Effects of PEG1000 and Sol Concentration on the Structural and Optical Properties of Sol–Gel ZnO Porous Thin Films
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials
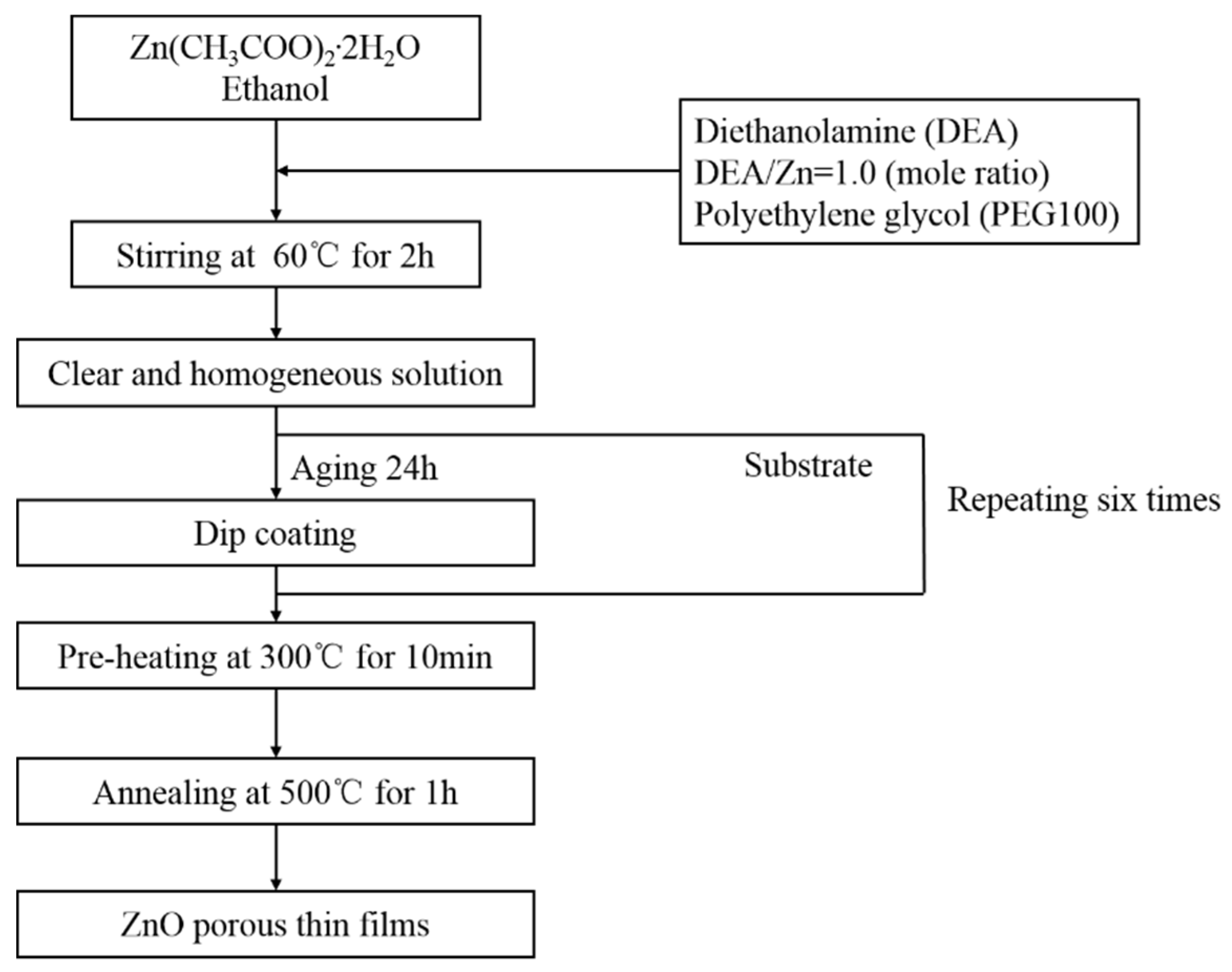
2.2. Sol Preparation and Deposition of Films
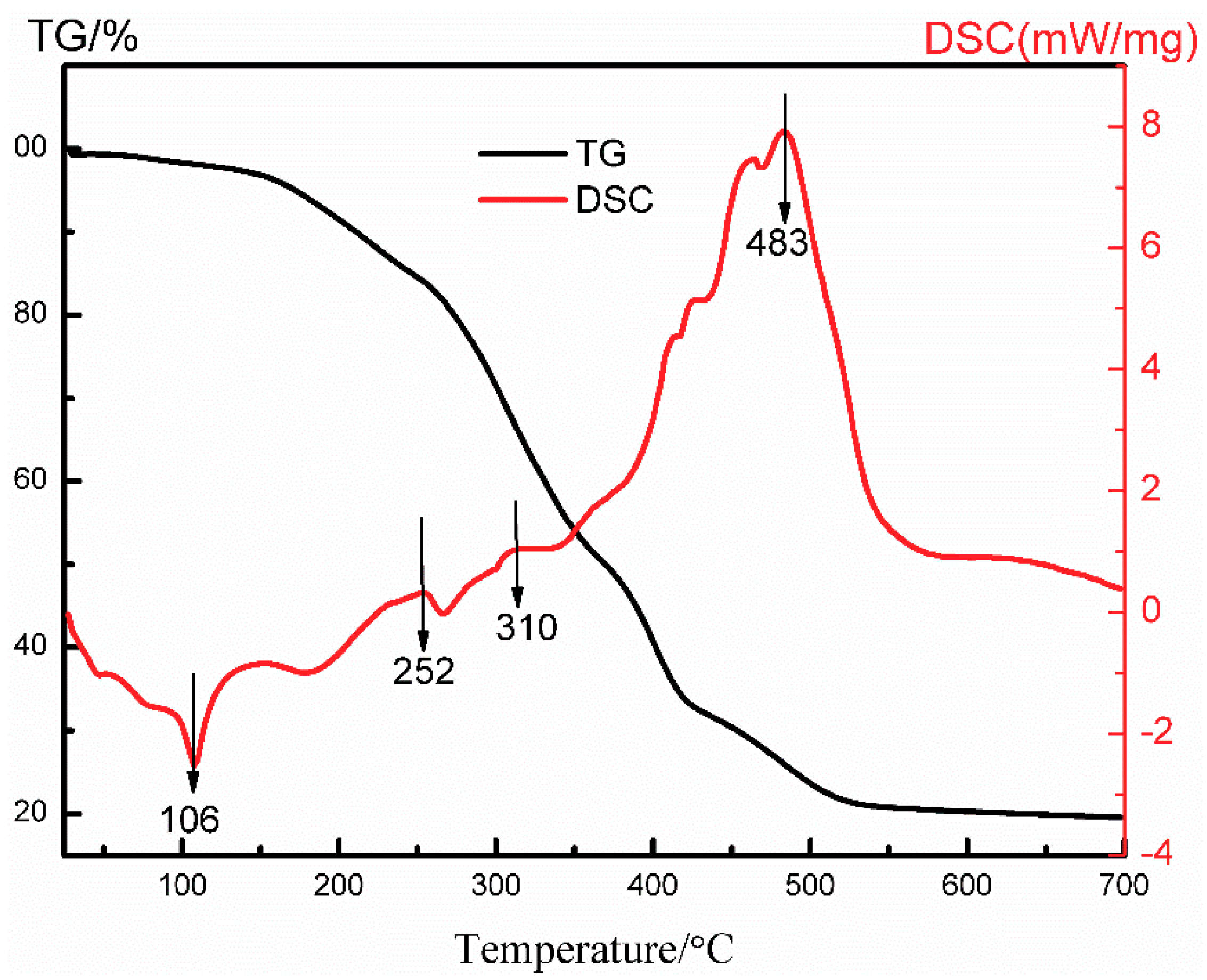
2.3. Characterization
3. Results and Discussion
3.1. Mechanism of Pore Formation
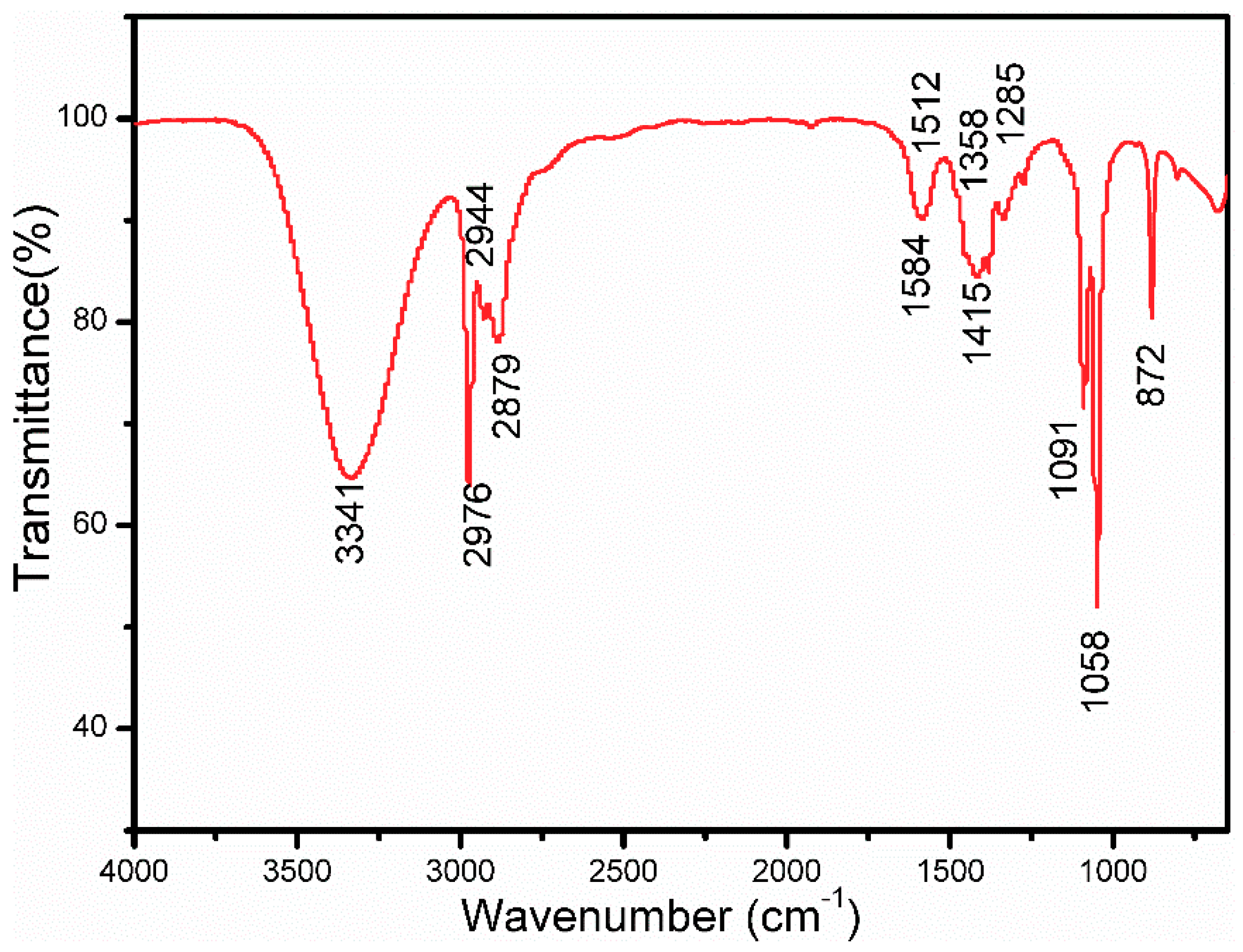
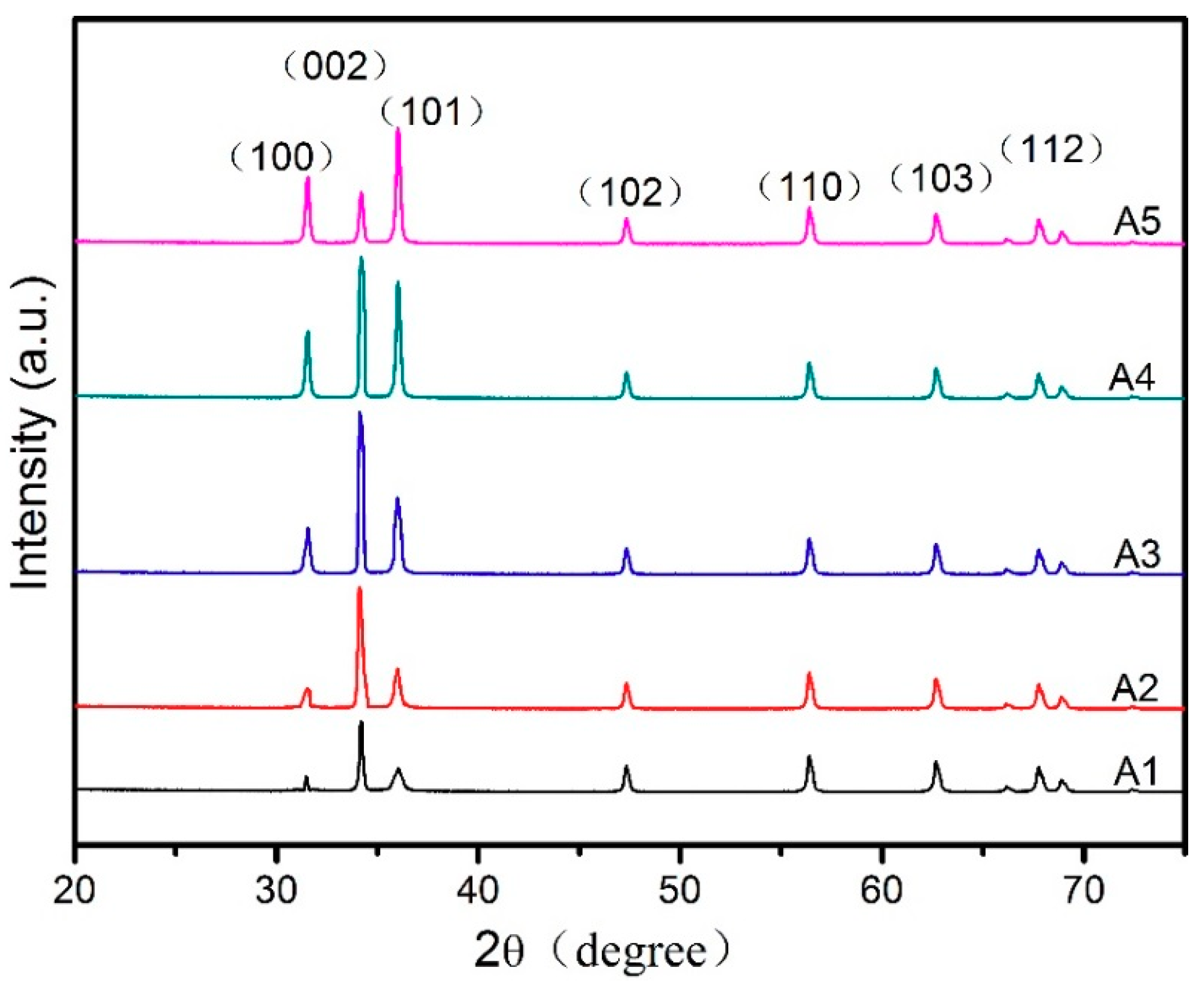
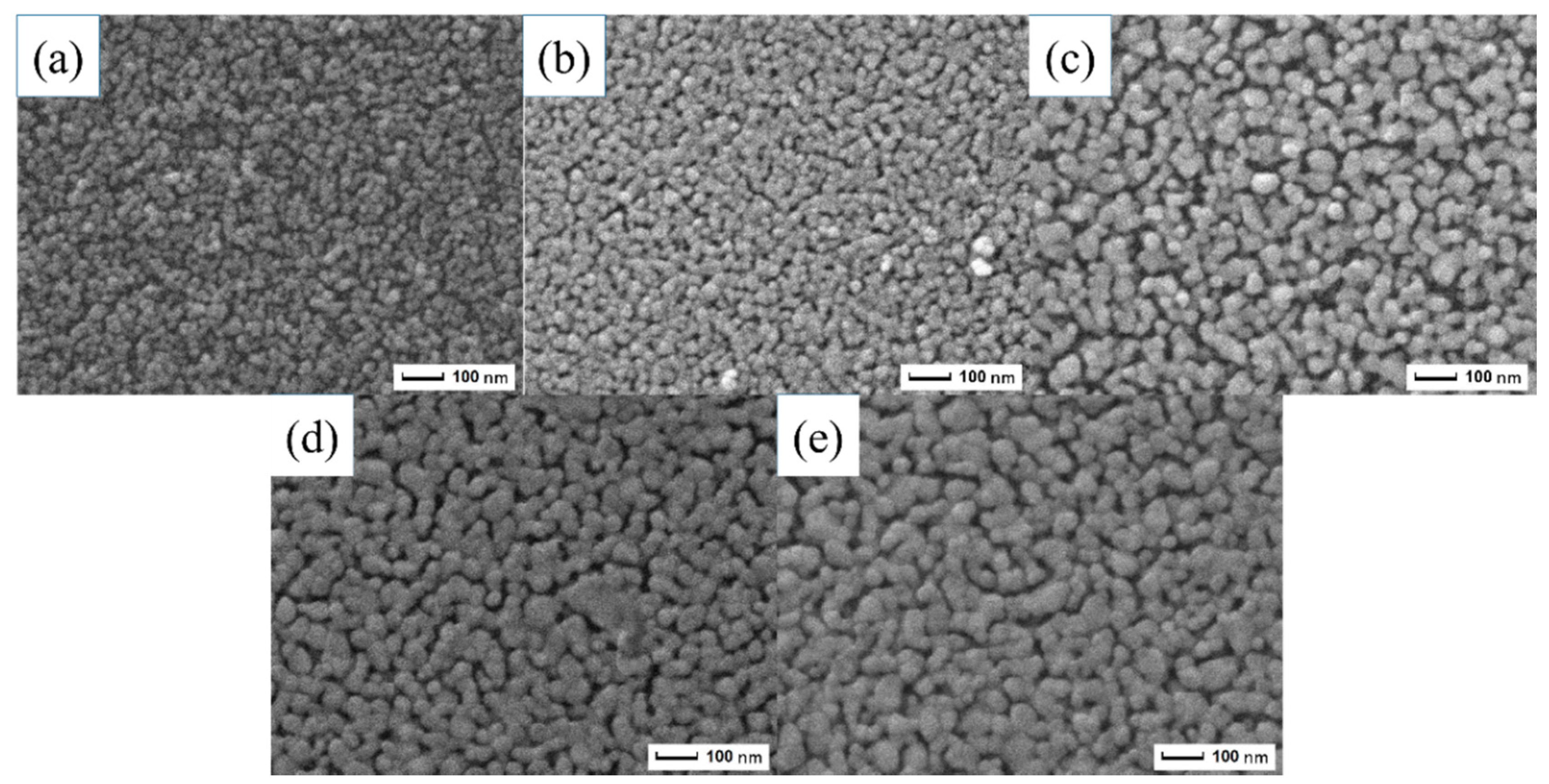
3.2. Characteristics of the ZnO Porous Thin Film
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Farrag, A.A.G.; Balboul, M.R. Nano ZnO thin films synthesis by sol-gel spin coating method as a transparent layer for solar cell applications. J. Sol-Gel Sci. Technol. 2017, 822, 269–279. [Google Scholar] [CrossRef]
- Su, B.Y.; Su, Y.K.; Tseng, Z.L.; Shih, M.F.; Cheng, C.Y.; Wu, T.H.; Wu, C.S.; Yeh, J.J.; Ho, P.Y. Antireflective and Radiation Resistant ZnO Thin Films for the Efficiency Enhancement of GaAs Photovoltaics. J. Electrochem. Soc. 2011, 158, H267–H270. [Google Scholar] [CrossRef]
- Xu, D.; Song, K.; Li, Y.L.; Jiao, L.; Zhong, S.J.; Ma, J.; Bao, L.; Zhang, L.; Song, J. Sc2O3 doped Bi2O3-ZnO thin films varistor prepared by sol-gel method. J. Alloys Compd. 2018, 746, 314–319. [Google Scholar] [CrossRef]
- Tiwari, N.; Shieh, H.P.D.; Liu, P.T. Structural, optical, and photoluminescence study of ZnO/IGZO thin film for thin film transistor application. Mater. Lett. 2015, 151, 53–56. [Google Scholar] [CrossRef]
- Mereu, B.; Caglar, O.; Cashmore, J.S.; Losio, P.A.; Salabas, A.; Sinicco, I. Window p-layer in amorphous pin solar cells using ZnO as Transparent Conductive Oxide. Sol. Energy Mater. Sol. Cells 2016, 152, 147–154. [Google Scholar] [CrossRef]
- Xu, Y.T.; Li, Y.; Zhang, H.; Jin, L.; Fang, X.; Shi, Y.L.; Xu, L.; Ma, X.H.; Zou, Y.G.; Yin, J.Z. Ultraviolet-enhanced electroluminescence from individual ZnO microwire/p-Si light-emitting diode by reverse tunneling effect. J. Mater. Chem. C 2017, 5, 6640–6646. [Google Scholar] [CrossRef]
- Khamsa, N.; Tahar, T.; Azeddine, C.; Lydia, O.; Djamel, D.; Boubekeur, B. Investigation of the effects of thickness on physical properties of AZO sol-gel films for photonic device applications. J. Alloys Compd. 2018, 735, 2236–2246. [Google Scholar] [CrossRef]
- Djurišić, A.; Ng, A.; Chen, X. ZnO nanostructures for optoelectronics: Material properties and device applications. Prog. Quantum Electron. 2010, 34, 191–259. [Google Scholar] [CrossRef]
- Yamamoto, N.; Makino, H.; Osone, S.; Ujihara, A.; Ito, T.; Hokari, H.; Maruyama, T.; Yamamoto, T. Development of Ga-doped ZnO transparent electrodes for liquid crystal display panels. Thin Solid Films 2012, 520, 4131–4138. [Google Scholar] [CrossRef]
- Makableh, Y.; Vasan, R.; Sarker, J.; Nusir, A.; Seal, S.; Manasreh, M. Enhancement of GaAs solar cell performance by using a ZnO sol-gel anti-reflection coating. Sol. Energy Mater. Sol. Cells 2014, 123, 178–182. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Lin, H.; Hu, X.Y.; Chen, C.X.; Xie, X.Z.; Yang, W.B.; Zhang, X.X.; Chen, H.X. Refractive-tunable and hydrophobic antireflective coatings by PMHS-Modification assisted sol-gel method. Mater. Lett. 2016, 186, 123–126. [Google Scholar] [CrossRef]
- Salem, M.; Alami, Z.Y.; Bessais, B.; Chahboun, A.; Gaidi, M. Structural and optical properties of ZnO nanoparticles deposited on porous silicon for mc-Si passivation. J. Nanopart. Res. 2015, 17, 137. [Google Scholar] [CrossRef]
- Li, S.F.; Jiao, S.J.; Wang, D.B.; Gao, S.Y.; Wang, J.Z. The influence of sputtering power on the structural, morphological and optical properties of beta-Ga2O3 thin films. J. Alloys Compd. 2018, 753, 186–191. [Google Scholar] [CrossRef]
- Biethan, J.P.; Sirkeli, V.; Considine, L.; Nedeoglo, D.; Pavlidis, D.; Hartnagel, H. Photoluminescence study of ZnO nanostructures grown on silicon by MOCVD. Mater. Sci. Eng. B 2012, 177, 594–599. [Google Scholar] [CrossRef]
- Khorramshahi, V.; Karamdel, J.; Yousefi, R. Acetic acid sensing of Mg-doped ZnO thin films fabricated by the sol–gel method. J. Mater. Sci. Mater. Electron. 2018, 29, 14679–14688. [Google Scholar] [CrossRef]
- Asikuzun, E.; Ozturk, O.; Arda, L.; Terzioglu, C. Preparation, growth and characterization of nonvacuum Cu-doped ZnO thin films. J. Mol. Struct. 2018, 1165, 1–7. [Google Scholar] [CrossRef]
- Jahn, R.; Löbmann, P. Microstructure and performance of AZO thin films prepared by sol–gel processing. J. Sol-Gel Sci. Technol. 2013, 66, 120–125. [Google Scholar] [CrossRef]
- Xi, J.Q.; Schubert, M.F.; Kim, J.K.; Schubert, E.F.; Chen, M.; Lin, S.Y.; Liu, W.; Smart, J.A. Optical thin-film materials with low refractive index for broadband elimination of Fresnel reflection. Nat. Photonics 2007, 1, 176–179. [Google Scholar] [CrossRef]
- Biswas, K.; Gangopadhyay, S.; Kim, H.C.; Miller, R.D. Nanoporous organosilicate films as antireflection coatings. Thin Solid Films 2006, 514, 350–354. [Google Scholar] [CrossRef]
- Rvenant, C.; Benwadih, M. Morphology of sol-gel porous In-Ga-Zn-O thin films as a function of annealing temperatures. Thin Solid Films 2016, 616, 643–648. [Google Scholar] [CrossRef]
- Revenant, C.; Benwadih, M.; Maret, M. Self-organized nanoclusters in solution-processed mesoporous In–Ga–Zn–O thin films. Chem. Commun. 2015, 51, 1218–1221. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.F.; Jin, Z.G.; Li, w.; Qiu, J.J. Preparation of ZnO porous thin films by sol–gel method using PEG template. Mater. Lett. 2005, 59, 3620–3625. [Google Scholar] [CrossRef]
- Liu, Z.F.; Li, J.W.; Ya, J.; Xin, J.; Jin, Z.G. Mechanism and characteristics of porous ZnO films by sol–gel method with PEG template. Mater. Lett. 2008, 62, 1190–1193. [Google Scholar] [CrossRef]
- Feng, J.B.; Huang, J.C. Improvement of Environmental stability of the antireflective coating by PEG modified SiO2 Sol. J. Mater. Sci. Chem. Eng. 2017, 5, 1. [Google Scholar] [CrossRef]
- Sun, J.H.; Fan, W.H.; Dong, W.; Sun, Y.H. Structure control of SiO2 sol-gels via addition of PEG. Stud. Surf. Sci. Catal. 1998, 118, 617–624. [Google Scholar] [CrossRef]
- Meshram, J.V.; Koli, V.B.; Kumbhar, S.G.; Borde, L.C.; Phadatare, M.R.; Pawar, S.H. Structural, spectroscopic and antimicrobial inspection of PEG capped ZnO nanoparticles for biomedical applications. Mater. Res. Express 2018, 5, 4. [Google Scholar] [CrossRef]
- Xiang, Y.L.; Li, X.G.; Du, A.; Wu, S.; Shen, J.; Zhou, B. Timing of polyethylene glycol addition for the control of SiO2 sol structure and sol–gel coating properties. J. Coat. Technol. Res. 2017, 14, 447–454. [Google Scholar] [CrossRef]
- Dutta, M.; Mridha, S.; Basak, D. Effect of sol concentration on the properties of ZnO thin films prepared by sol–gel technique. Appl. Surf. Sci. 2008, 254, 2743–2747. [Google Scholar] [CrossRef]
- Sengupta, J.; Sahoo, R.K.; Bardhan, K.K.; Mukherjee, C.D. Influence of annealing temperature on the structural, topographical and optical properties of sol-gel derived ZnO thin films. Mater. Lett. 2011, 65, 2572–2574. [Google Scholar] [CrossRef]
- Xu, L.H.; Li, X.Y.; Chen, Y.L.; Fei, X. Structural and optical properties of ZnO thin films prepared by sol-gel method with different thickness. Appl. Surf. Sci. 2011, 257, 4031–4037. [Google Scholar] [CrossRef]
- Bahadur, H.; Srivastava, A.; Chandra, S. Effect of sol strength on growth, faceting and orientation of sol-gel derived ZnO nanostructures. IEEE Sens. J. 2008, J8, 831–836. [Google Scholar] [CrossRef]
- Li, J.T.; Yang, D.Y.; Zhu, X.H. Effects of aging time and annealing temperature on structural and optical properties of sol-gel ZnO thin films. AIP Adv. 2017, 7, 259. [Google Scholar] [CrossRef]
- Chaitra, U.; Kekuda, D.; Rao, K.M. Dependence of solution molarity on structural, optical and electrical properties of spin coated ZnO thin films. J. Mater. Sci. Mater. Electron. 2016, 27, 7614–7621. [Google Scholar] [CrossRef]
- Li, J.T.; Yang, D.Y.; Zhu, X.H. Annealing temperature dependence of optical properties of sol-gel ZnO thin films with different sol aging time. J. Nano Res. 2017, 48, 211–217. [Google Scholar] [CrossRef]
- Li, D.Z.; Huang, F.Q.; Ding, S.J. Sol–gel preparation and characterization of nanoporous ZnO/SiO2 coatings with broadband antireflection properties. Appl. Surf. Sci. 2011, 257, 9752–9756. [Google Scholar] [CrossRef]
- Saidani, T.; Zaabat, M.; Aida, M.S.; Barille, R.; Rasheed, M.; Almohamed, Y. Influence of precursor source on sol–gel deposited ZnO thin films properties. J. Mater. Sci. Mater. Electron. 2017, 28, 9252–9257. [Google Scholar] [CrossRef]
- Li, J.T.; Yang, D.Y.; Zhu, X.H.; Sun, H.; Gao, X.Y.; Wangyang, P.H.; Tian, H.B. Effects of annealing temperature on the structural and optical properties of ZnO thin films prepared by sol–gel method. Funct. Mater. Lett. 2016, 9, 1750010. [Google Scholar] [CrossRef]
- Cruz, M.R.A.; Hernandez-Como, N.; Mejia, I.; Ortega-Zarzosa, G.; Quevedo-Lopez, M.A. Impact of the annealing atmosphere in the electrical and optical properties of ZnO thin films. J. Sol-Gel Sci. Technol. 2016, 79, 184–189. [Google Scholar] [CrossRef]
- Li, J.T.; Yang, D.Y.; Zhu, X.H.; Sun, H.; Gao, X.Y.; Wangyang, P.H.; Tian, H.B. Structural and optical properties of nano-crystalline ZnO thin films synthesized by sol–gel method. J. Sol-Gel Sci. Technol. 2017, 82, 563–568. [Google Scholar] [CrossRef]

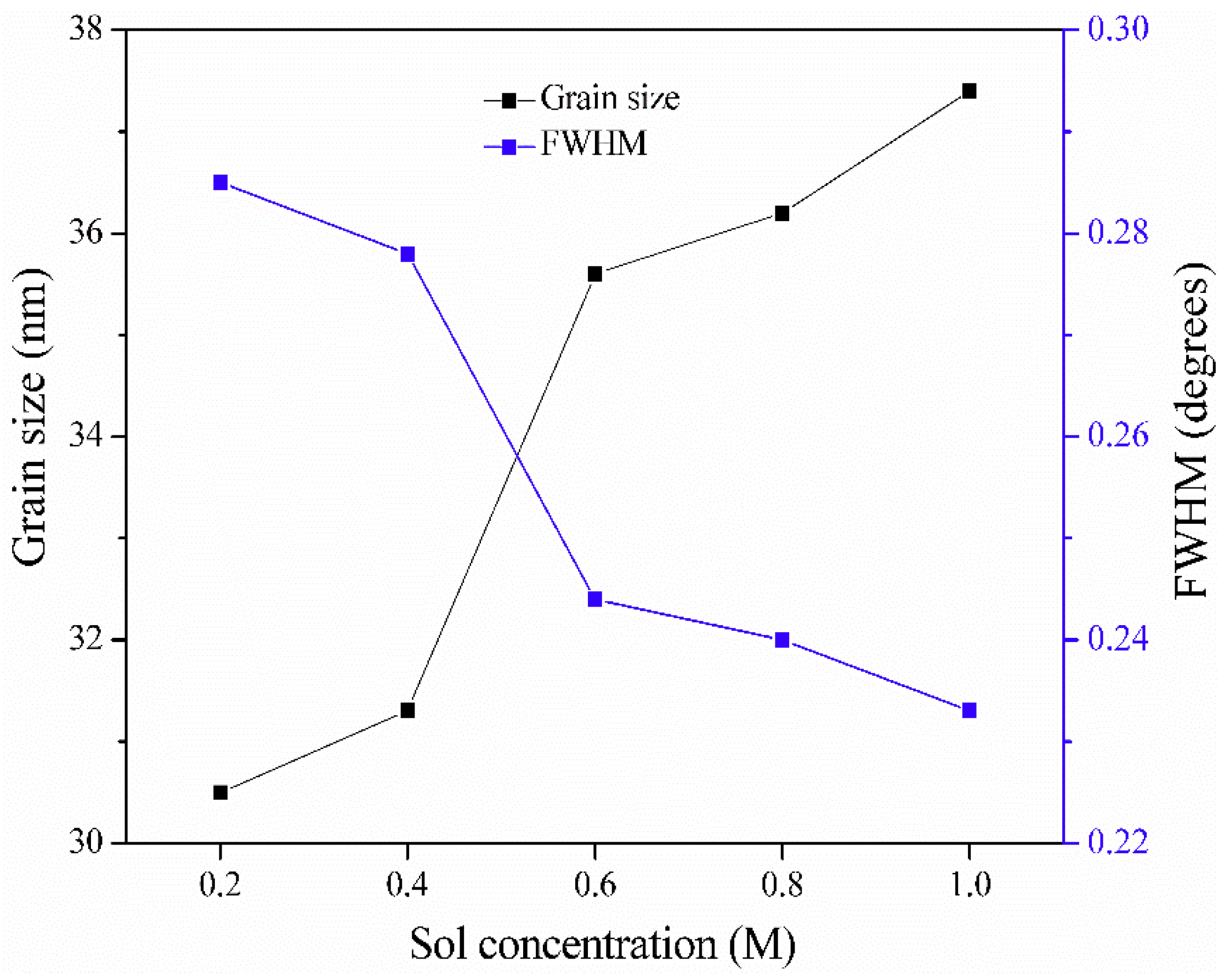


| Sol Concentration (M) | 2θ (002) (°) | FWHM (°) | d (nm) | D (nm) |
|---|---|---|---|---|
| 0.2 | 34.217 | 0.285 | 0.26184 | 30.5 |
| 0.4 | 34.278 | 0.278 | 0.26094 | 31.3 |
| 0.6 | 34.338 | 0.244 | 0.26006 | 35.6 |
| 0.8 | 34.398 | 0.240 | 0.25962 | 36.2 |
| 1.0 | 34.428 | 0.233 | 0.25874 | 37.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, D.; Yu, Q.; Chen, T.; Zhong, S.; Ma, J.; Bao, L.; Zhang, L.; Zhao, F.; Du, S. Effects of PEG1000 and Sol Concentration on the Structural and Optical Properties of Sol–Gel ZnO Porous Thin Films. Materials 2018, 11, 1840. https://doi.org/10.3390/ma11101840
Xu D, Yu Q, Chen T, Zhong S, Ma J, Bao L, Zhang L, Zhao F, Du S. Effects of PEG1000 and Sol Concentration on the Structural and Optical Properties of Sol–Gel ZnO Porous Thin Films. Materials. 2018; 11(10):1840. https://doi.org/10.3390/ma11101840
Chicago/Turabian StyleXu, Dong, Qian Yu, Taiyun Chen, Sujuan Zhong, Jia Ma, Li Bao, Lei Zhang, Feiwen Zhao, and Sanming Du. 2018. "Effects of PEG1000 and Sol Concentration on the Structural and Optical Properties of Sol–Gel ZnO Porous Thin Films" Materials 11, no. 10: 1840. https://doi.org/10.3390/ma11101840
APA StyleXu, D., Yu, Q., Chen, T., Zhong, S., Ma, J., Bao, L., Zhang, L., Zhao, F., & Du, S. (2018). Effects of PEG1000 and Sol Concentration on the Structural and Optical Properties of Sol–Gel ZnO Porous Thin Films. Materials, 11(10), 1840. https://doi.org/10.3390/ma11101840





