Pitting Corrosion and Microstructure of J55 Carbon Steel Exposed to CO2/Crude Oil/Brine Solution under 2–15 MPa at 30–80 °C
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
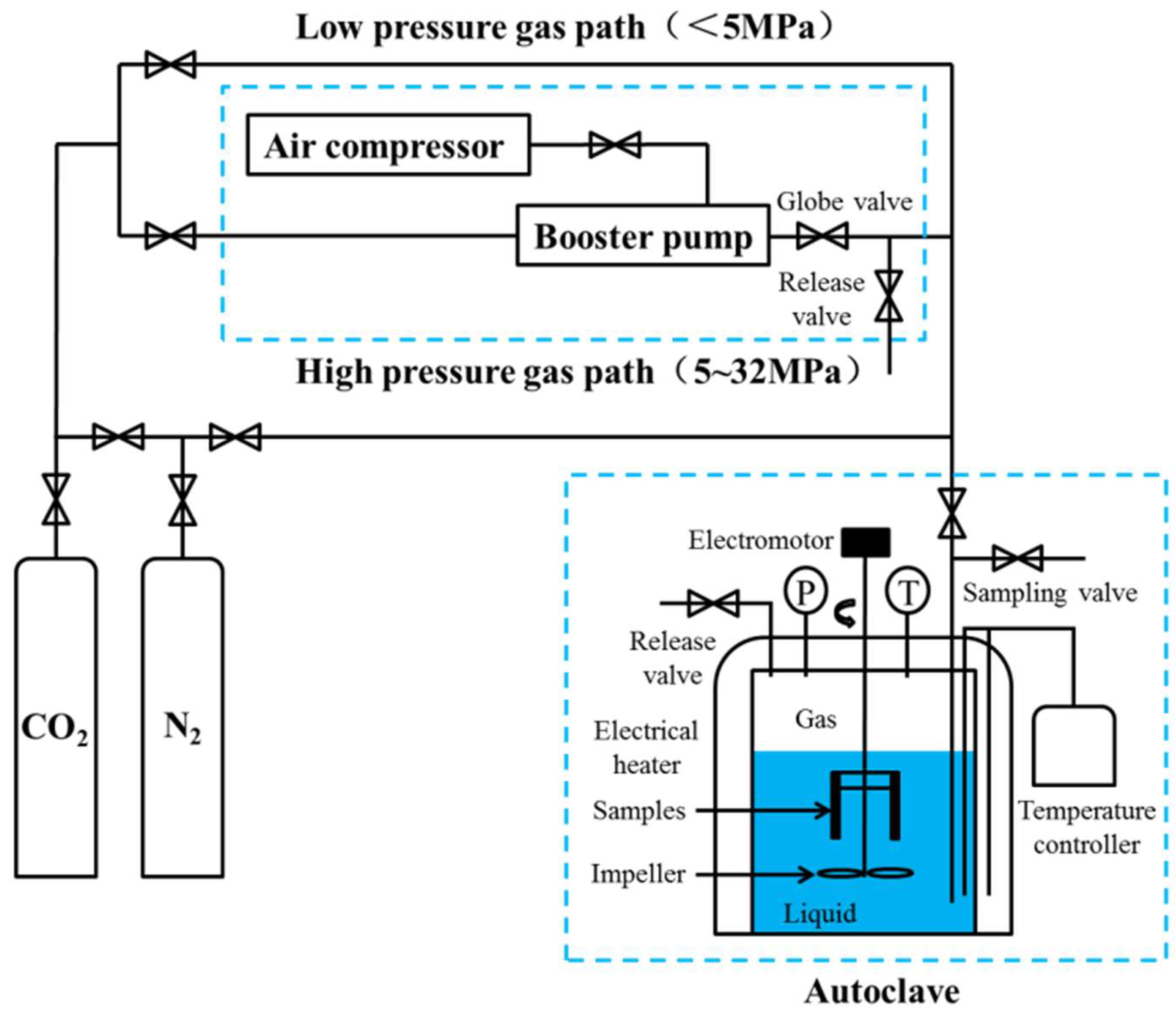
2.2. Weight-Loss Corrosion Test
2.3. Microstructure Observation
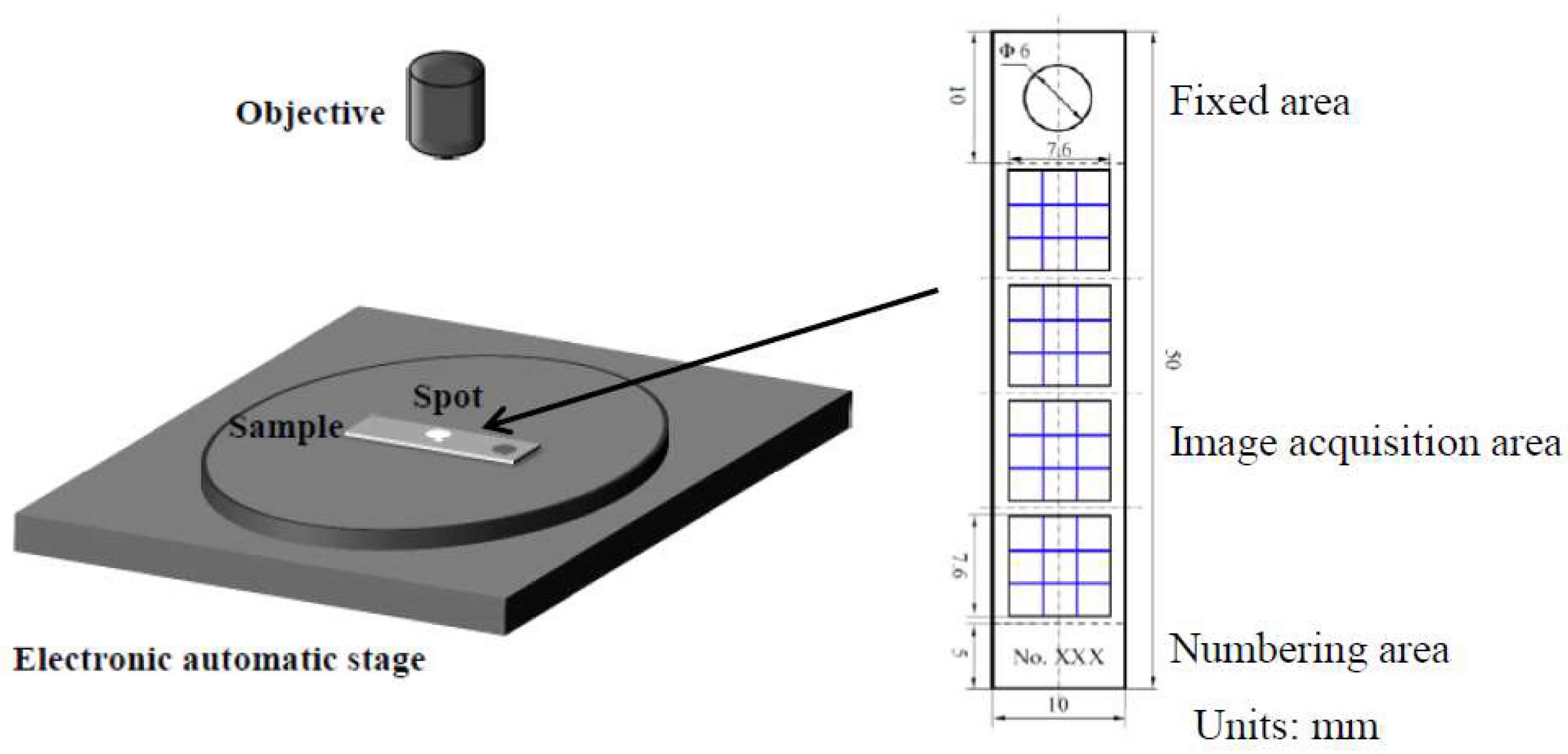
2.4. Statistics of Corrosion Depth Distribution
3. Results and Discussion
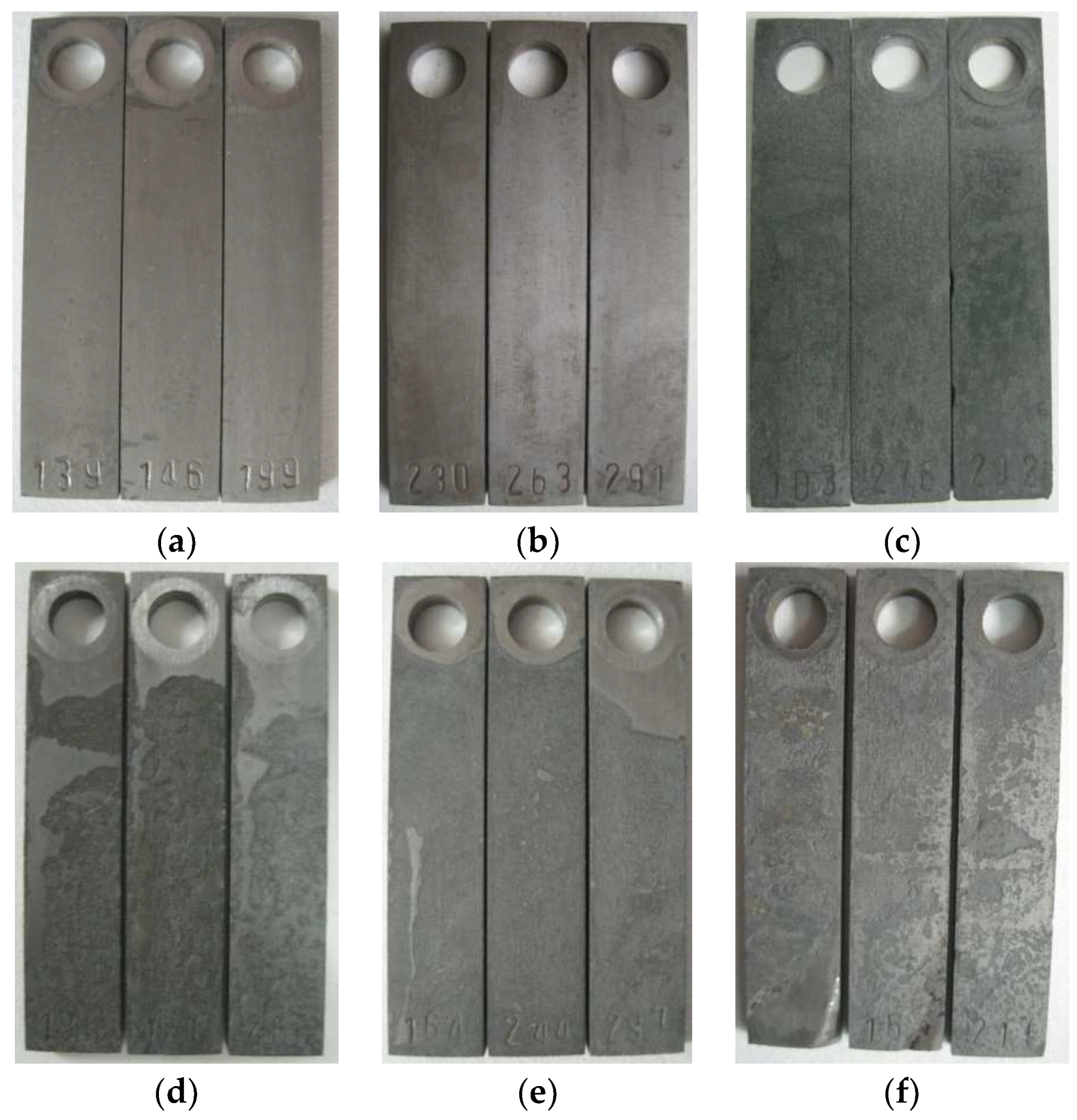
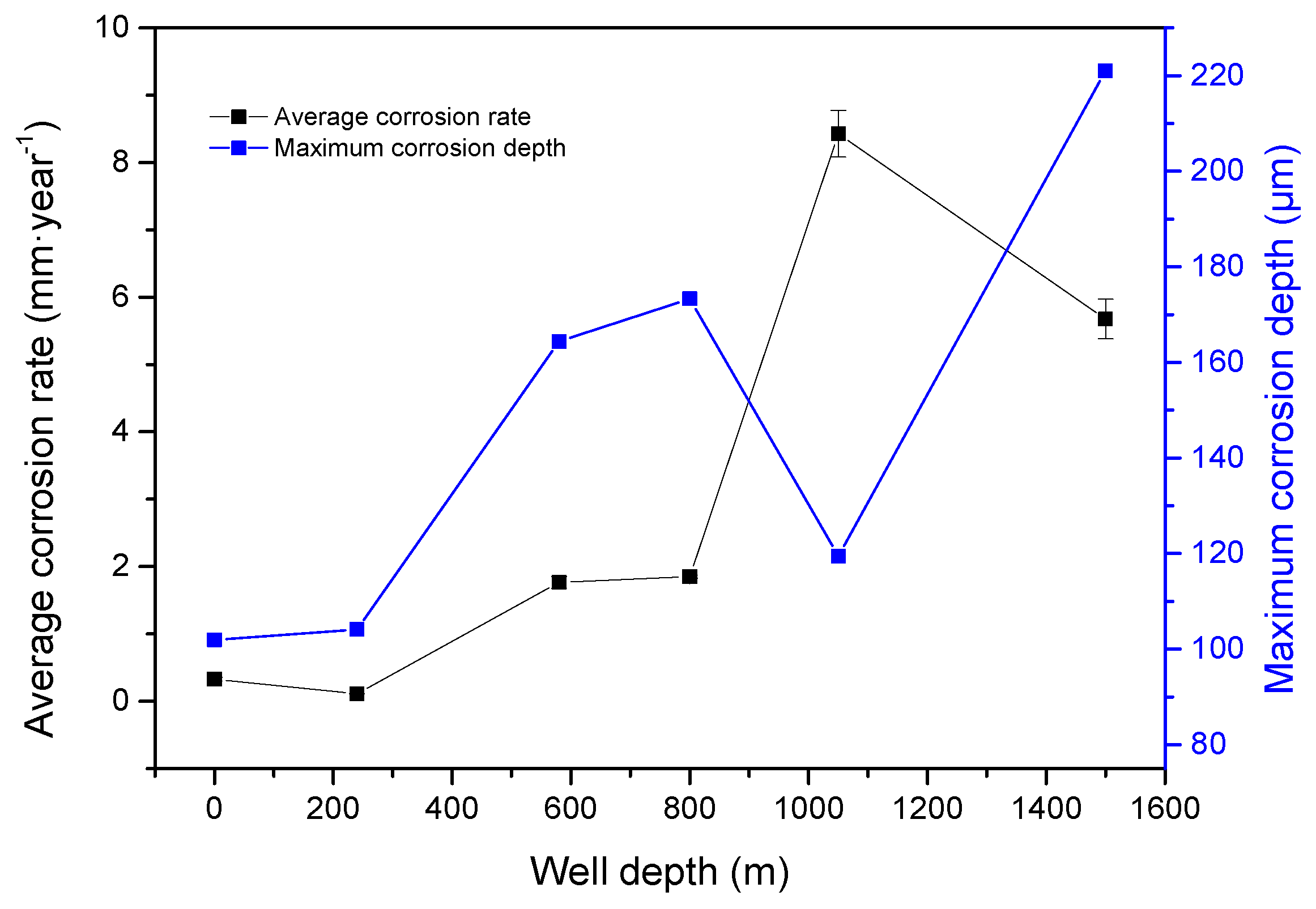
3.1. Corrosion Law of the Deepening Well
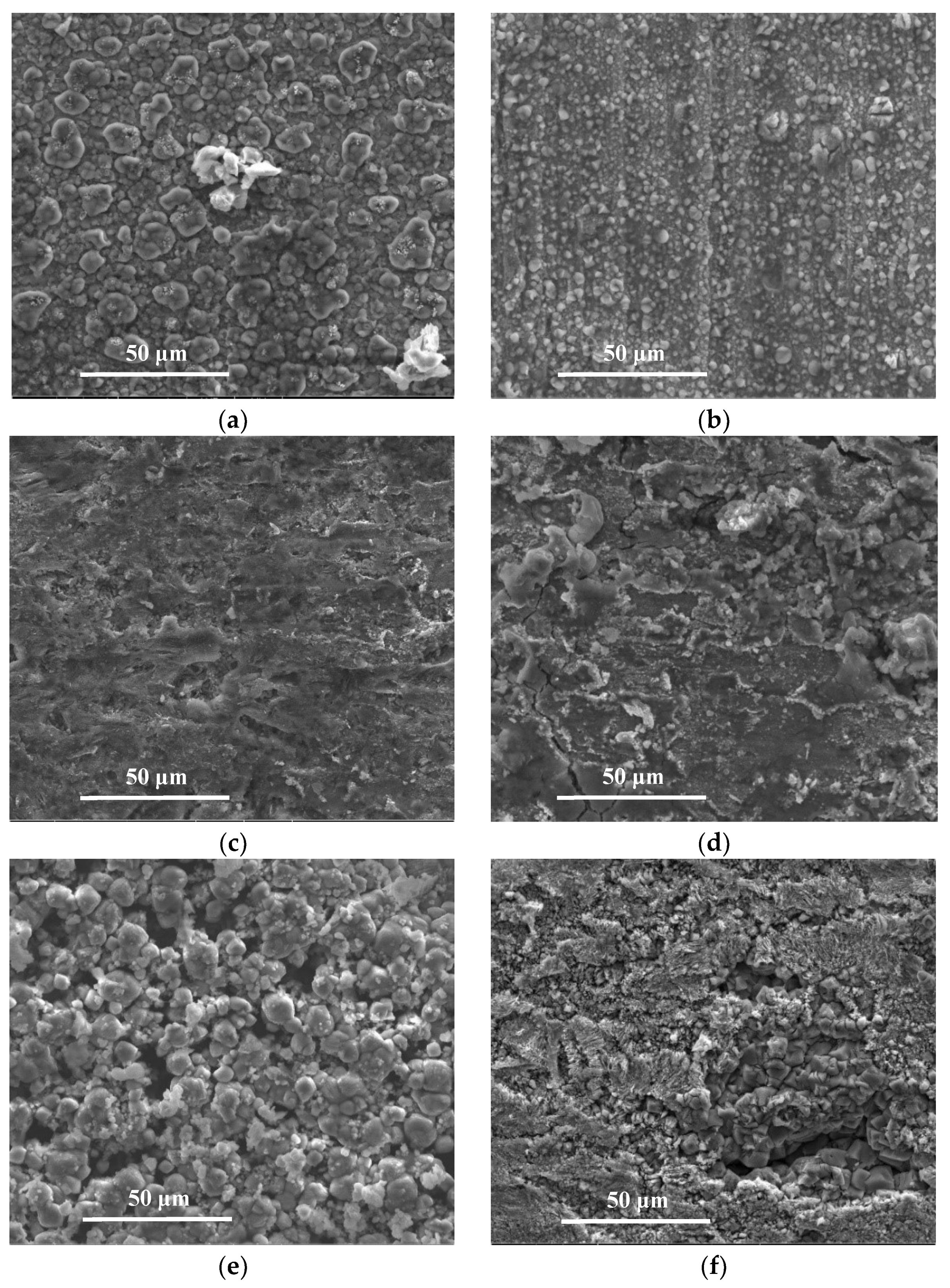
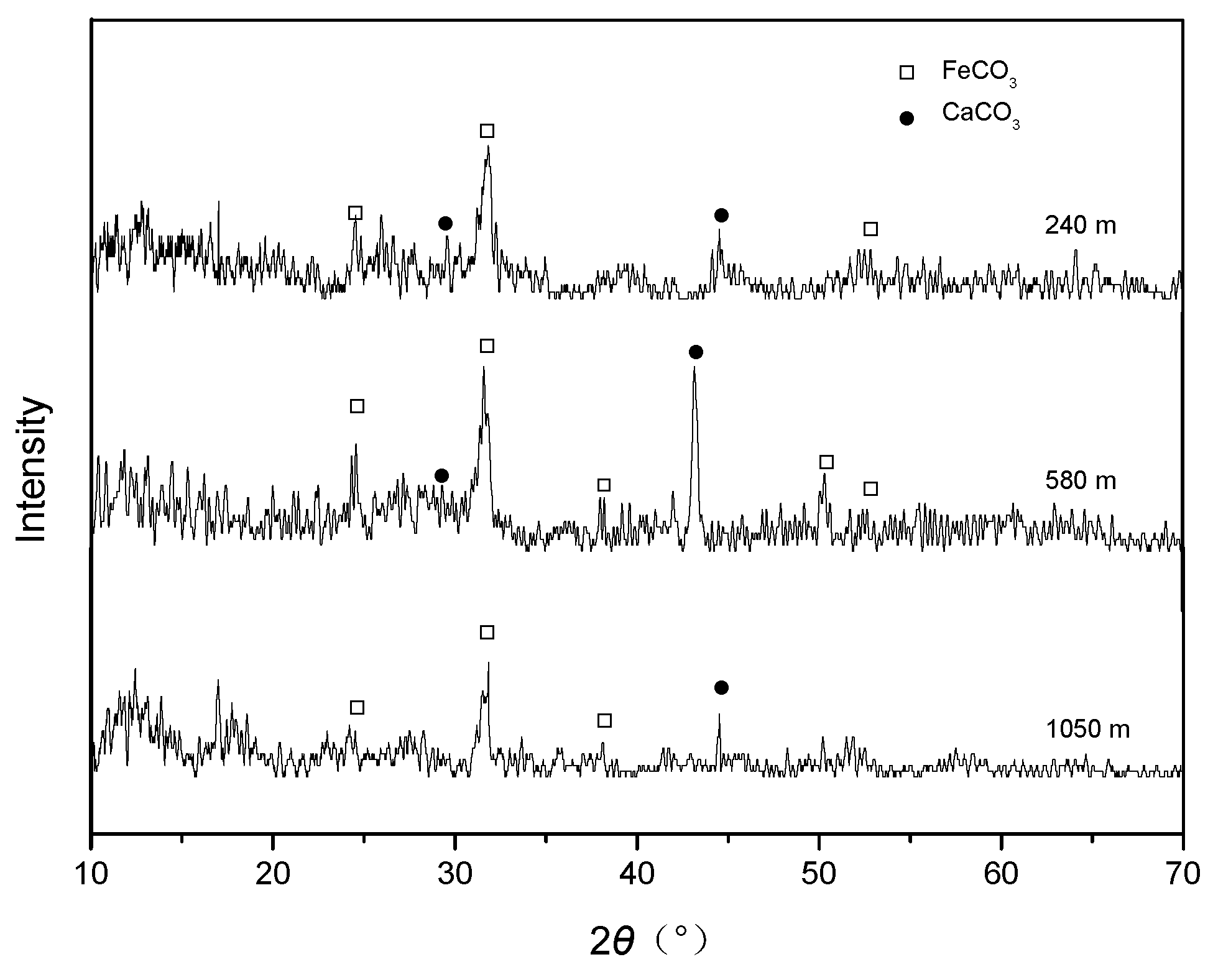
3.2. Microstructures and Compositions of Corrosion Scales
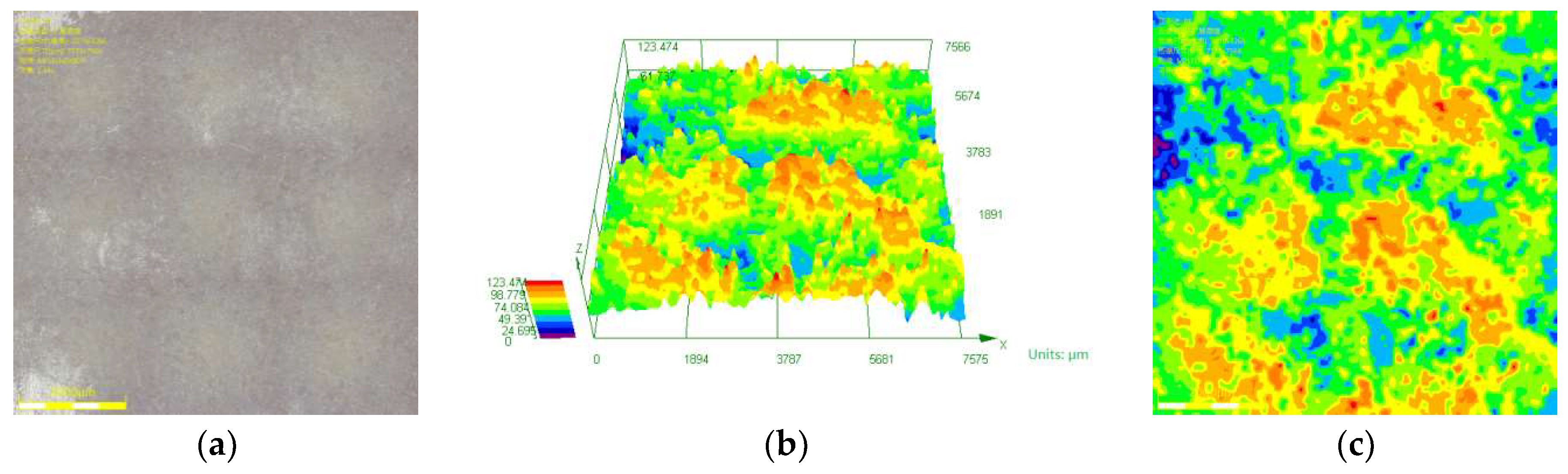
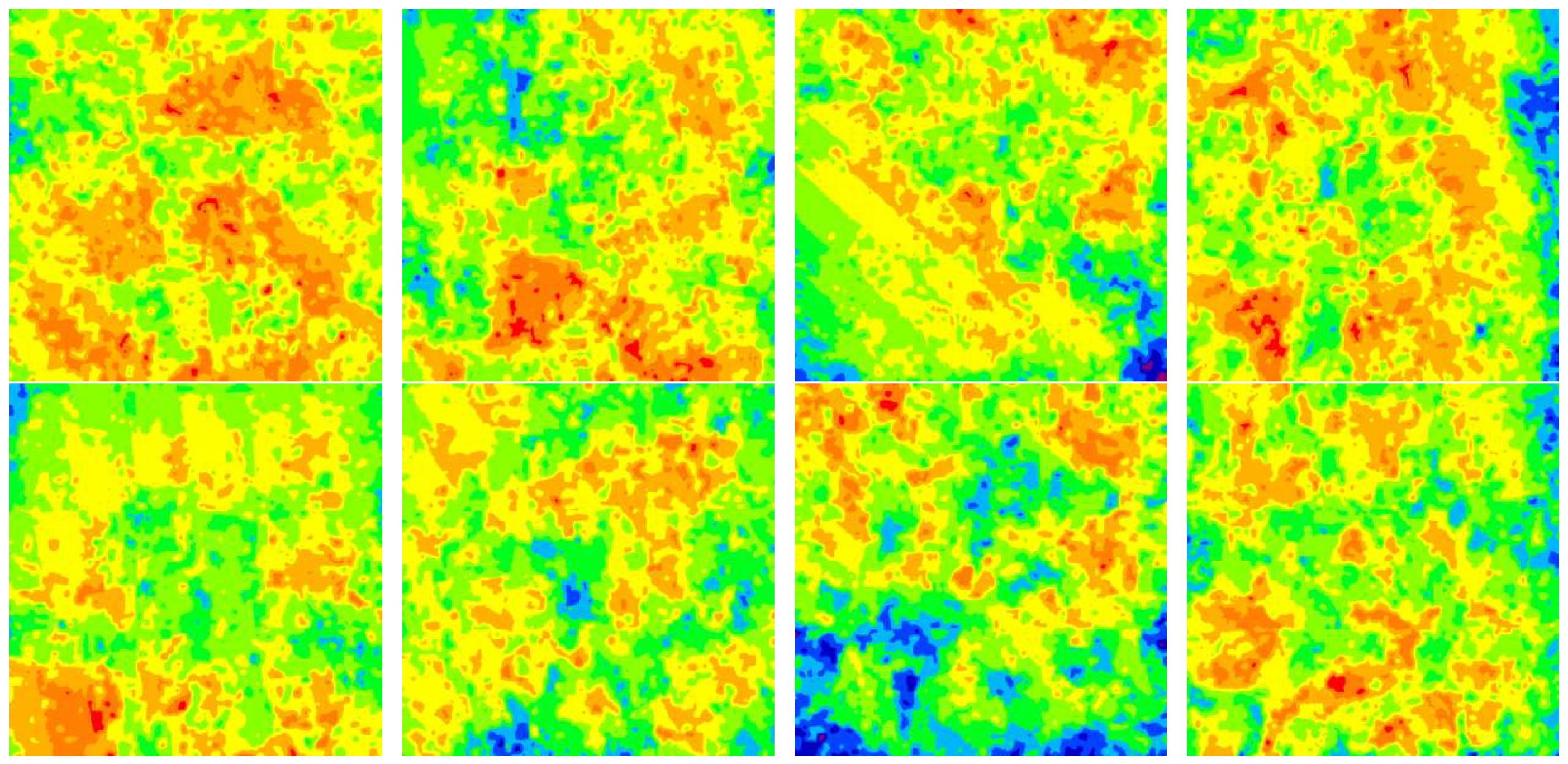
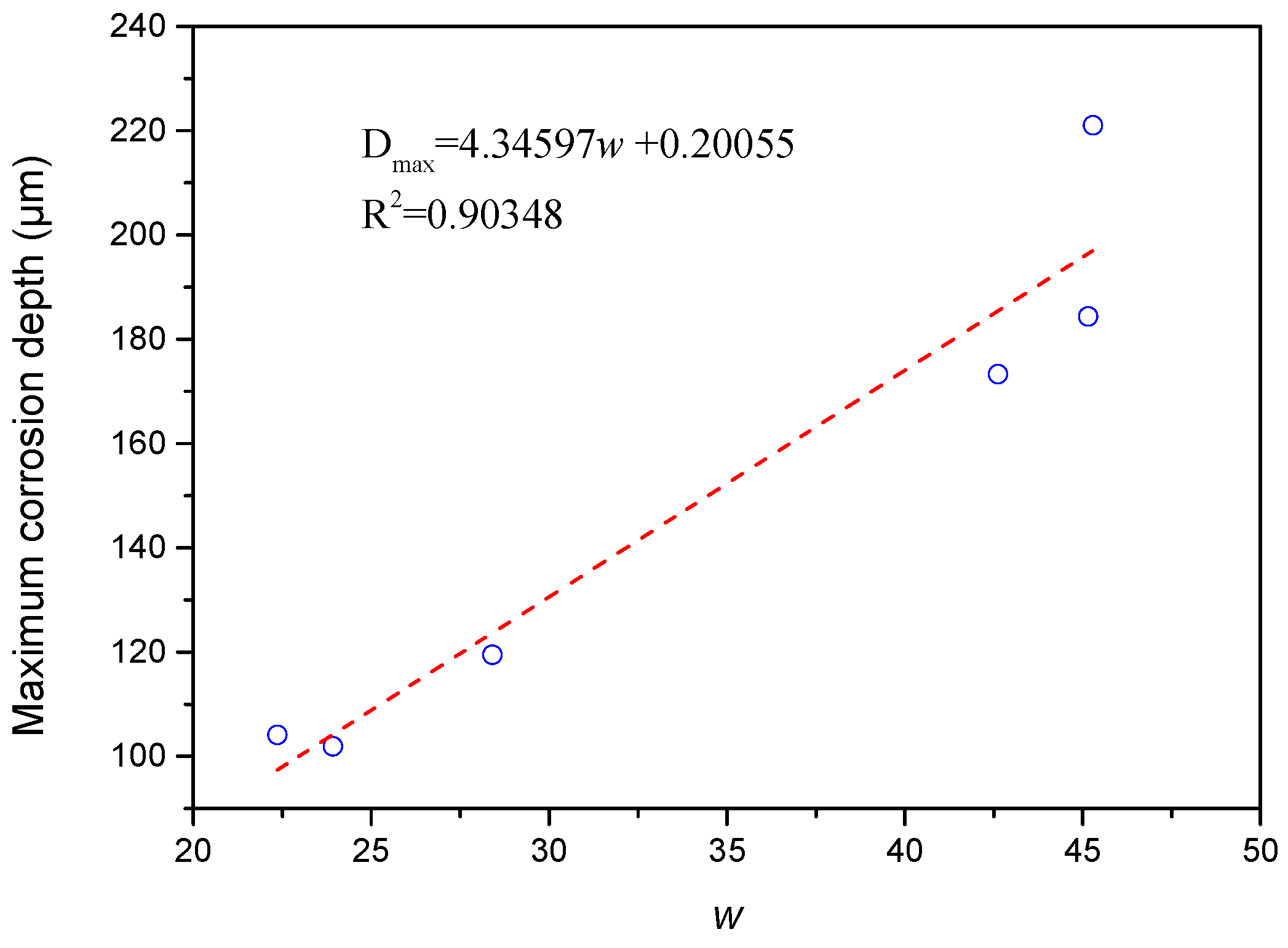
3.3. Law of Corrosion Depth Distribution
4. Conclusions
- (1)
- The average corrosion rate of J55 carbon steel initially increased and then decreased in the CO2/crude oil/brine environment as partial CO2 pressure increased. Corrosion type shifted from uniform to local corrosion;
- (2)
- The main corrosion products on the surfaces of J55 carbon steel were FeCO3 and CaCO3;
- (3)
- The distribution of corrosion depth obeyed Gaussian distribution, and w was positively correlated with the maximum corrosion depth.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kuramochi, T.; Ramírez, A.; Turkenburg, W.; Faaij, A. Effect of CO2 capture on the emissions of air pollutants from industrial processes. Int. J. Greenh. Gas. Control 2012, 10, 310–328. [Google Scholar] [CrossRef]
- Zeng, R.; Vincent, C.J.; Tian, X.; Stephenson, M.H.; Wang, S.; Xu, W. New potential carbon emission reduction enterprises in china: deep geological storage of CO2 emitted through industrial usage of coal in China. Greenh. Gases 2013, 3, 106–115. [Google Scholar] [CrossRef]
- Luhar, A.K.; Etheridge, D.M.; Leuning, R.; Loh, Z.M.; Jenkins, C.R.; Yee, E. Locating and quantifying greenhouse gas emissions at a geological CO2 storage site using atmospheric modeling and measurements. J. Geophys. Res. Atmos. 2015, 119, 10959–10979. [Google Scholar] [CrossRef]
- Uddin, M.; Jafari, A.; Perkins, E. Effects of mechanical dispersion on CO2 storage in Weyburn CO2-EOR field-numerical history match and prediction. Int. J. Greenh. Gas. Control 2013, 16, S35–S49. [Google Scholar] [CrossRef]
- Barnes, D.; Harrison, B.; Grammer, G.M.; Asmus, J. CO2-EOR and geological carbon storage resource potential in the Niagaran pinnacle reef trend, lower Michigan, USA. Energy Procedia 2013, 37, 6786–6799. [Google Scholar] [CrossRef]
- Choi, J.W.; Nicot, J.P.; Hosseini, S.A.; Clift, S.J.; Hovorka, S.D. CO2 recycling accounting and EOR operation scheduling to assist in storage capacity assessment at a U.S. gulf coast depleted reservoir. Int. J. Greenh. Gas. Control 2013, 18, 474–484. [Google Scholar] [CrossRef]
- Cui, Z.D.; Wu, S.L.; Li, C.F.; Zhu, S.L.; Yang, X.J. Corrosion behavior of oil tube steels under conditions of multiphase flow saturated with super-critical carbon dioxide. Mater. Lett. 2004, 68, 1035–1040. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Pang, X.L.; Qu, S.P.; Li, X.; Gao, K.W. The relationship between fracture toughness of CO2 corrosion scale and corrosion rate of X65 pipeline steel under supercritical CO2 condition. Int. J. Greenh. Gas. Control 2011, 5, 1643–1650. [Google Scholar] [CrossRef]
- Cui, Z.D.; Wu, S.L.; Zhu, S.L.; Yang, X.J. Study on corrosion properties of pipelines in simulated produced water saturated with supercritical CO2. Appl. Surf. Sci. 2006, 252, 2368–2374. [Google Scholar] [CrossRef]
- Li, J.L.; Zhu, S.D.; Qun, C.T. Abrasion resistances of CO2 corrosion scales formed at different temperatures and their relationship to corrosion behaviour. Br. Corros. J. 2014, 49, 73–79. [Google Scholar]
- Wei, L.; Pang, X.L.; Gao, K.W. Effects of crude oil on the corrosion behavior of pipeline steel under wet CO2 conditions. Mater. Perform. 2015, 54, 58–62. [Google Scholar]
- Efird, K.D.; Jasinski, R.J. Effect of the crude oil on corrosion of steel in crude oil/brine production. Corros. Eng. 1989, 2, 165–171. [Google Scholar] [CrossRef]
- Lin, G.; Bai, Z.; Zhao, X. Effect of temperature and pressure on the morphology of carbon dioxide corrosion scales. Corrosion 2006, 62, 501–507. [Google Scholar] [CrossRef]
- Choi, Y.S.; Farelas, F.; Neši, S.; Magalhães, A.A.O.; Andrade, C.D.A. Corrosion behavior of deep water oil production tubing material under supercritical CO2 environment: Part 1 effect of pressure and temperature. Corros. Sci. 2013, 77, 38–47. [Google Scholar]
- Nesic, S.; Lunde, L. Carbon dioxide corrosion of carbon steel in two-phase flow. Corros. Eng. 1994, 50, 717–727. [Google Scholar] [CrossRef]
- Kermani, M.B.; Morshed, A.B. Carbon dioxide corrosion in oil and gas production: a compendium. Corrosion 2003, 59, 659–683. [Google Scholar] [CrossRef]
- Farelas, F.; Choi, Y.S.; Nesic, S. Corrosion behavior of deep water oil production tubing material under supercritical CO2 environment: Part 2 effect of crude oil and flow. Corros. Sci. 2013, 70, 137–145. [Google Scholar] [CrossRef]
- Sun, J.B.; Sun, C.; Zhang, G.A.; Zhao, W.B.; Wang, Y. Effect of water cut on the localized corrosion behavior of P110 tube steel in supercritical CO2/oil/water environment. Corrosion 2016, 72, 1470–1482. [Google Scholar] [CrossRef]
- Zhang, G.A.; Liu, D.; Li, Y.Z.; Guo, X.P. Corrosion behavior of N80 carbon steel in formation water under dynamic supercritical CO2 condition. Corros. Sci. 2017, 120, 107–120. [Google Scholar] [CrossRef]
- ASTM G-03. Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens; ASTM International: West Conshohocken, PA, USA, 2011. [Google Scholar]
- Motte, R.A.D.; Barker, R.; Burkle, D.; Vargas, S.M.; Neville, A. T The early stages of FeCO3 scale formation kinetics in CO2 corrosion. Mater. Chem. Phys. 2018, 216, 102–111. [Google Scholar] [CrossRef]
- Yong, H.; Barker, R.; Neville, A. Comparison of corrosion behaviour for X65 carbon steel in supercritical CO2-saturated water and water-saturated/unsaturated supercritical CO2. J. Supercrit. Fluids 2015, 97, 224–237. [Google Scholar]
- Eliyan, F.F.; Alfantazi, A. On the theory of CO2 corrosion reactions—Investigating their interrelation with the corrosion products and API-X100 steel microstructure. Corros. Sci. 2014, 85, 380–393. [Google Scholar] [CrossRef]
- Tavares, L.M.; Costa, E.M.D.; Andrade, J.J.D.O.; Hubler, R.; Huet, B. Effect of calcium carbonate on low carbon steel corrosion behavior in saline CO2 high pressure environments. Appl. Surf. Sci. 2015, 359, 143–152. [Google Scholar] [CrossRef]
- Esmaeely, S.N.; Choi, Y.S.; Young, D.; Nesic, S. Effect of calcium on the formation and protectiveness of iron carbonate layer in CO2 corrosion. Corros. Sci. 2013, 69, 6310–6327. [Google Scholar]
- Dugstad, A.; Hemmer, H.; Seiersten, M. Effect of steel microstructure on corrosion rate and protective iron carbonate film formation. Corrosion 2000, 57, 369–378. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.; Ding, H.; Jin, W. Non-uniform distribution of a corrosion layer at a steel/concrete interface described by a gaussian model. Corros. Sci. 2016, 112, 1–12. [Google Scholar] [CrossRef]
- Lu, Q.; Yao, X. Clustering and learning gaussian distribution for continuous optimization. IEEE Trans. Syst. Man Cybern. Part C Appl. Rev. 2005, 35, 195–204. [Google Scholar] [CrossRef]



| Elements (wt.%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| C | Si | Mn | P | S | Cr | Ni | Cu | Fe |
| 0.34–0.39 | 0.2–0.35 | 1.25–1.5 | ≤0.020 | ≤0.015 | ≤0.15 | ≤0.20 | ≤0.20 | Bal. |
| Property | Units | Value |
|---|---|---|
| Density (20 °C) | kg·m−3 | 848.3 |
| Kinematic viscosity (65 °C) | mm2·s−1 | 7.254 |
| Acid value | mg KOH·g−1 | 0.107 |
| Sulfur content | wt.% | 0.08 |
| Wax content | wt.% | 12.86 |
| Colloid | wt.% | 2.31 |
| asphaltene | wt.% | 0.60 |
| Property | Units | Value |
|---|---|---|
| NaCl | g·L−1 | 18.5028 |
| CaCl2 | g·L−1 | 13.7338 |
| MgCl2 | g·L−1 | 0.5897 |
| Na2SO4 | g·L−1 | 0.2440 |
| NaHCO3 | g·L−1 | 0.0631 |
| Salinity | g·L−1 | 33.0000 |
| Well Depth/m | 0 | 240 | 580 | 800 | 1050 | 1500 | |
|---|---|---|---|---|---|---|---|
| Element | |||||||
| C K | 25.04 | 35.63 | 45.36 | 17.94 | 19.14 | 9.92 | |
| O K | 31.97 | 22.40 | 17.52 | 34.22 | 42.21 | 39.24 | |
| Cl K | 0.26 | / | / | / | 0.57 | 1.38 | |
| Ca K | 1.10 | / | / | 4.09 | 10.51 | 4.70 | |
| Mn K | 0.69 | / | 0.23 | / | 0.54 | 1.39 | |
| Fe K | 38.89 | 41.97 | 36.89 | 36.47 | 26.78 | 42.08 | |
| Cu K | 2.04 | / | / | 6.12 | / | 1.29 | |
| S K | / | / | / | 1.16 | 0.26 | / | |
| total content | 100 | 100 | 100 | 100 | 100 | 100 | |
| Well Depth/m | 0 | 240 | 580 | 800 | 1050 | 1500 |
|---|---|---|---|---|---|---|
| xc/μm | 40.250 | 27.680 | 71.189 | 67.189 | 47.268 | 52.028 |
| w | 23.923 | 22.363 | 45.154 | 42.617 | 28.408 | 45.292 |
| Statistics | DF | Sum of Squares | Mean Square | F Value | Prob > F | |
|---|---|---|---|---|---|---|
| Global | Regression | 12 | 8.11 × 10−3 | 6.76 × 10−4 | 719.55 | 0 |
| Residual | 54 | 5.07 × 10−5 | 9.39 × 10−7 | |||
| Uncorrected Total | 66 | 8.16 × 10−3 | ||||
| Corrected Total | 60 | 5.11 × 10−3 | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, H.; Wang, Y.; Ma, Y.; Ren, P.; Zhang, N. Pitting Corrosion and Microstructure of J55 Carbon Steel Exposed to CO2/Crude Oil/Brine Solution under 2–15 MPa at 30–80 °C. Materials 2018, 11, 2374. https://doi.org/10.3390/ma11122374
Bai H, Wang Y, Ma Y, Ren P, Zhang N. Pitting Corrosion and Microstructure of J55 Carbon Steel Exposed to CO2/Crude Oil/Brine Solution under 2–15 MPa at 30–80 °C. Materials. 2018; 11(12):2374. https://doi.org/10.3390/ma11122374
Chicago/Turabian StyleBai, Haitao, Yongqing Wang, Yun Ma, Peng Ren, and Ningsheng Zhang. 2018. "Pitting Corrosion and Microstructure of J55 Carbon Steel Exposed to CO2/Crude Oil/Brine Solution under 2–15 MPa at 30–80 °C" Materials 11, no. 12: 2374. https://doi.org/10.3390/ma11122374




