Tungsten-Embedded Graphene: Theoretical Study on a Potential High-Activity Catalyst toward CO Oxidation
Abstract
:1. Introduction
2. Computational Details
3. Results and Discussion
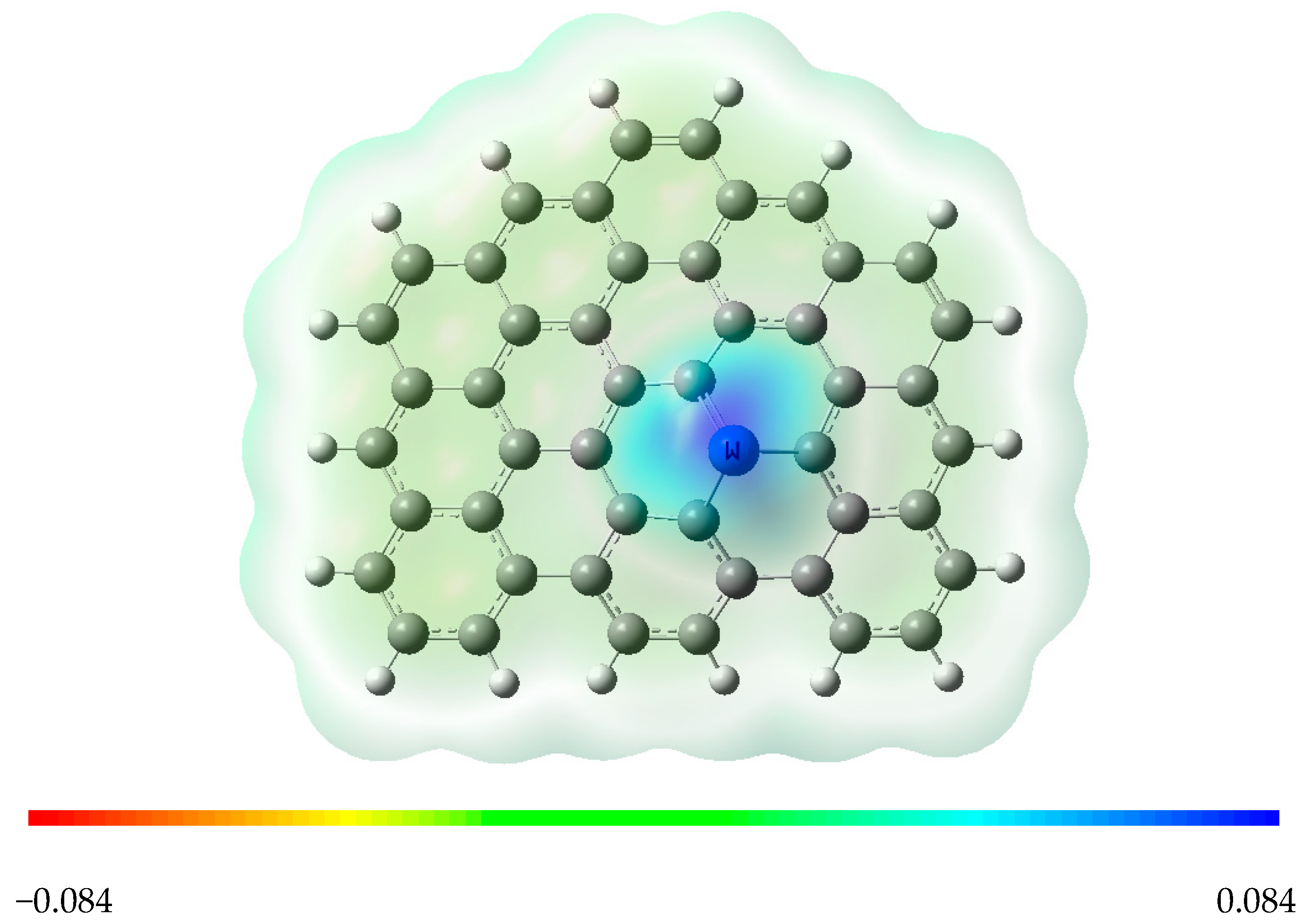
3.1. Tungsten-Embedded Graphene
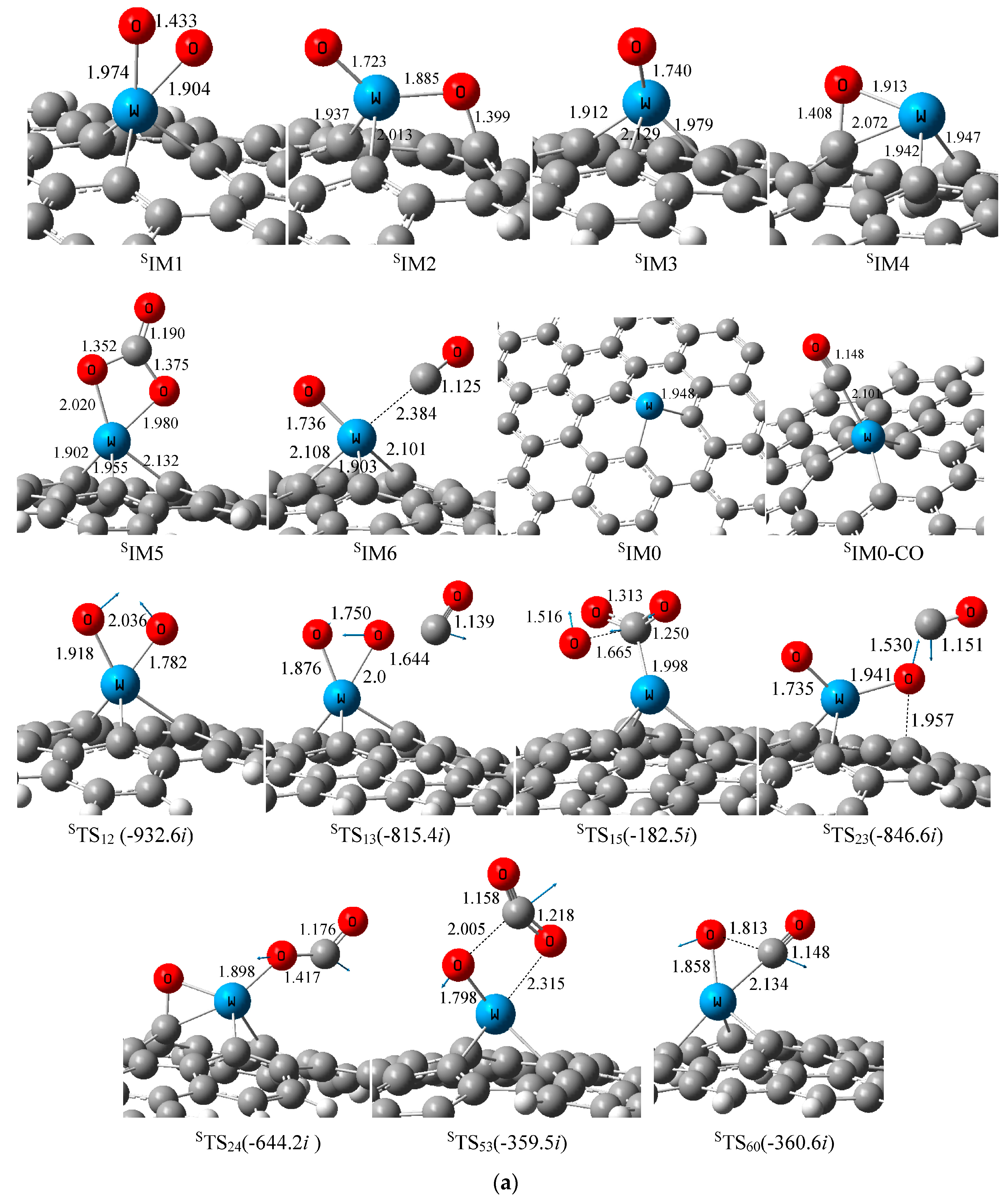
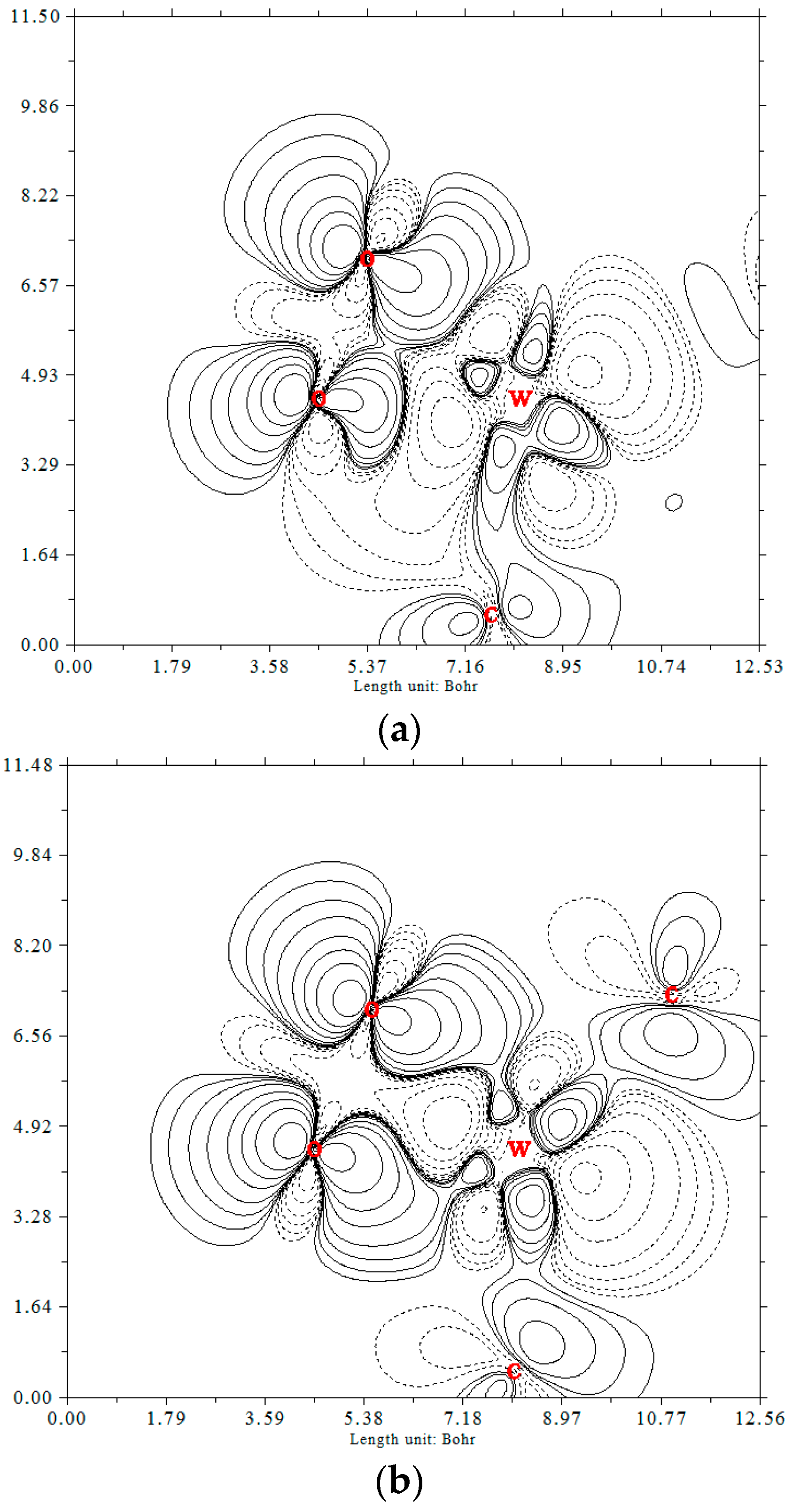
3.2. Adsorption of O2 and CO Species over W-Embedded Graphene
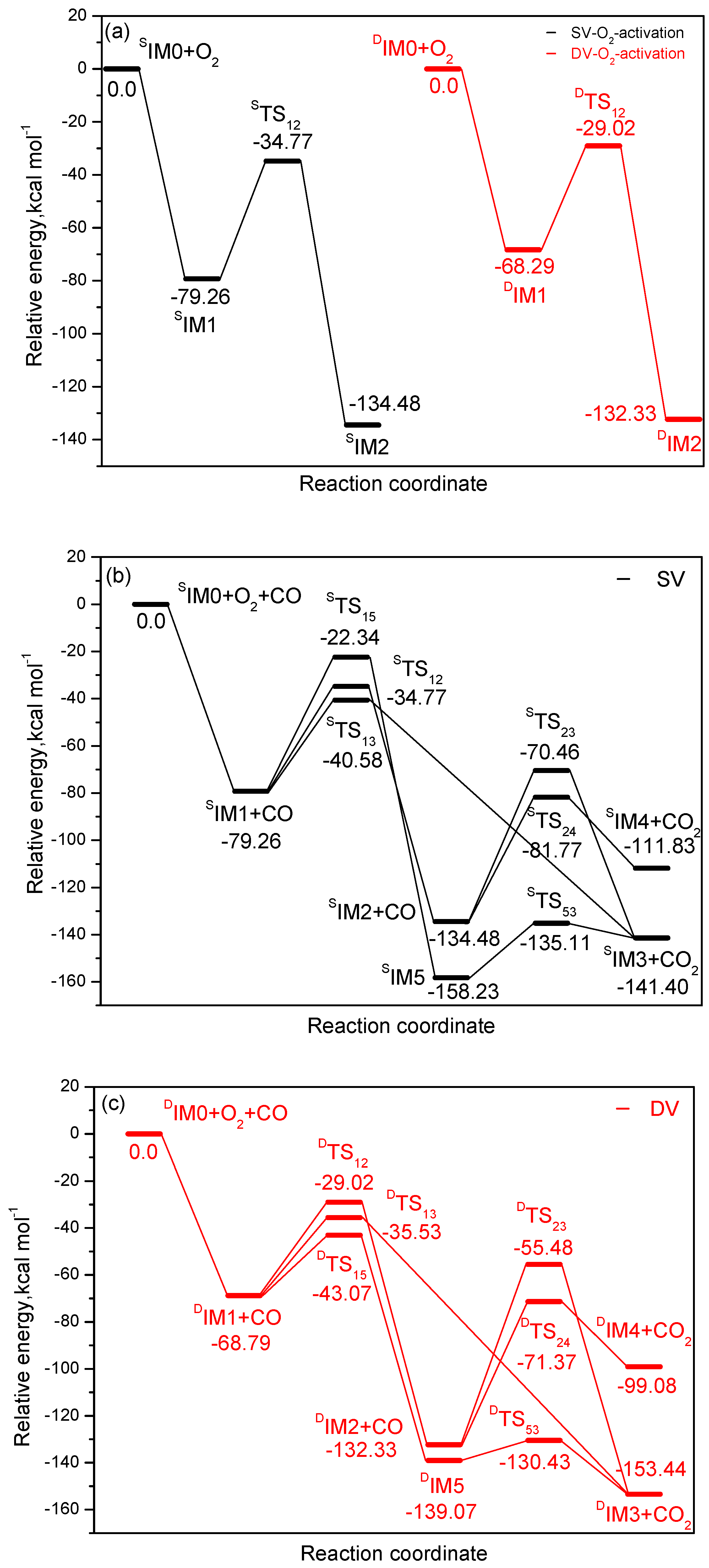
3.3. The CO Oxidation on Tungsten-Embedded Graphene through ER Mechanism
3.4. The CO Oxidation on Tungsten-Embedded Graphene through LH Mechanism
3.5. The CO Oxidation on Tungsten-Embedded Graphene by Oads
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Royer, S.; Duprez, D. Catalytic oxidation of carbon monoxide over transition metal oxides. ChemCatChem 2011, 3, 24–65. [Google Scholar] [CrossRef]
- Chen, M.S.; Cai, Y.; Yan, Z.; Gath, K.K.; Axnanda, S.; Goodman, D.W. Highly active surfaces for CO oxidation on Rh, Pd, and Pt. Surf. Sci. 2007, 601, 5326–5331. [Google Scholar] [CrossRef]
- Qu, L.T.; Liu, Y.; Baek, J.B.; Dai, L.M. Nitrogen-Doped Graphene as Efficient Metal-Free Electrocatalyst for Oxygen Reduction in Fuel Cells. ACS Nano 2010, 4, 1321–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasap, H.; Godin, R.; Jeay-Bizot, C.; Achilleos, D.S.; Fang, H.; Durrant, J.R.; Reisner, E. Interfacial Engineering of a Carbon Nitride-Graphene Oxide Molecular Ni Catalyst Hybrid for Enhanced Photocatalytic Activity. ACS Catal. 2018, 8, 6914–6926. [Google Scholar] [CrossRef]
- Fei, F.; Cseri, L.; Szekely, G.; Blanford, C.F. Robust Covalently Cross-linked Polybenzimidazole/Graphene Oxide Membranes for High-Flux Organic Solvent Nanofiltration. ACS Appl. Mater. Interfaces 2018, 10, 16140–16147. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.H.; Zhou, M.; Zhang, C.; Feng, Y.P. Metal-embedded graphene: A possible catalyst with high activity. J. Phys. Chem. C 2009, 113, 20156–20160. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Nematollahi, P.; Nurazar, R. Pd-embedded graphene: An efficient and highly active catalyst for oxidation of CO. Superlattice Microstruct. 2016, 92, 60–67. [Google Scholar] [CrossRef]
- Tang, Y.N.; Yang, Z.X.; Dai, X.Q. A theoretical simulation on the catalytic oxidation of CO on Pt/graphene. Phys. Chem. Chem. Phys. 2012, 14, 16566–16572. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.L.; Wang, R.; Yang, F.; Ma, D.W.; Yang, Z.X.; Lu, Z.S. CO oxidation on single Pd atom embedded defect-graphene via a new termolecular Eley-Rideal mechanism. Carbon 2017, 118, 35–42. [Google Scholar] [CrossRef]
- Li, Y.F.; Zhao, J.J.; Chen, Z.F. Fe-anchored graphene oxide: A low-cost and easily accessible catalyst for low-temperature CO oxidation. J. Phys. Chem. C 2012, 116, 2507–2514. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Z.; Yu, G.; Chen, W.; Chen, Z. CO catalytic oxidation on iron-embedded graphene: Computational quest for low-cost nanocatalysts. J. Phys. Chem. C 2010, 114, 6250–6254. [Google Scholar] [CrossRef]
- Wannakao, S.; Nongnual, T.; Khongpracha, P.; Maihom, T.; Limtrakul, J. Reaction mechanisms for CO catalytic oxidation by N2O on Fe-embedded graphene. J. Phys. Chem. C 2012, 116, 16992–16998. [Google Scholar] [CrossRef]
- Song, E.H.; Wen, Z.; Jiang, Q. CO catalytic oxidation on copper-embedded graphene. J. Phys. Chem. C 2011, 115, 3678–3683. [Google Scholar] [CrossRef]
- Esrafili, M.; Saeidi, N. Sn-embedded graphene: An active catalyst for CO oxidation to CO2? Phys. E Low-Dimens. Syst. Nanostruct. 2015, 74, 382–387. [Google Scholar] [CrossRef]
- Tang, Y.N.; Pan, L.J.; Chen, W.G.; Li, C.G.; Shen, Z.G.; Dai, X.Q. Reaction mechanisms for CO catalytic oxidation on monodisperse Mo atom-embedded graphene. Appl. Phys. A 2015, 119, 475–485. [Google Scholar] [CrossRef]
- Wang, M.G.; Wang, Z. Single Ni atom incorporated with pyridinic nitrogen graphene as an efficient catalyst for CO oxidation: First-principles investigation. RSC Adv. 2017, 7, 48819–48824. [Google Scholar] [CrossRef]
- Jiang, Q.G.; Ao, Z.M.; Li, S.; Wen, Z. Density functional theory calculations on the CO catalytic oxidation on Al-embedded graphene. RSC Adv. 2014, 4, 20290–20296. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.X.; Chen, Y.; Fu, H.G. Si-embedded graphene: An efficient and metal-free catalyst for CO oxidation by N2O or O2. Theor. Chem. Acc. 2012, 131, 1242–1252. [Google Scholar] [CrossRef]
- Tang, Y.N.; Liu, Z.Y.; Dai, X.Q.; Yang, Z.X.; Chen, W.G.; Ma, D.W.; Lu, Z.S. Theoretical study on the Si-doped graphene as an efficient metal-free catalyst for CO oxidation. Appl. Surf. Sci. 2014, 308, 402–407. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Saeidi, N.; Nematollahi, P. A DFT study on SO3 capture and activation over Si- or Al-doped graphene. Chem. Phys. Lett. 2016, 658, 146–151. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Nematollahi, P.; Abdollahpour, H. A comparative DFT study on the CO oxidation reaction over Al- and Ge-embedded graphene as efficient metal-free catalysts. Appl. Surf. Sci. 2016, 378, 418–425. [Google Scholar] [CrossRef]
- Bagsican, F.R.; Winchester, A.; Ghosh, S.; Zhang, X.; Ma, L.L.; Wang, M.J.; Murakami, H.; Talapatra, S.; Vajtai, R.; Ajayan, P.M.; et al. Adsorption energy of oxygen molecules on graphene and two-dimensional tungsten disulfide. Sci. Rep. 2017, 7, 1774–1783. [Google Scholar] [CrossRef] [PubMed]
- Razali, M.; Kim, J.F.; Attfield, M.; Budd, P.M.; Drioli, E.; Lee, Y.M.; Szekely, G. Sustainable wastewater treatment and recycling in membrane manufacturing. Green. Chem. 2015, 17, 5196–5205. [Google Scholar] [CrossRef] [Green Version]
- Robertson, A.W.; Allen, C.S.; Wu, Y.A.; He, K.; Olivier, J.; Neethling, J.; Kirkland, A.I.; Warner, J.H. Spatial control of defect creation in graphene at the nanoscale. Nat. Commun. 2012, 3, 1144–1150. [Google Scholar] [CrossRef] [PubMed]
- Krasheninnikov, A.V.; Lehtinen, P.O.; Foster, A.S.; Pyykko, P.; Nieminen, R.M. Embedding Transition-Metal Atoms in Graphene: Structure, Bonding, and Magnetism. Phys. Rev. Lett. 2009, 102, 126807–126810. [Google Scholar] [CrossRef] [PubMed]
- Jeevitha, G.; Abhinayaa, R.; Mangalaraj, D.; Ponpandian, N. Tungsten oxide-graphene oxide (WO3-GO) nanocomposite as an efficient photocatalyst, antibacterial and anticancer agent. J. Phys. Chem. Solids 2018, 116, 137–147. [Google Scholar] [CrossRef]
- Chang, X.T.; Zhou, Q.; Sun, S.B.; Shao, C.Z.; Lei, Y.H.; Liu, T.; Dong, L.H.; Yin, Y.S. Graphene-tungsten oxide nanocomposites with highly enhanced gassensing performance. J. Alloy. Compd. 2017, 705, 659–667. [Google Scholar] [CrossRef]
- Dong, L.L.; Chen, W.G.; Zheng, C.H.; Deng, N. Microstructure and properties characterization of tungstene-copper composite materials doped with graphene. J. Alloy. Compd. 2017, 695, 1637–1646. [Google Scholar] [CrossRef]
- Jeong, B.; Ye, B.; Kim, E.S.; Kim, H.D. Characteristics of selective catalytic reduction (SCR) catalyst adding graphene-tungsten nanocomposite. Catal. Commun. 2017, 93, 15–19. [Google Scholar] [CrossRef]
- Yan, Y.; Xia, B.Y.; Qi, X.Y.; Wang, H.B.; Xu, R.; Wang, J.Y.; Zhang, H.; Wang, X. Nano-tungsten carbide decorated graphene as co-catalysts for enhanced hydrogen evolution on molybdenum disulfide. Chem. Commun. 2013, 49, 4884–4886. [Google Scholar] [CrossRef] [PubMed]
- Luan, H.X.; Zhang, C.W.; Li, S.S.; Zhang, R.W.; Wang, P.J. First-principles study on ferromagnetism in W-doped graphene. RSC Adv. 2013, 3, 26261–26265. [Google Scholar] [CrossRef]
- Sun, M.L.; Tang, W.C.; Ren, Q.Q.; Zhao, Y.M.; Wang, S.K.; Yu, J.; Du, Y.H.; Hao, Y.T. Electronic and magnetic behaviors of graphene with 5d series transition metal atom substitutions: A first-principles study. Phys. E Low-Dimens. Syst. Nanostruct. 2016, 80, 142–148. [Google Scholar] [CrossRef]
- Jin, H.; Zhou, H.G.; Zhang, Y.F. Insight into the Mechanism of CO Oxidation on WO3(001) Surfaces for Gas Sensing: A DFT Study. Sensors 2017, 17, 1898. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. A new mixing of Hartree-Fock and local density-functional theories. J. Chem. Phys. 1993, 98, 1372–1377. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F.W. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Fodi, T.; Didaskalou, C.; Kupai, J.; Balogh, G.T.; Huszthy, P.; Szekely, G. Nanofiltration-Enabled In Situ Solvent and Reagent Recycle for Sustainable Continuous-Flow Synthesis. ChemSusChem 2017, 10, 3435–3444. [Google Scholar] [CrossRef] [PubMed]



| Species | W | O1 | O2 | Sum(O2) | C | O | Sum(CO) | Sum(Graphene) |
|---|---|---|---|---|---|---|---|---|
| SIM0 | 0.778 | – | – | – | – | – | – | −0.778 |
| SIM0 + CO | 0.484 | – | – | – | 0.512 | −0.424 | 0.088 | −0.572 |
| SCO | – | – | – | 0.517 | −0.517 | 0 | – | |
| SIM1 | 1.072 | −0.330 | −0.248 | −0.578 | – | – | – | −0.494 |
| SIM2 | 1.256 | −0.549 | −0.588 | −1.137 | – | – | – | −0.119 |
| SIM3 | 1.022 | −0.531 | – | – | – | – | – | −0.491 |
| SIM4 | 1.002 | – | −0.603 | – | – | – | – | −0.399 |
| SIM5 | 1.108 | −0.601 | −0.658 | −1.259 | 1.034 | −0.580 | 0.454 | −0.303 |
| SIM6 | 0.712 | −0.486 | – | – | 0.700 | −0.376 | 0.324 | −0.550 |
| STS12 | 1.213 | −0.289 | −0.484 | −0.773 | – | – | – | −0.440 |
| STS13 | 1.273 | −0.475 | −0.458 | −0.933 | 0.725 | −0.457 | 0.268 | −0.608 |
| STS15 | 0.813 | −0.294 | −0.346 | −0.640 | 0.795 | −0.595 | 0.200 | −0.373 |
| STS23 | 1.230 | −0.552 | −0.593 | −1.145 | 0.713 | −0.468 | 0.245 | −0.330 |
| STS24 | 1.268 | −0.662 | −0.556 | −1.218 | 0.638 | −0.538 | 0.100 | −0.150 |
| STS53 | 1.115 | −0.619 | −0.649 | −1.268 | 1.112 | −0.479 | 0.633 | −0.480 |
| STS60 | 0.852 | −0.630 | – | – | 0.709 | −0.440 | 0.269 | −0.491 |
| Species | W | O1 | O2 | Sum(O2) | C | O | Sum(CO) | Sum(Graphene) |
|---|---|---|---|---|---|---|---|---|
| DIM0 | 0.875 | – | – | – | – | – | – | −0.875 |
| DIM0 + CO | 0.438 | – | – | – | 0.661 | −0.415 | 0.246 | −0.684 |
| DCO | – | – | – | – | 0.517 | −0.517 | 0 | – |
| DIM1 | 1.290 | −0.312 | −0.310 | −0.622 | – | – | – | −0.668 |
| DIM2 | 1.440 | −0.530 | −0.583 | −1.113 | – | – | – | −0.327 |
| DIM3 | 1.321 | −0.535 | – | – | – | – | – | −0.786 |
| DIM4 | 1.020 | – | −0.611 | – | – | – | – | −0.409 |
| DIM5 | 1.291 | −0.665 | −0.660 | −1.325 | 1.083 | −0.589 | 0.494 | −0.460 |
| DIM6 | 1.298 | −0.537 | – | – | 0.518 | −0.507 | 0.011 | −0.772 |
| DTS12 | 1.226 | −0.474 | −0.276 | −0.750 | – | – | – | −0.476 |
| DTS13 | 1.274 | −0.473 | −0.396 | −0.869 | 0.711 | −0.464 | 0.247 | −0.652 |
| DTS15 | 0.797 | −0.319 | −0.306 | −0.625 | 0.753 | −0.475 | 0.278 | −0.450 |
| DTS23 | 1.302 | −0.504 | −0.575 | −1.079 | 0.689 | −0.537 | 0.152 | −0.375 |
| DTS24 | 1.258 | −0.647 | −0.585 | −1.232 | 0.753 | −0.533 | 0.220 | −0.246 |
| DTS53 | 1.299 | −0.627 | −0.659 | −1.286 | 1.114 | −0.519 | 0.595 | −0.608 |
| DTS60 | 1.077 | −0.623 | – | – | 0.732 | −0.502 | 0.230 | −0.684 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, G.; Chen, L.; Zhao, X. Tungsten-Embedded Graphene: Theoretical Study on a Potential High-Activity Catalyst toward CO Oxidation. Materials 2018, 11, 1848. https://doi.org/10.3390/ma11101848
Dai G, Chen L, Zhao X. Tungsten-Embedded Graphene: Theoretical Study on a Potential High-Activity Catalyst toward CO Oxidation. Materials. 2018; 11(10):1848. https://doi.org/10.3390/ma11101848
Chicago/Turabian StyleDai, Guoliang, Lei Chen, and Xin Zhao. 2018. "Tungsten-Embedded Graphene: Theoretical Study on a Potential High-Activity Catalyst toward CO Oxidation" Materials 11, no. 10: 1848. https://doi.org/10.3390/ma11101848
APA StyleDai, G., Chen, L., & Zhao, X. (2018). Tungsten-Embedded Graphene: Theoretical Study on a Potential High-Activity Catalyst toward CO Oxidation. Materials, 11(10), 1848. https://doi.org/10.3390/ma11101848




