CrossRef]
- Chen, F.W.; Huang, X.B.; Wang, Y.; Zhang, Y.; Hu, Z.Q. Investigation on foam ceramic filter to remove inclusions in revert superalloy. Mater. Lett. 1998, 34, 372–376. [Google Scholar] [CrossRef]
- Shen, J.; Xia, T.D.; Wang, X.J.; Feng, X.C. Characterization and analysis of non-metallic inclusions in Ni42-Fe expansive alloy. Trans. Nonferrous Met. Soc. China 2007, 17, S1165–S1171. [Google Scholar]
- Zhang, H.Y.; Zhang, S.H.; Cheng, M. Effect of δ phase on the tensile deformation behavior of GH4169 alloy at high temperature. Acta Metall. Sin. 2013, 49, 483–488. [Google Scholar] [CrossRef]
- Liu, X.L.; Chen, X.; Hou, X.Q.; Tao, C.H. Damage and fracture speciality of FGH95 powder superalloy. Rare Met. Mater. Eng. 2009, 38, 1179–1183. [Google Scholar]
- Jackman, L.A.; Manurer, G.E.; Widge, S. New knowledge about ‘white spots’ in superalloys. Adv. Mater. Process. 1993, 143, 18–25. [Google Scholar]
- Grignard, J.F.; Soller, A.; Jourdan, J.; Bellot, J.P.; Jardy, A. On the formation of white-spot defects in a superalloy VAR ingot. Adv. Eng. Mater. 2011, 7, 563–569. [Google Scholar] [CrossRef]
- Wang, L.; Fan, X.; Luo, L.; Xu, J.; Chen, R.; Yi, H.; Zhou, G.; Wang, N.; Zhang, J.; Su, Y. Influence of traveling magnetic field on solidification defects and mechanical properties of ZL205A alloy sheet casts. Rare Met. Mater. Eng. 2016, 45, 3177–3180. [Google Scholar]
- Campbell, J. Melting, remelting, and casting for clean steel. Steel Res. Int. 2017, 88, 1600093. [Google Scholar] [CrossRef]





| Cr | C | Co | Mo | Al | Ti | S | Ni |
|---|---|---|---|---|---|---|---|
| 18.0–21.0 | 0.03–0.1 | 12.0–15.0 | 3.5–5.0 | 1.2–1.6 | 2.75–3.25 | <0.015 | Balance |
| Melting Processes | Al | Ti | C | N | S | O |
|---|---|---|---|---|---|---|
| VIM (#1) | 1.47 | 2.97 | 0.07 | 0.0067 | 0.0021 | 0.0017 |
| VAR (#1) | 1.45 | 2.96 | 0.07 | 0.0057 | 0.0020 | 0.0012 |
| VIM (#2) | 1.46 | 2.96 | 0.071 | 0.0066 | 0.0021 | 0.0017 |
| ESR (#2) | 1.38 | 2.94 | 0.071 | 0.0065 | 0.0007 | 0.0008 |
| VAR (#2) | 1.37 | 2.94 | 0.07 | 0.0056 | 0.0007 | 0.0008 |
| Standard requirement | 1.2–1.6 | 2.75–3.25 | 0.03–0.1 | ≤0.01 | ≤0.015 | ≤0.005 |
| Inclusions | VIM + VAR(#1) | VIM + ESR + VAR(#2) |
|---|---|---|
| Ti(C, N)-MoS | 4299 | 2873 |
| Ti(C, N) | 524 | 458 |
| Ce-MoS | 409 | —— |
| SiC | 398 | —— |
| Others | 133 | 81 |
| Total Inclusions | 5763 | 3412 |
| Average Inclusion Size (μm) | 3.1 | 2.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Yang, S.; Qu, J.; Li, J.; Dong, A.; Gu, Y. Effects of Different Melting Technologies on the Purity of Superalloy GH4738. Materials 2018, 11, 1838. https://doi.org/10.3390/ma11101838
Chen Z, Yang S, Qu J, Li J, Dong A, Gu Y. Effects of Different Melting Technologies on the Purity of Superalloy GH4738. Materials. 2018; 11(10):1838. https://doi.org/10.3390/ma11101838
Chicago/Turabian StyleChen, Zhengyang, Shufeng Yang, Jinglong Qu, Jingshe Li, Anping Dong, and Yu Gu. 2018. "Effects of Different Melting Technologies on the Purity of Superalloy GH4738" Materials 11, no. 10: 1838. https://doi.org/10.3390/ma11101838




