Recent Progress on Microelectrodes in Neural Interfaces
Abstract
:1. Introduction
2. Microelectrode Array (MEA) for In Vitro Applications
2.1. History
2.2. Type of MEA
2.2.1. MEA for Extracellular Recording
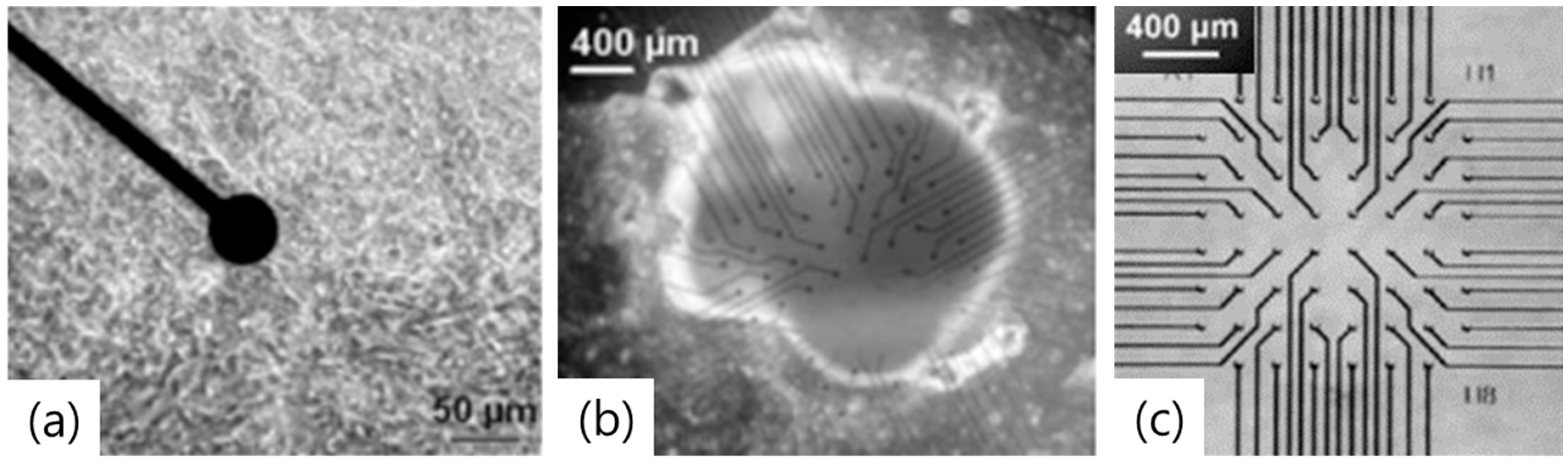
Planar Type MEA
Biomimetic MEA
2.2.2. MEA for Intracellular Recording
Sharp Glass Electrodes/Patch-Clamp Electrodes
Vertical Electrodes Using Nanostructure (Nanowire/Nanotube)
2.3. Challenges
3. Penetrating Electrode for In Vivo Applications
3.1. History
3.2. Shape of Each Electrode
3.2.1. Microwire Type
3.2.2. Microelectrodes
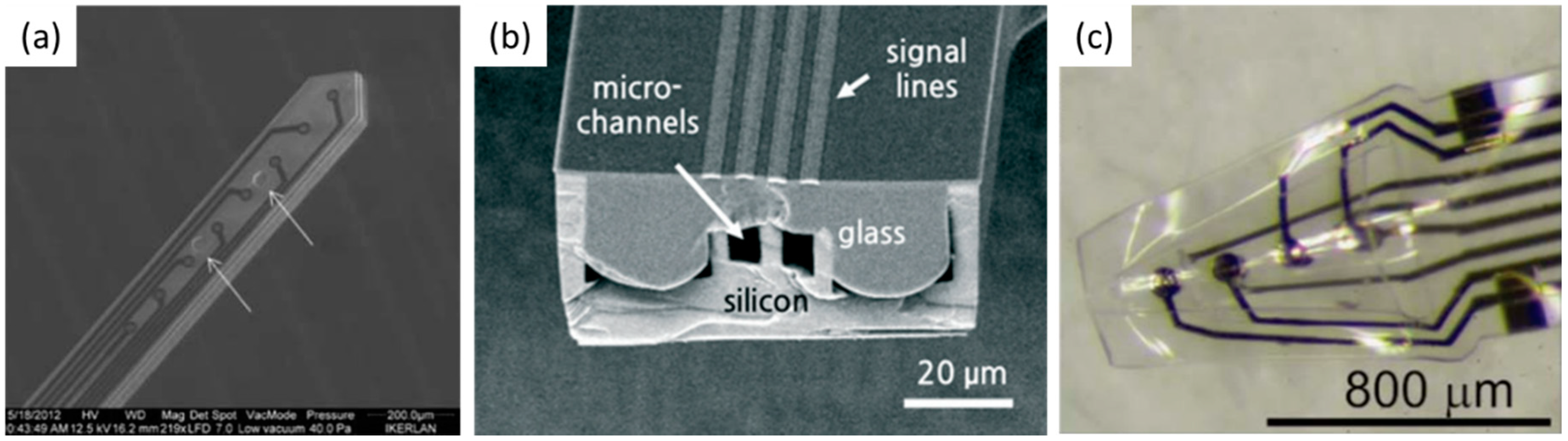
3.2.3. Polymer Electrode
3.2.4. Multifunctional Electrode
3.3. Requirements and Future Directions
4. Non-Penetrating Electrodes
4.1. EEG Electrode
4.1.1. History
4.1.2. EEG Electrodes
Wet EEG Electrodes
Dry EEG Electrodes
4.2. ECoG Electrode
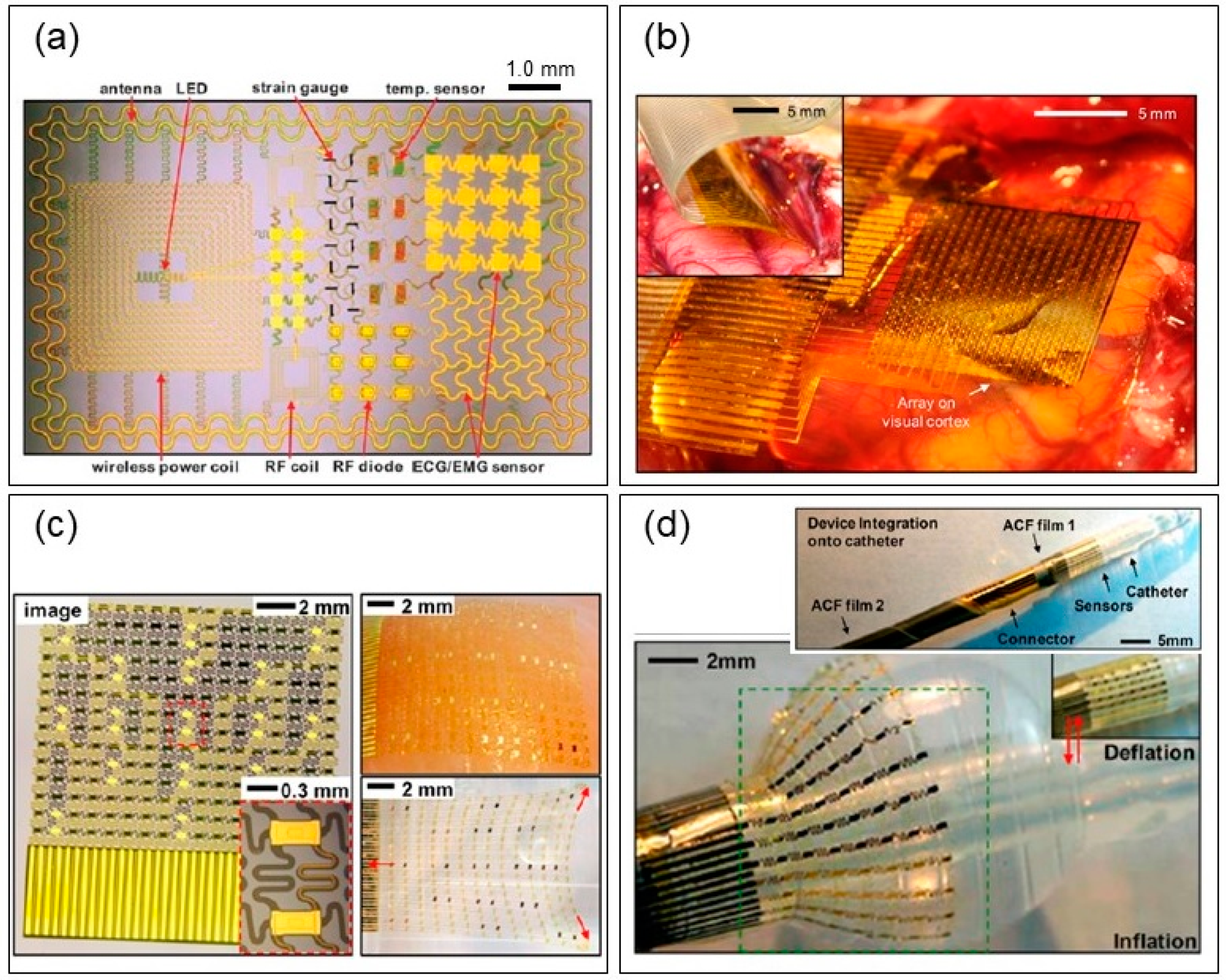
4.3. Recent Neural Electrodes with Flexible and Stretchable Characteristics
5. The Selection of Materials and Surface Modification
5.1. The Materials of Substrate and Electrode Parts
5.2. Surface Modification for Enhancing Electrode Impedance
6. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Polikov, V.S.; Tresco, P.A.; Reichert, W.M. Response of brain tissue to chronically implanted neural electrodes. J. Neurosci. Methods 2005, 148, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Niparko, J.K.; Altschuler, R.A.; Wiler, J.A.; Xue, X.; Anderson, D.J. Surgical Implantation and Biocompatibility of Central Nervous System Auditory Prostheses Surgical Implantation and Biocompatibility of Central Nervous System Auditory Prostheses. Ann. Otol. Rhinol. Laryngol. 1989, 98, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Torsi, L.; Magliulo, M.; Manoli, K.; Palazzo, G. Organic field-effect transistor sensors: A tutorial review. Chem. Soc. Rev. 2013, 42, 8612–8628. [Google Scholar] [CrossRef] [PubMed]
- A Review of Organic and Inorganic Biomaterials for Neural Interfaces—Fattahi—2014—Advanced Materials—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/adma.201304496 (accessed on 12 July 2018).
- Cogan, S.F. Neural Stimulation and Recording Electrodes. Annu. Rev. Biomed. Eng. 2008, 10, 275–309. [Google Scholar] [CrossRef] [PubMed]
- Fenno, L.; Yizhar, O.; Deisseroth, K. The development and application of optogenetics. Annu. Rev. Neurosci. 2011, 34, 389–412. [Google Scholar] [CrossRef] [PubMed]
- Richter, C.-P.; Matic, A.I.; Wells, J.D.; Jansen, E.D.; Walsh, J.T. Neural stimulation with optical radiation. Laser Photonics Rev. 2011, 5, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Spira, M.E.; Hai, A. Multi-electrode array technologies for neuroscience and cardiology. Nat. Nanotechnol. 2013, 8, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.T.; Jorgolli, M.; Park, H. Nanowire electrodes for high-density stimulation and measurement of neural circuits. Front. Neural Circuits 2013, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hai, A.; Shappir, J.; Spira, M.E. In-cell recordings by extracellular microelectrodes. Nat. Methods 2010, 7, 200–202. [Google Scholar] [CrossRef] [PubMed]
- Lacour, S.P.; Benmerah, S.; Tarte, E.; FitzGerald, J.; Serra, J.; McMahon, S.; Fawcett, J.; Graudejus, O.; Yu, Z.; Morrison, B. Flexible and stretchable micro-electrodes for in vitro and in vivo neural interfaces. Med. Biol. Eng. Comput. 2010, 48, 945–954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodgkin, A.L.; Huxley, A.F. Action Potentials Recorded from Inside a Nerve Fibre. Nature 1939, 144, 710–711. [Google Scholar] [CrossRef]
- Robinson, D.A. The electrical properties of metal microelectrodes. Proc. IEEE 1968, 56, 1065–1071. [Google Scholar] [CrossRef]
- Thomas, C.A.; Springer, P.A.; Loeb, G.E.; Berwald-Netter, Y.; Okun, L.M. A miniature microelectrode array to monitor the bioelectric activity of cultured cells. Exp. Cell Res. 1972, 74, 61–66. [Google Scholar] [CrossRef]
- Pine, J. Recording action potentials from cultured neurons with extracellular microcircuit electrodes. J. Neurosci. Methods 1980, 2, 19–31. [Google Scholar] [CrossRef]
- Gabay, T.; Jakobs, E.; Ben-Jacob, E.; Hanein, Y. Engineered self-organization of neural networks using carbon nanotube clusters. Phys. Stat. Mech. Its Appl. 2005, 350, 611–621. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Rutten, W.L.C. Selective Electrical Interfaces with the Nervous System. Annu. Rev. Biomed. Eng. 2002, 4, 407–452. [Google Scholar] [CrossRef] [PubMed]
- Massobrio, P.; Massobrio, G.; Martinoia, S. Interfacing Cultured Neurons to Microtransducers Arrays: A Review of the Neuro-Electronic Junction Models. Front. Neurosci. 2016, 10. [Google Scholar] [CrossRef] [PubMed]
- Stett, A.; Egert, U.; Guenther, E.; Hofmann, F.; Meyer, T.; Nisch, W.; Haemmerle, H. Biological application of microelectrode arrays in drug discovery and basic research. Anal. Bioanal. Chem. 2003, 377, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Spira, M.E.; Kamber, D.; Dormann, A.; Cohen, A.; Bartic, C.; Borghs, G.; Langedijk, J.P.M.; Yitzchaik, S.; Shabthai, K.; Shappir, J. Improved Neuronal Adhesion to the Surface of Electronic Device by Engulfment of Protruding Micro-Nails Fabricated on the Chip Surface. IEEE Trans. Biomed. Circuits Syst. 2007, 1247–1250. [Google Scholar] [CrossRef]
- Roelandse, M.; Welman, A.; Wagner, U.; Hagmann, J.; Matus, A. Focal motility determines the geometry of dendritic spines. Neuroscience 2003, 121, 39–49. [Google Scholar] [CrossRef]
- Akaike, N.; Harata, N. Nystatin Perforated Patch Recording and Its Applications to Analyses of Intracellular Mechanisms. Jpn. J. Physiol. 1994, 44, 433–473. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Krishtal, O.A.; Petersen, O.H. From Galvani to patch clamp: The development of electrophysiology. Pflüg. Arch. 2006, 453, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Martina, M.; Luk, C.; Py, C.; Martinez, D.; Comas, T.; Monette, R.; Denhoff, M.; Syed, N.; Mealing, G.A.R. Recordings of cultured neurons and synaptic activity using patch-clamp chips. J. Neural Eng. 2011, 8, 034002. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Bauer, S.; von der Mark, K.; Schmuki, P. Nanosize and Vitality: TiO2 Nanotube Diameter Directs Cell Fate. Nano Lett. 2007, 7, 1686–1691. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.W.; Herrup, K. The Control of Neuron Number. Annu. Rev. Neurosci. 1988, 11, 423–453. [Google Scholar] [CrossRef] [PubMed]
- Ghane-Motlagh, B.; Sawan, M. A review of Microelectrode Array technologies: Design and implementation challenges. In Proceedings of the 2013 2nd International Conference on Advances in Biomedical Engineering, Tripoli, Lebanon, 11–13 September 2013; pp. 38–41. [Google Scholar]
- Robinson, J.T.; Jorgolli, M.; Shalek, A.K.; Yoon, M.-H.; Gertner, R.S.; Park, H. Vertical nanowire electrode arrays as a scalable platform for intracellular interfacing to neuronal circuits. Nat. Nanotechnol. 2012, 7, 180–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eick, S.; Wallys, J.; Hofmann, B.; van Ooyen, A.; Schnakenberg, U.; Ingebrandt, S.; Offenhäusser, A. Iridium Oxide Microelectrode Arrays for In Vitro Stimulation of Individual Rat Neurons from Dissociated Cultures. Front. Neuroeng. 2009, 2. [Google Scholar] [CrossRef] [PubMed]
- Buitenweg, J.R.; Rutten, W.L.C.; Marani, E. Geometry-based finite-element modeling of the electrical contact between a cultured neuron and a microelectrode. IEEE Trans. Biomed. Eng. 2003, 50, 501–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dotti, C.G.; Sullivan, C.A.; Banker, G.A. The establishment of polarity by hippocampal neurons in culture. J. Neurosci. 1988, 8, 1454–1468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bi, G.; Poo, M. Distributed synaptic modification in neural networks induced by patterned stimulation. Nature 1999, 401, 792–796. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.M.; Blurton-Jones, M.; Rhee, S.W.; Cribbs, D.H.; Cotman, C.W.; Jeon, N.L. A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nat. Methods 2005, 2, 599–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valor, L.M.; Charlesworth, P.; Humphreys, L.; Anderson, C.N.G.; Grant, S.G.N. Network activity-independent coordinated gene expression program for synapse assembly. Proc. Natl. Acad. Sci. USA 2007, 104, 4658–4663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slutzky, M.W.; Jordan, L.R.; Lindberg, E.W.; Lindsay, K.E.; Miller, L.E. Decoding rat forelimb movement direction from epidural and intracortical field potentials. J. Neural Eng. 2011, 8, 036013. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, A.; Lee, K.H.; Garmestani, H.; Lim, C.P. (Eds.) Emerging Trends in Neuro Engineering and Neural Computation; Series in BioEngineering; Springer: Singapore, 2017; ISBN 978-981-10-3955-3. [Google Scholar]
- Du, Z.J.; Kolarcik, C.L.; Kozai, T.D.Y.; Luebben, S.D.; Sapp, S.A.; Zheng, X.S.; Nabity, J.A.; Cui, X.T. Ultrasoft microwire neural electrodes improve chronic tissue integration. Acta Biomater. 2017, 53, 46–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferguson, J.E.; Boldt, C.; Redish, A.D. Creating low-impedance tetrodes by electroplating with additives. Sens. Actuators Phys. 2009, 156, 388–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desai, S.A.; Rolston, J.D.; Guo, L.; Potter, S.M. Improving Impedance of Implantable Microwire Multi-Electrode Arrays by Ultrasonic Electroplating of Durable Platinum Black. Front. Neuroeng. 2010, 3. [Google Scholar] [CrossRef] [PubMed]
- Berger, H. Über das elektrenkephalogramm des menschen. Arch. Psychiatr Nervenkr 1929, 87, 527–570. [Google Scholar] [CrossRef]
- Williams, D.; Parsons-Smith, G. The spontaneous electrical activity of the human thalamus. Brain 1949, 72, 450–482. [Google Scholar] [CrossRef] [PubMed]
- Hubel, D.H.; Wiesel, T.N. Receptive fields of single neurones in the cat’s striate cortex. J. Physiol. 1959, 148, 574–591. [Google Scholar] [CrossRef] [PubMed]
- Marg, E.; Adams, J.E. Indwelling multiple micro-electrodes in the brain. Electroencephalogr. Clin. Neurophysiol. 1967, 23, 277–280. [Google Scholar] [CrossRef]
- Bertrand, G.; Jasper, H. Microelectrode Recording of Unit Activity in the Human Thalamus. Stereotact. Funct. Neurosurg. 1965, 26, 205–208. [Google Scholar] [CrossRef]
- Wise, K.D.; Angell, J.B.; Starr, A. An Integrated-Circuit Approach to Extracellular Microelectrodes. IEEE Trans. Biomed. Eng. 1970, BME-17, 238–247. [Google Scholar] [CrossRef]
- Krüger, J.; Bach, M. Simultaneous recording with 30 microelectrodes in monkey visual cortex. Exp. Brain Res. 1981, 41, 191–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halgren, E.; Babb, T.L.; Crandall, P.H. Activity of human hippocampal formation and amygdala neurons during memory testing. Electroencephalogr. Clin. Neurophysiol. 1978, 45, 585–601. [Google Scholar] [CrossRef]
- Georgopoulos, A.; Schwartz, A.; Kettner, R. Neuronal population coding of movement direction. Science 1986, 233, 1416–1419. [Google Scholar] [CrossRef] [PubMed]
- Ojemann, G.A.; Creutzfeldt, O.; Lettich, E.; Haglund, M.M. Neuronal activity in human lateral temporal cortex related to short-term verbal memory, naming and reading. Brain 1988, 111, 1383–1403. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.K.; Jones, K.E.; Huber, R.J.; Horch, K.W.; Normann, R.A. A silicon-based, three-dimensional neural interface: Manufacturing processes for an intracortical electrode array. IEEE Trans. Biomed. Eng. 1991, 38, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Carbon-Nanotube-Modified Electrodes for Highly Efficient Acute Neural Recording—Shin—2014—Advanced Healthcare Materials—Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/epdf/10.1002/adhm.201300183 (accessed on 8 July 2018).
- Gray, C.M.; Maldonado, P.E.; Wilson, M.; McNaughton, B. Tetrodes markedly improve the reliability and yield of multiple single-unit isolation from multi-unit recordings in cat striate cortex. J. Neurosci. Methods 1995, 63, 43–54. [Google Scholar] [CrossRef]
- Pei, W.; Zhao, H.; Zhao, S.; Fang, X.; Chen, S.; Gui, Q.; Tang, R.; Chen, Y.; Hong, B.; Gao, X.; et al. Silicon-based wire electrode array for neural interfaces. J. Micromech. Microeng. 2014, 24, 095015. [Google Scholar] [CrossRef]
- Woldring, S.; Dirken, M.N. Spontaneous unit-activity in the superficial cortical layers. Acta Physiol. Pharmacol. Neerlandica 1950, 1, 369–379. [Google Scholar]
- Dowben, R.M.; Rose, J.E. A Metal-Filled Microelectrodel. Science 1953, 118, 22–24. [Google Scholar] [CrossRef] [PubMed]
- Green, J.D. A Simple Microelectrode for recording from the Central Nervous System. Nature 1958, 182, 962. [Google Scholar] [CrossRef] [PubMed]
- Dymond, A.M.; Kaechele, L.E.; Jurist, J.M.; Crandall, P.H. Brain tissue reaction to some chronically implanted metals. J. Neurosurg. 1970, 33, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Wolbarsht, M.L.; MacNichol, E.F.; Wagner, H.G. Glass Insulated Platinum Microelectrode. Science 1960, 132, 1309–1310. [Google Scholar] [CrossRef] [PubMed]
- Abeles, M.; Goldstein, M.H. Multispike train analysis. Proc. IEEE 1977, 65, 762–773. [Google Scholar] [CrossRef]
- Prasad, A.; Xue, Q.-S.; Sankar, V.; Nishida, T.; Shaw, G.; Streit, W.J.; Sanchez, J.C. Comprehensive characterization and failure modes of tungsten microwire arrays in chronic neural implants. J. Neural Eng. 2012, 9, 056015. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.C.; Rennaker, R.L.; Kipke, D.R. Long-term neural recording characteristics of wire microelectrode arrays implanted in cerebral cortex. Brain Res. Protoc. 1999, 4, 303–313. [Google Scholar] [CrossRef]
- Krüger, J.; Caruana, F.; Volta, R.D.; Rizzolatti, G. Seven Years of Recording from Monkey Cortex with a Chronically Implanted Multiple Microelectrode. Front. Neuroeng. 2010, 3. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, T.; Kawai, K.; Uno, T.; Kunii, N.; Miyakawa, N.; Usami, K.; Kawasaki, K.; Hasegawa, I.; Saito, N. Simultaneous Recording of Single-Neuron Activities and Broad-Area Intracranial Electroencephalography: Electrode Design and Implantation Procedure. Oper. Neurosurg. 2013, 73, ons146–ons154. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.P.; Rajdev, P.; Ellison, C.; Irazoqui, P.P. Toward a comparison of microelectrodes for acute and chronic recordings. Brain Res. 2009, 1282, 183–200. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, C.W.; Reswick, J.B. A Percutaneous Wire Electrode for Chronic Research Use. IEEE Trans. Biomed. Eng. 1975, BME-22, 429–432. [Google Scholar] [CrossRef]
- Chen, R.; Canales, A.; Anikeeva, P. Neural recording and modulation technologies. Nat. Rev. Mater. 2017, 2. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Q.; Li, Y.; Wang, Y.; Zhu, J.; Zhang, S.; Zheng, X. Long-term decoding stability of local field potentials from silicon arrays in primate motor cortex during a 2D center out task. J. Neural Eng. 2014, 11, 036009. [Google Scholar] [CrossRef] [PubMed]
- House, P.A.; MacDonald, J.D.; Tresco, P.A.; Normann, R.A. Acute microelectrode array implantation into human neocortex: Preliminary technique and histological considerations. Neurosurg. Focus 2006, 20, 1–4. [Google Scholar] [CrossRef]
- Normann, R.A.; Maynard, E.M.; Rousche, P.J.; Warren, D.J. A neural interface for a cortical vision prosthesis. Vis. Res. 1999, 39, 2577–2587. [Google Scholar] [CrossRef] [Green Version]
- Kindlundh, M.; Norlin, P.; Hofmann, U.G. A neural probe process enabling variable electrode configurations. Sens. Actuators B Chem. 2004, 102, 51–58. [Google Scholar] [CrossRef]
- Bérces, Z.; Tóth, K.; Márton, G.; Pál, I.; Kováts-Megyesi, B.; Fekete, Z.; Ulbert, I.; Pongrácz, A. Neurobiochemical changes in the vicinity of a nanostructured neural implant. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.K.; Park, D.J.; Skousen, J.L.; Hess-Dunning, A.E.; Tyler, D.J.; Rowan, S.J.; Weder, C.; Capadona, J.R. Mechanically-compliant intracortical implants reduce the neuroinflammatory response. J. Neural Eng. 2014, 11, 056014. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Yen, S.-C.; Xue, N.; Sun, T.; Tsang, W.M.; Zhang, S.; Liao, L.-D.; Thakor, N.V.; Lee, C. Ultra-thin flexible polyimide neural probe embedded in a dissolvable maltose-coated microneedle. J. Micromech. Microeng. 2014, 24, 065015. [Google Scholar] [CrossRef]
- Hsu, J.; Rieth, L.; Normann, R.A.; Tathireddy, P.; Solzbacher, F. Encapsulation of an Integrated Neural Interface Device with Parylene C. IEEE Trans. Biomed. Eng. 2009, 56, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Altuna, A.; Bellistri, E.; Cid, E.; Aivar, P.; Gal, B.; Berganzo, J.; Gabriel, G.; Guimerà, A.; Villa, R.; Fernández, L.J.; et al. SU-8 based microprobes for simultaneous neural depth recording and drug delivery in the brain. Lab Chip 2013, 13, 1422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitale, F.; Vercosa, D.G.; Rodriguez, A.V.; Pamulapati, S.S.; Seibt, F.; Lewis, E.; Yan, J.S.; Badhiwala, K.; Adnan, M.; Royer-Carfagni, G.; et al. Fluidic Microactuation of Flexible Electrodes for Neural Recording. Nano Lett. 2018, 18, 326–335. [Google Scholar] [CrossRef] [PubMed]
- John, J.; Li, Y.; Zhang, J.; Loeb, J.A.; Xu, Y. Microfabrication of 3D neural probes with combined electrical and chemical interfaces. J. Micromech. Microeng. 2011, 21, 105011. [Google Scholar] [CrossRef]
- Wu, F.; Im, M.; Yoon, E. A flexible fish-bone-shaped neural probe strengthened by biodegradable silk coating for enhanced biocompatibility. In Proceedings of the 16th International Solid-State Sensors, Actuators and Microsystems Conference, Beijing, China, 5–9 June 2011; pp. 966–969. [Google Scholar]
- Lu, Y.; Lyu, H.; Richardson, A.G.; Lucas, T.H.; Kuzum, D. Flexible Neural Electrode Array Based-on Porous Graphene for Cortical Microstimulation and Sensing. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Son, Y.; Kim, J.; Lee, C.J.; Yoon, E.-S.; Cho, I.-J. A multichannel neural probe with embedded microfluidic channels for simultaneous in vivo neural recording and drug delivery. Lab Chip 2015, 15, 1590–1597. [Google Scholar] [CrossRef] [PubMed]
- Kuo, J.T.W.; Kim, B.J.; Hara, S.A.; Lee, C.D.; Gutierrez, C.A.; Hoang, T.Q.; Meng, E. Novel flexible Parylene neural probe with 3D sheath structure for enhancing tissue integration. Lab Chip 2013, 13, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Fekete, Z.; Pálfi, E.; Márton, G.; Handbauer, M.; Bérces, Z.; Ulbert, I.; Pongrácz, A.; Négyessy, L. Combined in vivo recording of neural signals and iontophoretic injection of pathway tracers using a hollow silicon microelectrode. Sens. Actuators B Chem. 2016, 236, 815–824. [Google Scholar] [CrossRef] [Green Version]
- Boehler, C.; Kleber, C.; Martini, N.; Xie, Y.; Dryg, I.; Stieglitz, T.; Hofmann, U.G.; Asplund, M. Actively controlled release of dexamethasone from neural microelectrodes in a chronic in vivo study. Biomaterials 2017, 129, 176–187. [Google Scholar]
- Huffman, M.L.; Venton, B.J. Carbon-fiber microelectrodes for in vivo applications. Analyst 2009, 134, 18–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruther, P.; Paul, O. New approaches for CMOS-based devices for large-scale neural recording. Curr. Opin. Neurobiol. 2015, 32, 31–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horváth, D.; Fiáth, R.; Kerekes, B.P.; Dombovári, B.; Acsády, L.; Seidl, K.; Herwik, S.; Paul, O.; Ruther, P.; Neves, H.P.; et al. High channel count electrode system to investigate thalamocortical interactions. Procedia Comput. Sci. 2011, 7, 178–179. [Google Scholar] [CrossRef] [Green Version]
- Geddes, L.A.; Roeder, R. Criteria for the Selection of Materials for Implanted Electrodes. Ann. Biomed. Eng. 2003, 31, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Saha, R.; Jackson, N.; Patel, C.; Muthuswamy, J. Highly Doped Polycrystalline Silicon Microelectrodes Reduce Noise in Neuronal Recordings In Vivo. IEEE Trans. Neural Syst. Rehabil. Eng. 2010, 18, 489–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.-H.; Wiler, J.A.; Anderson, D.J.; Kipke, D.R.; Martin, D.C. Conducting polymers on hydrogel-coated neural electrode provide sensitive neural recordings in auditory cortex. Acta Biomater. 2010, 6, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, K.A.; Uram, J.D.; Yang, J.; Martin, D.C.; Kipke, D.R. Chronic neural recordings using silicon microelectrode arrays electrochemically deposited with a poly(3,4-ethylenedioxythiophene) (PEDOT) film. J. Neural Eng. 2006, 3, 59–70. [Google Scholar] [CrossRef] [PubMed]
- George, P.M.; Lyckman, A.W.; LaVan, D.A.; Hegde, A.; Leung, Y.; Avasare, R.; Testa, C.; Alexander, P.M.; Langer, R.; Sur, M. Fabrication and biocompatibility of polypyrrole implants suitable for neural prosthetics. Biomaterials 2005, 26, 3511–3519. [Google Scholar] [CrossRef] [PubMed]
- Dimaki, M.; Vazquez, P.; Olsen, M.H.; Sasso, L.; Rodriguez-Trujillo, R.; Vedarethinam, I.; Svendsen, W.E. Fabrication and Characterization of 3D Micro- and Nanoelectrodes for Neuron Recordings. Sensors 2010, 10, 10339–10355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, D.M.; Charkhkar, H.; John, C.S.; Rajendran, S.; Kang, T.; Reit, R.; Arreaga-Salas, D.; McHail, D.G.; Knaack, G.L.; Sloan, A.; et al. Design and demonstration of an intracortical probe technology with tunable modulus. J. Biomed. Mater. Res. A 2017, 105, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Jackson, N.; Sridharan, A.; Anand, S.; Baker, M.; Okandan, M.; Muthuswamy, J. Long-Term Neural Recordings Using MEMS Based Movable Microelectrodes in the Brain. Front. Neuroeng. 2010, 3. [Google Scholar] [CrossRef]
- Cham, J.G.; Branchaud, E.A.; Nenadic, Z.; Greger, B.; Andersen, R.A.; Burdick, J.W. Semi-Chronic Motorized Microdrive and Control Algorithm for Autonomously Isolating and Maintaining Optimal Extracellular Action Potentials. J. Neurophysiol. 2005, 93, 570–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehring, C.; Rickert, J.; Vaadia, E.; de Oliveira, S.C.; Aertsen, A.; Rotter, S. Inference of hand movements from local field potentials in monkey motor cortex. Nat. Neurosci. 2003, 6, 1253–1254. [Google Scholar] [CrossRef] [PubMed]
- Waldert, S.; Preissl, H.; Demandt, E.; Braun, C.; Birbaumer, N.; Aertsen, A.; Mehring, C. Hand Movement Direction Decoded from MEG and EEG. J. Neurosci. 2008, 28, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Waldert, S. Invasive vs. Non-Invasive Neuronal Signals for Brain-Machine Interfaces: Will One Prevail? Front. Neurosci. 2016, 10. [Google Scholar] [CrossRef] [PubMed]
- Waterstraat, G.; Burghoff, M.; Fedele, T.; Nikulin, V.; Scheer, H.J.; Curio, G. Non-invasive single-trial EEG detection of evoked human neocortical population spikes. NeuroImage 2015, 105, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Freeman, W.J.; Rogers, L.J.; Holmes, M.D.; Silbergeld, D.L. Spatial spectral analysis of human electrocorticograms including the alpha and gamma bands. J. Neurosci. Methods 2000, 95, 111–121. [Google Scholar] [CrossRef]
- Slutzky, M.W.; Jordan, L.R.; Krieg, T.; Chen, M.; Mogul, D.J.; Miller, L.E. Optimal Spacing of Surface Electrode Arrays for Brain Machine Interface Applications. J. Neural Eng. 2010, 7, 26004. [Google Scholar] [CrossRef] [PubMed]
- Freeman, W.J.; Holmes, M.D.; Burke, B.C.; Vanhatalo, S. Spatial spectra of scalp EEG and EMG from awake humans. Clin. Neurophysiol. 2003, 114, 1053–1068. [Google Scholar] [CrossRef] [Green Version]
- Ball, T.; Kern, M.; Mutschler, I.; Aertsen, A.; Schulze-Bonhage, A. Signal quality of simultaneously recorded invasive and non-invasive EEG. NeuroImage 2009, 46, 708–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coenen, A.; Fine, E.; Zayachkivska, O. Adolf Beck: A forgotten pioneer in electroencephalography. J. Hist. Neurosci. 2014, 23, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Haas, L. Hans Berger (1873–1941), Richard Caton (1842–1926), and electroencephalography. J. Neurol. Neurosurg. Psychiatry 2003, 74, 9. [Google Scholar] [CrossRef] [PubMed]
- Bronzino, J.D. The Biomedical Engineering Handbook, Second Edition, Two Volume Set; CRC Press: Boca Raton, FL, USA, 1999; ISBN 978-1-4200-4951-0. [Google Scholar]
- Bozinovski, S.; Sestakov, M.; Bozinovska, L. Using EEG alpha rhythm to control a mobile robot. In Proceedings of the 10th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, New Orleans, LA, USA, 4–7 November 1988. [Google Scholar] [CrossRef]
- Millan, J.R.; Renkens, F.; Mourino, J.; Gerstner, W. Noninvasive brain-actuated control of a mobile robot by human EEG. IEEE Trans. Biomed. Eng. 2004, 51, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Shedeed, H.A.; Issa, M.F.; El-sayed, S.M. Brain EEG signal processing for controlling a robotic arm. In Proceedings of the 2013 8th International Conference on Computer Engineering Systems (ICCES), Cairo, Egypt, 26–27 November 2013; pp. 152–157. [Google Scholar]
- Tallgren, P.; Vanhatalo, S.; Kaila, K.; Voipio, J. Evaluation of commercially available electrodes and gels for recording of slow EEG potentials. Clin. Neurophysiol. 2005, 116, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Nunez, P.L.; Srinivasan, R. Electric Fields of the Brain: The Neurophysics of EEG, 2nd ed.; Oxford University Press: Oxford, UK; New York, NY, USA, 2005; ISBN 978-0-19-505038-7. [Google Scholar]
- Griss, P.; Enoksson, P.; Tolvanen-Laakso, H.K.; Merilainen, P.; Ollmar, S.; Stemme, G. Micromachined electrodes for biopotential measurements. J. Microelectromech. Syst. 2001, 10, 10–16. [Google Scholar] [CrossRef]
- Chiou, J.-C.; Ko, L.-W.; Lin, C.-T.; Hong, C.-T.; Jung, T.-P.; Liang, S.-F.; Jeng, J.-L. Using novel MEMS EEG sensors in detecting drowsiness application. In Proceedings of the 2006 IEEE Biomedical Circuits and Systems Conference, London, UK, 29 November–1 December 2006; pp. 33–36. [Google Scholar]
- Ruffini, G.; Dunne, S.; Fuentemilla, L.; Grau, C.; Farres, E.; Marco-Pallares, J.; Watts, P.C.P.; Silva, S.R.P. First human trials of a dry electrophysiology sensor using a carbon nanotube array interface. Sens. Actuators A Phys. 2008, 144, 275–279. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-H.; de Beeck, M.O.; Vanderheyden, L.; Carrette, E.; Mihajlović, V.; Vanstreels, K.; Grundlehner, B.; Gadeyne, S.; Boon, P.; Van Hoof, C. Soft, comfortable polymer dry electrodes for high quality ECG and EEG recording. Sensors 2014, 14, 23758–23780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvo, P.; Raedt, R.; Carrette, E.; Schaubroeck, D.; Vanfleteren, J.; Cardon, L. A 3D printed dry electrode for ECG/EEG recording. Sens. Actuators Phys. 2012, 174, 96–102. [Google Scholar] [CrossRef]
- Grozea, C.; Voinescu, C.D.; Fazli, S. Bristle-sensors—Low-cost flexible passive dry EEG electrodes for neurofeedback and BCI applications. J. Neural Eng. 2011, 8, 025008. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.-L.; Liu, J.-Q.; Dong, Y.-Z.; Yang, B.; Chen, X.; Yang, C.-S. Parylene-based flexible dry electrode for bioptential recording. Sens. Actuators B Chem. 2016, 231, 1–11. [Google Scholar] [CrossRef]
- Yu, Y.H.; Lu, S.W.; Liao, L.D.; Lin, C.T. Design, Fabrication, and Experimental Validation of Novel Flexible Silicon-Based Dry Sensors for Electroencephalography Signal Measurements. IEEE J. Transl. Eng. Health Med. 2014, 2, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Leuthardt, E.C.; Schalk, G.; Wolpaw, J.R.; Ojemann, J.G.; Moran, D.W. A brain-computer interface using electrocorticographic signals in humans. J. Neural Eng. 2004, 1, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Hinterberger, T.; Widman, G.; Lal, T.N.; Hill, J.; Tangermann, M.; Rosenstiel, W.; Schölkopf, B.; Elger, C.; Birbaumer, N. Voluntary brain regulation and communication with electrocorticogram signals. Epilepsy Behav. 2008, 13, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Collinger, J.L.; Degenhart, A.D.; Tyler-Kabara, E.C.; Schwartz, A.B.; Moran, D.W.; Weber, D.J.; Wodlinger, B.; Vinjamuri, R.K.; Ashmore, R.C.; et al. An Electrocorticographic Brain Interface in an Individual with Tetraplegia. PLoS ONE 2013, 8, e55344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bullara, L.A.; Agnew, W.F.; Yuen, T.G.; Jacques, S.; Pudenz, R.H. Evaluation of electrode array material for neural prostheses. Neurosurgery 1979, 5, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Schalk, G.; Leuthardt, E.C. Brain-computer interfaces using electrocorticographic signals. IEEE Rev. Biomed. Eng. 2011, 4, 140–154. [Google Scholar] [CrossRef] [PubMed]
- Blakely, T.; Miller, K.J.; Zanos, S.P.; Rao, R.P.N.; Ojemann, J.G. Robust, long-term control of an electrocorticographic brain-computer interface with fixed parameters. Neurosurg. Focus 2009, 27, E13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kellis, S.S.; House, P.A.; Thomson, K.E.; Brown, R.; Greger, B. Human neocortical electrical activity recorded on nonpenetrating microwire arrays: Applicability for neuroprostheses. Neurosurg. Focus 2009, 27, E9. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Guvanasen, G.S.; Liu, X.; Tuthill, C.; Nichols, T.R.; DeWeerth, S.P. A PDMS-based integrated stretchable microelectrode array (isMEA) for neural and muscular surface interfacing. IEEE Trans. Biomed. Circuits Syst. 2013, 7, 1–10. [Google Scholar] [CrossRef]
- Rubehn, B.; Bosman, C.; Oostenveld, R.; Fries, P.; Stieglitz, T. A MEMS-based flexible multichannel ECoG-electrode array. J. Neural Eng. 2009, 6, 036003. [Google Scholar] [CrossRef] [PubMed]
- Toda, H.; Suzuki, T.; Sawahata, H.; Majima, K.; Kamitani, Y.; Hasegawa, I. Simultaneous recording of ECoG and intracortical neuronal activity using a flexible multichannel electrode-mesh in visual cortex. NeuroImage 2011, 54, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Garnier, F.; Hajlaoui, R.; Yassar, A.; Srivastava, P. All-Polymer Field-Effect Transistor Realized by Printing Techniques. Science 1994, 265, 1684–1686. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Feng, Y.; Dodabalapur, A.; Raju, V.R.; Lovinger, A.J. High-Performance Plastic Transistors Fabricated by Printing Techniques. Chem. Mater. 1997, 9, 1299–1301. [Google Scholar] [CrossRef]
- Reuss, R.H.; Chalamala, B.R.; Moussessian, A.; Kane, M.G.; Kumar, A.; Zhang, D.C.; Rogers, J.A.; Hatalis, M.; Temple, D.; Moddel, G.; et al. Macroelectronics: Perspectives on Technology and Applications. Proc. IEEE 2005, 93, 1239–1256. [Google Scholar] [CrossRef]
- Rogers, J.A.; Bao, Z.; Baldwin, K.; Dodabalapur, A.; Crone, B.; Raju, V.R.; Kuck, V.; Katz, H.; Amundson, K.; Ewing, J.; et al. Paper-like electronic displays: Large-area rubber-stamped plastic sheets of electronics and microencapsulated electrophoretic inks. Proc. Natl. Acad. Sci. USA 2001, 98, 4835–4840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gelinck, G.H.; Huitema, H.E.A.; van Veenendaal, E.; Cantatore, E.; Schrijnemakers, L.; van der Putten, J.B.P.H.; Geuns, T.C.T.; Beenhakkers, M.; Giesbers, J.B.; Huisman, B.-H.; et al. Flexible active-matrix displays and shift registers based on solution-processed organic transistors. Nat. Mater. 2004, 3, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.A.; Someya, T.; Huang, Y. Materials and Mechanics for Stretchable Electronics. Science 2010, 327, 1603–1607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.-H.; Lu, N.; Ma, R.; Kim, Y.-S.; Kim, R.-H.; Wang, S.; Wu, J.; Won, S.M.; Tao, H.; Islam, A.; et al. Epidermal Electronics. Science 2011, 333, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yeo, W.-H.; Liu, Y.; Rogers, J.A. Epidermal differential impedance sensor for conformal skin hydration monitoring. Biointerphases 2012, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Yeo, W.-H.; Kim, Y.-S.; Lee, J.; Ameen, A.; Shi, L.; Li, M.; Wang, S.; Ma, R.; Jin, S.H.; Kang, Z.; et al. Multifunctional Epidermal Electronics Printed Directly Onto the Skin. Adv. Mater. 2013, 25, 2773–2778. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Viventi, J.; Amsden, J.J.; Xiao, J.; Vigeland, L.; Kim, Y.-S.; Blanco, J.A.; Panilaitis, B.; Frechette, E.S.; Contreras, D.; et al. Dissolvable films of silk fibroin for ultrathin conformal bio-integrated electronics. Nat. Mater. 2010, 9, 511–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viventi, J.; Kim, D.-H.; Vigeland, L.; Frechette, E.S.; Blanco, J.A.; Kim, Y.-S.; Avrin, A.E.; Tiruvadi, V.R.; Hwang, S.-W.; Vanleer, A.C.; et al. Flexible, foldable, actively multiplexed, high-density electrode array for mapping brain activity in vivo. Nat. Neurosci. 2011, 14, 1599–1605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, B.; Akhtar, A.; Liu, Y.; Chen, H.; Yeo, W.-H.; Park, S.I.; Boyce, B.; Kim, H.; Yu, J.; Lai, H.-Y.; et al. An Epidermal Stimulation and Sensing Platform for Sensorimotor Prosthetic Control, Management of Lower Back Exertion, and Electrical Muscle Activation. Adv. Mater. 2016, 28, 4462–4471. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Ghaffari, R.; Lu, N.; Wang, S.; Lee, S.P.; Keum, H.; D’Angelo, R.; Klinker, L.; Su, Y.; Lu, C.; et al. Electronic sensor and actuator webs for large-area complex geometry cardiac mapping and therapy. Proc. Natl. Acad. Sci. USA 2012, 109, 19910–19915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viventi, J.; Kim, D.-H.; Moss, J.D.; Kim, Y.-S.; Blanco, J.A.; Annetta, N.; Hicks, A.; Xiao, J.; Huang, Y.; Callans, D.J.; et al. A Conformal, Bio-interfaced Class of Silicon Electronics for Mapping Cardiac Electrophysiology. Sci. Transl. Med. 2010, 2, 24ra22. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Lu, N.; Ghaffari, R.; Kim, Y.-S.; Lee, S.P.; Xu, L.; Wu, J.; Kim, R.-H.; Song, J.; Liu, Z.; et al. Materials for multifunctional balloon catheters with capabilities in cardiac electrophysiological mapping and ablation therapy. Nat. Mater. 2011, 10, 316–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.-H.; Wang, S.; Keum, H.; Ghaffari, R.; Kim, Y.-S.; Tao, H.; Panilaitis, B.; Li, M.; Kang, Z.; Omenetto, F.; et al. Thin, Flexible Sensors and Actuators as ‘Instrumented’ Surgical Sutures for Targeted Wound Monitoring and Therapy. Small 2012, 8, 3263–3268. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.T.; Chen, Y.C.; Huang, T.Y.; Chiu, T.T.; Ko, L.W.; Liang, S.F.; Hsieh, H.Y.; Hsu, S.H.; Duann, J.R. Development of Wireless Brain Computer Interface With Embedded Multitask Scheduling and its Application on Real-Time Driver’s Drowsiness Detection and Warning. IEEE Trans. Biomed. Eng. 2008, 55, 1582–1591. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.T.; Ko, L.W.; Chiou, J.C.; Duann, J.R.; Huang, R.S.; Liang, S.F.; Chiu, T.W.; Jung, T.P. Noninvasive Neural Prostheses Using Mobile and Wireless EEG. Proc. IEEE 2008, 96, 1167–1183. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.M.; Byeon, H.J.; Lee, J.H.; Baek, D.H.; Lee, K.H.; Hong, J.S.; Lee, S.-H. Self-adhesive epidermal carbon nanotube electronics for tether-free long-term continuous recording of biosignals. Sci. Rep. 2014, 4, 6074. [Google Scholar] [CrossRef] [PubMed]
- Page, N.G.; Gresty, M.A. Motorist’s vestibular disorientation syndrome. J. Neurol. Neurosurg. Psychiatry 1985, 48, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Norton, J.J.S.; Lee, D.S.; Lee, J.W.; Lee, W.; Kwon, O.; Won, P.; Jung, S.-Y.; Cheng, H.; Jeong, J.-W.; Akce, A.; et al. Soft, curved electrode systems capable of integration on the auricle as a persistent brain–computer interface. Proc. Natl. Acad. Sci. USA 2015, 112, 3920–3925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, S.H.; Lu, H.M.; Cauller, L.; Romero-Ortega, M.I.; Lee, J.B.; Hughes, G.A. Biocompatible SU-8-Based Microprobes for Recording Neural Spike Signals From Regenerated Peripheral Nerve Fibers. IEEE Sens. J. 2008, 8, 1830–1836. [Google Scholar] [CrossRef]
- Altuna, A.; Menendez de la Prida, L.; Bellistri, E.; Gabriel, G.; Guimerá, A.; Berganzo, J.; Villa, R.; Fernández, L.J. SU-8 based microprobes with integrated planar electrodes for enhanced neural depth recording. Biosens. Bioelectron. 2012, 37, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.-M.; Im, C.; Lee, W.R. Plateau-Shaped Flexible Polymer Microelectrode Array for Neural Recording. Polymers 2017, 9, 690. [Google Scholar] [CrossRef]
- Lee, K.-K.; He, J.; Singh, A.; Massia, S.; Ehteshami, G.; Kim, B.; Raupp, G. Polyimide-based intracortical neural implant with improved structural stiffness. J. Micromech. Microeng. 2004, 14, 32. [Google Scholar] [CrossRef]
- Shin, J.H.; Kim, G.B.; Lee, E.J.; An, T.; Shin, K.; Lee, S.E.; Choi, W.; Lee, S.; Latchoumane, C.; Shin, H.-S.; et al. Carbon-Nanotube-Modified Electrodes for Highly Efficient Acute Neural Recording. Adv. Healthc. Mater. 2014, 3, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Hwang, G.-T.; Im, D.; Lee, S.E.; Lee, J.; Koo, M.; Park, S.Y.; Kim, S.; Yang, K.; Kim, S.J.; Lee, K.; et al. In Vivo Silicon-Based Flexible Radio Frequency Integrated Circuits Monolithically Encapsulated with Biocompatible Liquid Crystal Polymers. ACS Nano 2013, 7, 4545–4553. [Google Scholar] [CrossRef] [PubMed]
- Hess, A.E.; Capadona, J.R.; Shanmuganathan, K.; Hsu, L.; Rowan, S.J.; Weder, C.; Tyler, D.J.; Zorman, C.A. Development of a stimuli-responsive polymer nanocomposite toward biologically optimized, MEMS-based neural probes. J. Micromech. Microeng. 2011, 21, 054009. [Google Scholar] [CrossRef] [Green Version]
- Jackson, A.; Fetz, E.E. Compact Movable Microwire Array for Long-Term Chronic Unit Recording in Cerebral Cortex of Primates. J. Neurophysiol. 2007, 98, 3109–3118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aflalo, T.; Kellis, S.; Klaes, C.; Lee, B.; Shi, Y.; Pejsa, K.; Shanfield, K.; Hayes-Jackson, S.; Aisen, M.; Heck, C.; et al. Decoding motor imagery from the posterior parietal cortex of a tetraplegic human. Science 2015, 348, 906–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perge, J.A.; Homer, M.L.; Malik, W.Q.; Cash, S.; Eskandar, E.; Friehs, G.; Donoghue, J.P.; Hochberg, L.R. Intra-day signal instabilities affect decoding performance in an intracortical neural interface system. J. Neural Eng. 2013, 10, 036004. [Google Scholar] [CrossRef] [PubMed]
- Bensmaia, S.J.; Miller, L.E. Restoring sensorimotor function through intracortical interfaces: Progress and looming challenges. Nat. Rev. Neurosci. 2014, 15, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.-M.; Hong, G.; Zhou, T.; Schuhmann, T.G.; Viveros, R.D.; Lieber, C.M. Stable long-term chronic brain mapping at the single-neuron level. Nat. Methods 2016, 13, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Bickford, R.G.; Fischer, G.; Sayre, G.P. Histologic changes in the cat’s brain after introduction of metallic and plastic coated wire used in electro-encephalography. Proc. Staff Meet. Mayo Clin. 1957, 32, 14–21. [Google Scholar] [PubMed]
- Kim, R.; Hong, N.; Nam, Y. Gold nanograin microelectrodes for neuroelectronic interfaces. Biotechnol. J. 2013, 8, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Kang, G.; Nam, Y.; Choi, Y.-K. Surface-modified microelectrode array with flake nanostructure for neural recording and stimulation. Nanotechnology 2010, 21, 085303. [Google Scholar] [CrossRef] [PubMed]
- The Fabrication of Low-Impedance Nanoporous Gold Multiple-Electrode Arrays for Neural Electrophysiology Studies—IOPscience. Available online: http://iopscience.iop.org/article/10.1088/0957-4484/21/12/125504/meta (accessed on 6 July 2018).
- Czeschik, A.; Offenhäusser, A.; Wolfrum, B. Fabrication of MEA-based nanocavity sensor arrays for extracellular recording of action potentials. Phys. Status Solidi A 2014, 211, 1462–1466. [Google Scholar] [CrossRef]
- Xie, C.; Lin, Z.; Hanson, L.; Cui, Y.; Cui, B. Intracellular recording of action potentials by nanopillar electroporation. Nat. Nanotechnol. 2012, 7, 185–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathieson, K.; Kachiguine, S.; Adams, C.; Cunningham, W.; Gunning, D.; O’Shea, V.; Smith, K.M.; Chichilnisky, E.J.; Litke, A.M.; Sher, A.; et al. Large-area microelectrode arrays for recording of neural signals. IEEE Trans. Nucl. Sci. 2004, 51, 2027–2031. [Google Scholar] [CrossRef]
- Jun, S.B.; Hynd, M.R.; Dowell-Mesfin, N.; Smith, K.L.; Turner, J.N.; Shain, W.; Kim, S.J. Low-density neuronal networks cultured using patterned poly-l-lysine on microelectrode arrays. J. Neurosci. Methods 2007, 160, 317–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.; Song, Y.J.; Boo, H.; Chung, T.D. Nanoporous Pt Microelectrode for Neural Stimulation and Recording: In Vitro Characterization. J. Phys. Chem. C 2010, 114, 8721–8726. [Google Scholar] [CrossRef]
- Takayama, Y.; Moriguchi, H.; Kotani, K.; Suzuki, T.; Mabuchi, K.; Jimbo, Y. Network-wide integration of stem cell-derived neurons and mouse cortical neurons using microfabricated co-culture devices. Biosystems 2012, 107, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Beder, O.E.; Eade, G. An investigation of tissue tolerance to titanium metal implants in dogs. Surgery 1956, 39, 470–473. [Google Scholar] [PubMed]
- Robinson, F.R.; Johnson, M.T. Histopathological studies of tissue reactions to various metals implanted in cat brains. ASD Tech. Rep. 1961, 61, 13. [Google Scholar] [PubMed]
- Zátonyi, A.; Borhegyi, Z.; Srivastava, M.; Cserpán, D.; Somogyvári, Z.; Kisvárday, Z.; Fekete, Z. Functional brain mapping using optical imaging of intrinsic signals and simultaneous high-resolution cortical electrophysiology with a flexible, transparent microelectrode array. Sens. Actuators B Chem. 2018, 273, 519–526. [Google Scholar] [CrossRef]
- Park, D.-W.; Brodnick, S.K.; Ness, J.P.; Atry, F.; Krugner-Higby, L.; Sandberg, A.; Mikael, S.; Richner, T.J.; Novello, J.; Kim, H.; et al. Fabrication and utility of a transparent graphene neural electrode array for electrophysiology, in vivo imaging, and optogenetics. Nat. Protoc. 2016, 11, 2201–2222. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.D.; Cogan, S.F.; Nguyen, T.H.; Rauh, R.D. Electrodeposited iridium oxide for neural stimulation and recording electrodes. IEEE Trans. Neural Syst. Rehabil. Eng. 2001, 9, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.B.; Li, G.; Sun, X.N.; Zhu, Z.H.; Jin, Q.H.; Zhao, J.L.; Ren, Q.S. Integration of Au Nanorods with Flexible Thin-Film Microelectrode Arrays for Improved Neural Interfaces. J. Microelectromech. Syst. 2009, 18, 88–96. [Google Scholar] [CrossRef]
- Keefer, E.W.; Botterman, B.R.; Romero, M.I.; Rossi, A.F.; Gross, G.W. Carbon nanotube coating improves neuronal recordings. Nat. Nanotechnol. 2008, 3, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Gabay, T.; Ben-David, M.; Kalifa, I.; Sorkin, R.; Abrams, Z.R.; Ben-Jacob, E.; Hanein, Y. Electro-chemical and biological properties of carbon nanotube based multi-electrode arrays. Nanotechnology 2007, 18, 035201. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Fishman, H.A.; Dai, H.; Harris, J.S. Neural Stimulation with a Carbon Nanotube Microelectrode Array. Nano Lett. 2006, 6, 2043–2048. [Google Scholar] [CrossRef] [PubMed]
- Ansaldo, A.; Castagnola, E.; Maggiolini, E.; Fadiga, L.; Ricci, D. Superior Electrochemical Performance of Carbon Nanotubes Directly Grown on Sharp Microelectrodes. Available online: https://pubs.acs.org/doi/abs/10.1021/nn103445d (accessed on 6 July 2018).
- Kim, G.H.; Kim, K.; Nam, H.; Shin, K.; Choi, W.; Shin, J.H.; Lim, G. CNT-Au nanocomposite deposition on gold microelectrodes for improved neural recordings. Sens. Actuators B Chem. 2017, 252, 152–158. [Google Scholar] [CrossRef]






| Electrode Material | Young’s Modulus (GPa) | Toxicity | Reference |
|---|---|---|---|
| SU-8 | 2.0 | Non-toxic | [152,153] |
| PDMS | 0.00132–0.00297 | Non-toxic | [154] |
| PI | 2.5 | Non-toxic | [155] |
| Parylene C | 2.76 | Non-toxic | [75,156] |
| Electrode Material | Electrical Conductivity (at 20 °C, S/m) | Toxicity | Reference |
|---|---|---|---|
| Copper (Cu) | 5.96 × 107 | Toxic | [164] |
| Gold (Au) | 4.10 × 107 | Non-toxic | [165,166,167] |
| Platinum (Pt) | 9.43 × 106 | Non-toxic | [168,169,170,171,172,173] |
| Silver (Ag) | 6.30 × 107 | Toxic | [58] |
| Titanium (Ti) | 2.38 × 106 | Non-toxic | [174] |
| Tungsten (W) | 1.79 × 107 | Non-toxic | [175] |
| Indium-tin-oxide | 1.3 × 104 | Non-toxic | [176] |
| Graphene | 1.0 × 102 | Non-toxic | [177] |
| Material | Surface Modification Technique | References |
|---|---|---|
| Inorganic | Metal coating | [170,178] |
| Metal nanostructure coating | [165,167,169,179] | |
| Organic | Carbon based material coating | [156,180,181,182,183] |
| Hybrid | Composite material coating | [184] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.H.; Kim, K.; Lee, E.; An, T.; Choi, W.; Lim, G.; Shin, J.H. Recent Progress on Microelectrodes in Neural Interfaces. Materials 2018, 11, 1995. https://doi.org/10.3390/ma11101995
Kim GH, Kim K, Lee E, An T, Choi W, Lim G, Shin JH. Recent Progress on Microelectrodes in Neural Interfaces. Materials. 2018; 11(10):1995. https://doi.org/10.3390/ma11101995
Chicago/Turabian StyleKim, Geon Hwee, Kanghyun Kim, Eunji Lee, Taechang An, WooSeok Choi, Geunbae Lim, and Jung Hwal Shin. 2018. "Recent Progress on Microelectrodes in Neural Interfaces" Materials 11, no. 10: 1995. https://doi.org/10.3390/ma11101995
APA StyleKim, G. H., Kim, K., Lee, E., An, T., Choi, W., Lim, G., & Shin, J. H. (2018). Recent Progress on Microelectrodes in Neural Interfaces. Materials, 11(10), 1995. https://doi.org/10.3390/ma11101995





