Influence of the Heat Treatment on the Particles Size and on the Crystalline Phase of TiO2 Synthesized by the Sol-Gel Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
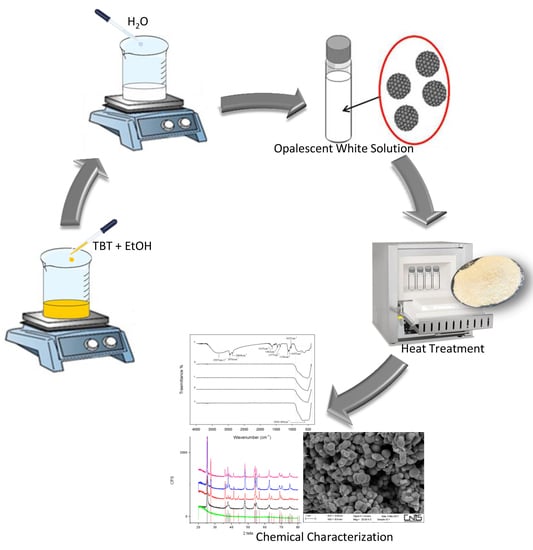
2.2. Sol–Gel Synthesis
2.3. Characterization
2.3.1. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.2. Scanning Electron Microscopy (SEM) and X-ray Diffraction (XRD)
3. Results
3.1. Nanoparticle Synthesis
3.2. Chemical Characterization
3.2.1. XRD Analysis
3.2.2. FTIR Analysis
3.2.3. SEM Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rahman, I.A.; Padavettan, V. Synthesis of silica nanoparticles by sol-gel: Size-dependent properties, surface modification, and applications in silica-polymer nanocomposites—A review. J. Nanomater. 2012, 2012, 8. [Google Scholar] [CrossRef]
- Ognibene, G.; Cristaldi, D.; Fiorenza, R.; Blanco, I.; Cicala, G.; Scire, S.; Fragala, M. Photoactivity of hierarchically nanostructured ZnO–PES fibre mats for water treatments. RSC Adv. 2016, 6, 42778–42785. [Google Scholar] [CrossRef]
- Catauro, M.; Pagliuca, C.; Lisi, L.; Ruoppolo, G. Synthesis of alkoxide-derived V-Nb catalysts prepared by sol-gel route. Thermochim. Acta 2002, 381, 65–72. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Papale, F.; Ferrara, C.; Mustarelli, P. Silica-polyethylene glycol hybrids synthesized by sol-gel: Biocompatibility improvement of titanium implants by coating. Mater. Sci. Eng. C 2015, 55, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Dell’Era, A.; Mura, F.; Pasquali, M.; Pozio, A.; Zaza, F. Synthesis and characterization of TiO2 nanotubes as anodic material in lithium-ion batteries. Nuovo Cimento Soc. Ital. Fis. C 2013, 36, 65–72. [Google Scholar]
- Scaramuzzo, F.A.; Pasqualia, M.; Muraa, F.; Poziob, A.; Dell’Eraa, A.; Curullic, A. TiO2 nanotubes photo-anode: An innovative cell design. Chem. Eng. 2014, 41. [Google Scholar] [CrossRef]
- Kulkarni, M.; Mazare, A.; Gongadze, E.; Perutkova, Š.; Kralj-Iglič, V.; Milošev, I.; Schmuki, P.; Iglič, A.; Mozetič, M. Titanium nanostructures for biomedical applications. Nanotechnology 2015, 26, 062002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petković, J.; Žegura, B.; Stevanović, M.; Drnovšek, N.; Uskoković, D.; Novak, S.; Filipič, M. DNA damage and alterations in expression of DNA damage responsive genes induced by TiO2 nanoparticles in human hepatoma HepG2 cells. Nanotoxicology 2011, 5, 341–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stankic, S.; Suman, S.; Haque, F.; Vidic, J. Pure and multi metal oxide nanoparticles: Synthesis, antibacterial and cytotoxic properties. J. Nanobiotechnol. 2016, 14, 73. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, C.; Zhao, H.; Qu, S.; Li, X.; Li, Y. New developments of Ti-based alloys for biomedical applications. Materials 2014, 7, 1709–1800. [Google Scholar] [CrossRef] [PubMed]
- Park, E.-J.; Yi, J.; Chung, K.-H.; Ryu, D.-Y.; Choi, J.; Park, K. Oxidative stress and apoptosis induced by titanium dioxide nanoparticles in cultured BEAS-2B cells. Toxicol. Lett. 2008, 180, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Song, L.; Hu, X.; Liu, C.; Shi, J.; Wang, H.; Zhan, L.; Song, H. Inhibition of Epithelial–Mesenchymal Transition and Tissue Regeneration by Waterborne Titanium Dioxide Nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 3449–3458. [Google Scholar] [CrossRef] [PubMed]
- Salata, O.V. Applications of nanoparticles in biology and medicine. J. Nanobiotechnol. 2004, 2, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taziwa, R.; Meyer, E. Fabrication of TiO2 Nanoparticles and Thin Films by Ultrasonic Spray Pyrolysis: Design and Optimization. In Pyrolysis; InTech: London, UK, 2017. [Google Scholar]
- Do Kim, K.; Kim, H.T. Synthesis of TiO2 nanoparticles by hydrolysis of TEOT and decrease of particle size using a two-stage mixed method. Powder Technol. 2001, 119, 164–172. [Google Scholar] [CrossRef]
- Kim, E.J.; Hahn, S.-H. Microstructure and photoactivity of titania nanoparticles prepared in nonionic W/O microemulsions. Mater. Sci. Eng. A 2001, 303, 24–29. [Google Scholar] [CrossRef]
- Vargas, M.A.; Rodríguez-Páez, J.E. Amorphous TiO2 nanoparticles: Synthesis and antibacterial capacity. J. Non-Cryst. Solids 2017, 459, 192–205. [Google Scholar] [CrossRef]
- Catauro, M.; Laudisio, G.; Costantini, A.; Fresa, R.; Branda, F. Low Temperature Synthesis, Structure and Bioactivity of 2CaO·3SiO2 Glass. J. Sol-Gel Sci. Technol. 1997, 10, 231–237. [Google Scholar] [CrossRef]
- Su, C.; Hong, B.-Y.; Tseng, C.-M. Sol–gel preparation and photocatalysis of titanium dioxide. Catal. Today 2004, 96, 119–126. [Google Scholar] [CrossRef]
- Macwan, D.; Dave, P.N.; Chaturvedi, S. A review on nano-TiO2 sol–gel type syntheses and its applications. J. Mater. Sci. 2011, 46, 3669–3686. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, Y.; Wang, J.; Guo, Q.; Li, J. Size-controlled synthesis of dispersed equiaxed amorphous TiO2 nanoparticles. Ceram. Int. 2015, 41, 9057–9062. [Google Scholar] [CrossRef]
- Rao, K.S.; El-Hami, K.; Kodaki, T.; Matsushige, K.; Makino, K. A novel method for synthesis of silica nanoparticles. J. Colloid Interface Sci. 2005, 289, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; Vejayakumaran, P.; Sipaut, C.; Ismail, J.; Bakar, M.A.; Adnan, R.; Chee, C. An optimized sol–gel synthesis of stable primary equivalent silica particles. Colloids Surf. A Physicochem. Eng. Asp. 2007, 294, 102–110. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Renella, R.A.; Papale, F. Sol-gel synthesis of SiO2–CaO–P2O5 glasses: Influence of the heat treatment on their bioactivity and biocompatibility. Ceram. Int. 2015, 41, 12578–12588. [Google Scholar] [CrossRef]
- Jin, C.; Tang, Y.; Yang, F.G.; Li, X.L.; Xu, S.; Fan, X.Y.; Huang, Y.Y.; Yang, Y.J. Cellular toxicity of TiO2 nanoparticles in anatase and rutile crystal phase. Biol. Trace Elem. Res. 2011, 141, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, S.; Yu, Q.; Yin, W. The effects of activated carbon supports on the structure and properties of TiO2 nanoparticles prepared by a sol–gel method. Appl. Surf. Sci. 2007, 253, 9254–9258. [Google Scholar] [CrossRef]
- Nguyen, K.; Garcia, A.; Sani, M.A.; Diaz, D.; Dubey, V.; Clayton, D.; Dal Poggetto, G.; Cornelius, F.; Payne, R.J.; Separovic, F.; et al. Interaction of N-terminal peptide analogues of the Na+, K+-ATPase with membranes. Biochim. Biophys. Acta 2018, 1860, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Dell’Era, A.; Vecchio Ciprioti, S. Synthesis, structural, spectroscopic and thermoanalytical study of sol-gel derived SiO2–CaO–P2O5 gel and ceramic materials. Thermochim. Acta 2016, 625, 20–27. [Google Scholar] [CrossRef]
- Vecchio Ciprioti, S.; Catauro, M. Synthesis, structural and thermal behavior study of four Ca-containing silicate gel-glasses. J. Therm. Anal. Calorim. 2016, 123, 2091–2101. [Google Scholar] [CrossRef]
- Catauro, M.; Renella, R.A.; Papale, F.; Vecchio Ciprioti, S. Investigation of bioactivity, biocompatibility and thermal behavior of sol-gel silica glass containing a high PEG percentage. Mater. Sci. Eng. C 2016, 61, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Vecchio Ciprioti, S.; Bollino, F.; Tranquillo, E.; Catauro, M. Synthesis, thermal behavior and physicochemical characterization of ZrO2/PEG inorganic/organic hybrid materials via sol–gel technique. J. Therm. Anal. Calorim. 2017, 130, 535–540. [Google Scholar] [CrossRef]
- Bogush, G.; Tracy, M.; Zukoski Iv, C. Preparation of monodisperse silica particles: Control of size and mass fraction. J. Non-Cryst. Solids 1988, 104, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Bogush, G.; Zukoski Iv, C. Studies of the kinetics of the precipitation of uniform silica particles through the hydrolysis and condensation of silicon alkoxides. J. Colloid Interface Sci. 1991, 142, 1–18. [Google Scholar] [CrossRef]
- Jafarzadeh, M.; Rahman, I.; Sipaut, C. Synthesis of silica nanoparticles by modified sol–gel process: The effect of mixing modes of the reactants and drying techniques. J. Sol-Gel Sci. Technol. 2009, 50, 328–336. [Google Scholar] [CrossRef]
- So, W.W.; Park, S.B.; Kim, K.J.; Moon, S.J. Phase transformation behavior at low temperature in hydrothermal treatment of stable and unstable titania sol. J. Colloid Interface Sci. 1997, 191, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Scaramuzzo, F.A.; Dell’Era, A.; Tarquini, G.; Caminiti, R.; Ballirano, P.; Pasquali, M. Phase Transition of TiO2 Nanotubes: An X-ray Study as a Function of Temperature. J. Phys. Chem. C 2017, 121, 24871–24876. [Google Scholar] [CrossRef]
- Picquart, M.; López, T.; Gómez, R.; Torres, E.; Moreno, A.; Garcia, J. Dehydration and crystallization process in sol-gel zirconia. J. Therm. Anal. Calorim. 2004, 76, 755–761. [Google Scholar] [CrossRef]
- Wachsman, E.; Henn, F.; Jiang, N.; Leezenberg, P.; Buchanan, R.; Frank, C.; Stevenson, D. Luminescence of anion vacancies and dopant-vacancy associates in stabilised Zirconia. In Proceedings of the International Conference on Science and Technology of Zirconia V, Melbourne, Australia, 16–21 August 1992. [Google Scholar]
- Yin, H.; Wada, Y.; Kitamura, T.; Kambe, S.; Murasawa, S.; Mori, H.; Sakata, T.; Yanagida, S. Hydrothermal synthesis of nanosized anatase and rutile TiO2 using amorphous phase TiO2. J. Mater. Chem. 2001, 11, 1694–1703. [Google Scholar] [CrossRef]
- Mardare, D.; Tasca, M.; Delibas, M.; Rusu, G. On the structural properties and optical transmittance of TiO2 rf sputtered thin films. Appl. Surf. Sci. 2000, 156, 200–206. [Google Scholar] [CrossRef]
- Wang, Y.-D.; Ma, C.-L.; Sun, X.-D.; Li, H.-D. Synthesis and characterization of amorphous TiO2 with wormhole-like framework mesostructure. J. Non-Cryst. Solids 2003, 319, 109–116. [Google Scholar] [CrossRef]
- Zeitler, V.A.; Brown, C.A. The Infrared Spectra of Some Ti–O–Si, Ti–O–Ti and Si–O–Si Compounds. J. Phys. Chem. 1957, 61, 1174–1177. [Google Scholar] [CrossRef]
- Farmer, V.C. Infrared Spectra of Minerals; Mineralogical Society: Chantilly, VA, USA, 1974. [Google Scholar]
- Gaber, A.; Abdel-Rahim, M.; Abdel-Latief, A.; Abdel-Salam, M.N. Influence of calcination temperature on the structure and porosity of nanocrystalline SnO2 synthesized by a conventional precipitation method. Int. J. Electrochem. Sci. 2014, 9, 81–95. [Google Scholar]
- Hao, R.; Jiang, B.; Li, M.; Xie, Y.; Fu, H. Fabrication of mixed-crystalline-phase spindle-like TiO2 for enhanced photocatalytic hydrogen production. Sci. China Mater. 2015, 58, 363–369. [Google Scholar] [CrossRef]
- Hanaor, D.A.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef]
- Jongprateep, O.; Puranasamriddhi, R.; Palomas, J. Nanoparticulate titanium dioxide synthesized by sol–gel and solution combustion techniques. Ceram. Int. 2015, 41, 1691. [Google Scholar] [CrossRef]
- Prasad, K.; Pinjari, D.; Pandit, A.; Mhaske, S. Synthesis of titanium dioxide by ultrasound assisted sol–gel technique: Effect of amplitude (power density) variation. Ultrason. Sonochem. 2010, 17, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Ghamsari, M.S.; Radiman, S.; Hamid, M.A.A.; Mahshid, S.; Rahmani, S. Room temperature synthesis of highly crystalline TiO2 nanoparticles. Mater. Lett. 2013, 92, 287–290. [Google Scholar] [CrossRef]
- Mahshid, S.; Askari, M.; Ghamsari, M.S.; Afshar, N.; Lahuti, S. Mixed-phase TiO2 nanoparticles preparation using sol–gel method. J. Alloys Compd. 2009, 478, 586–589. [Google Scholar] [CrossRef]
- Do Kim, K.; Kim, S.H.; Kim, H.T. Applying the Taguchi method to the optimization for the synthesis of TiO2 nanoparticles by hydrolysis of TEOT in micelles. Colloids Surf. A Physicochem. Eng. Asp. 2005, 254, 99–105. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, C.; Tian, H.; Wu, G.; Li, J. Martensitic transformation of Ni2FeGa ferromagnetic shape-memory alloy studied via transmission electron microscopy and electron energy-loss spectroscopy. Phys. Rev. B 2008, 77, 214106. [Google Scholar] [CrossRef]
- Vijayalakshmi, R.; Rajendran, V. Synthesis and characterization of nano-TiO2 via different methods. Arch. Appl. Sci. Res. 2012, 4, 1183–1190. [Google Scholar]






| Samples | Heat Treatments |
|---|---|
| TiO2-1 | 60 °C-72 h |
| TiO2-2 | 25 °C–400 °C-2 h at 9 °C/min |
| TiO2-3 | 25 °C–600 °C-2 h at 9 °C/min |
| TiO2-4 | 600 °C-1 h |
| TiO2-5 | wet precipitates at 600 °C-1 h |
| Samples | Heat TREATMENTS | Anatase % | Rutile % |
|---|---|---|---|
| TiO2-1 | T = 60 °C for 72 h | - | - |
| TiO2-2 | From 25 to 400 °C at 9 °C/min + T = 400 °C for 2 h | 100 | - |
| TiO2-3 | From 25 to 600 °C at 9 °C/min + T = 600 °C for 2 h | 30 | 70 |
| TiO2-4 | T = 600 °C for 1 h | 85 | 15 |
| TiO2-5 | wet precipitates T = 600 °C for 1 h | 68 | 32 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catauro, M.; Tranquillo, E.; Dal Poggetto, G.; Pasquali, M.; Dell’Era, A.; Vecchio Ciprioti, S. Influence of the Heat Treatment on the Particles Size and on the Crystalline Phase of TiO2 Synthesized by the Sol-Gel Method. Materials 2018, 11, 2364. https://doi.org/10.3390/ma11122364
Catauro M, Tranquillo E, Dal Poggetto G, Pasquali M, Dell’Era A, Vecchio Ciprioti S. Influence of the Heat Treatment on the Particles Size and on the Crystalline Phase of TiO2 Synthesized by the Sol-Gel Method. Materials. 2018; 11(12):2364. https://doi.org/10.3390/ma11122364
Chicago/Turabian StyleCatauro, Michelina, Elisabetta Tranquillo, Giovanni Dal Poggetto, Mauro Pasquali, Alessandro Dell’Era, and Stefano Vecchio Ciprioti. 2018. "Influence of the Heat Treatment on the Particles Size and on the Crystalline Phase of TiO2 Synthesized by the Sol-Gel Method" Materials 11, no. 12: 2364. https://doi.org/10.3390/ma11122364
APA StyleCatauro, M., Tranquillo, E., Dal Poggetto, G., Pasquali, M., Dell’Era, A., & Vecchio Ciprioti, S. (2018). Influence of the Heat Treatment on the Particles Size and on the Crystalline Phase of TiO2 Synthesized by the Sol-Gel Method. Materials, 11(12), 2364. https://doi.org/10.3390/ma11122364