Facile Oil Removal from Water-in-Oil Stable Emulsions Using PU Foams
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of Treated PU Foams (PUT)
2.3. Preparation of Emulsions
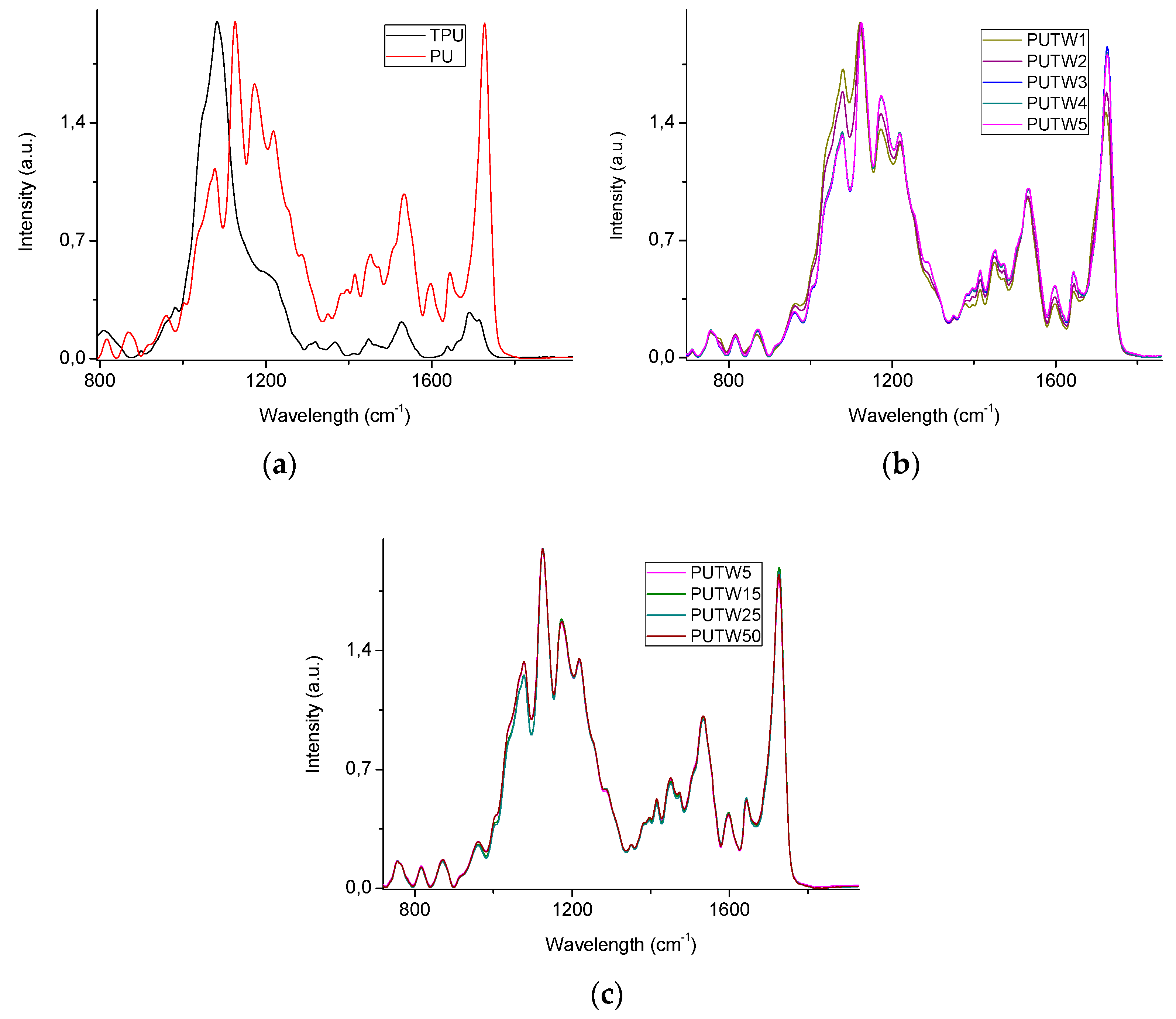
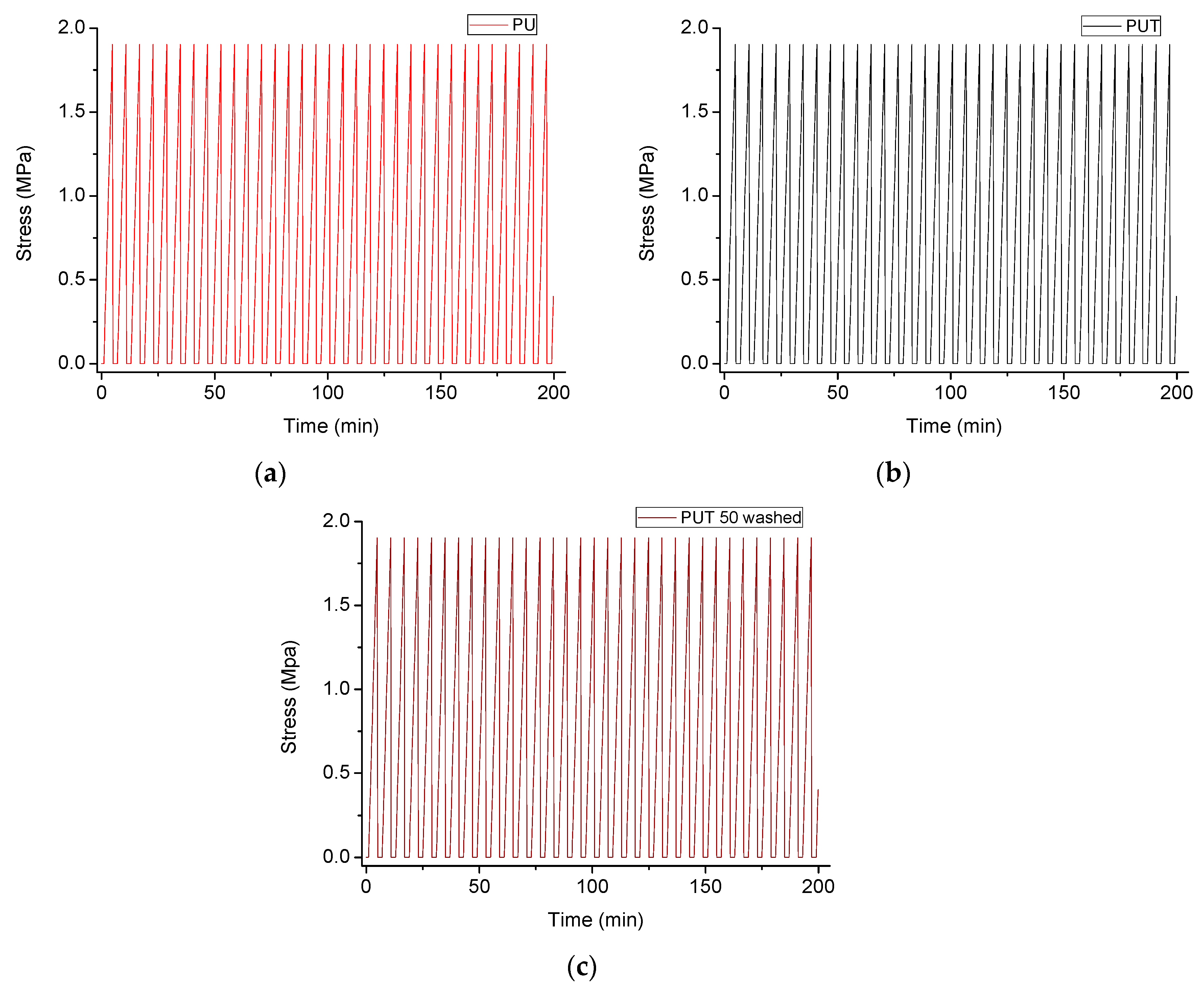
2.4. Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gibson, L.J.; Ashby, M.F. Cellular Solids: Structure and Properties; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Notario, B.; Pinto, J.; Rodriguez-Perez, M.A. Nanoporous polymeric materials: A new class of materials with enhanced properties. Prog. Mater. Sci. 2016, 78–79, 93–139. [Google Scholar] [CrossRef]
- Antunes, M.; Velasco, J.I. Multifunctional polymer foams with carbon nanoparticles. Prog. Polym. Sci. 2014, 39, 486–509. [Google Scholar] [CrossRef]
- Wang, H.; Li, T.-T.; Wu, L.; Lou, C.-W.; Lin, J.-H. Multifunctional, Polyurethane-Based Foam Composites Reinforced by a Fabric Structure: Preparation, Mechanical, Acoustic, and EMI Shielding Properties. Materials 2018, 11, 2085. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Jia, S.; Lv, G.; Yu, D. A Unique Strategy for Polyethylene Glycol/Hybrid Carbon Foam Phase Change Materials: Morphologies, Thermal Properties, and Energy Storage Behavior. Materials 2018, 11, 2011. [Google Scholar] [CrossRef] [PubMed]
- WHO. Drinking-Water. Available online: http://www.who.int/mediacentre/factsheets/fs391/en/ (accessed on 7 February 2018).
- Chavan, A.A.; Pinto, J.; Liakos, I.; Bayer, I.S.; Lauciello, S.; Athanassiou, A.; Fragouli, D. Spent Coffee Bioelastomeric Composite Foams for the Removal of Pb2+ and Hg2+ from Water. ACS Sustain. Chem. Eng. 2016, 4, 5495–5502. [Google Scholar] [CrossRef]
- Chavan, A.A.; Li, H.; Scarpellini, A.; Marras, S.; Manna, L.; Athanassiou, A.; Fragouli, D. Elastomeric nanocomposite foams for the removal of heavy metal ions from water. ACS Appl. Mater. Interfaces 2015, 7, 14778–14784. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.; Magrì, D.; Valentini, P.; Palazon, F.; Heredia-Guerrero, J.A.; Lauciello, S.; Barroso-Solares, S.; Ceseracciu, L.; Pompa, P.P.; Athanassiou, A.; et al. Antibacterial Melamine Foams Decorated with in Situ Synthesized Silver Nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 16095–16104. [Google Scholar] [CrossRef] [PubMed]
- Phong, N.T.P.; Thanh, N.V.K.; Phuong, P.H. Fabrication of antibacterial water filter by coating silver nanoparticles on flexible polyurethane foams. J. Phys. Conf. Ser. 2009, 187, 12079. [Google Scholar] [CrossRef]
- Deng, C.H.; Gong, J.L.; Zhang, P.; Zeng, G.M.; Song, B.; Liu, H.Y. Preparation of melamine sponge decorated with silver nanoparticles-modified graphene for water disinfection. J. Colloid Interface Sci. 2017, 488, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Sportelli, M.C.; Picca, R.A.; Ronco, R.; Bonerba, E.; Tantillo, G.; Pollini, M.; Sannino, A.; Valentini, A.; Cataldi, T.R.I.; Cioffi, N. Investigation of industrial polyurethane foams modified with antimicrobial copper nanoparticles. Materials 2016, 9, 544. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.; Athanassiou, A.; Fragouli, D. Surface modification of polymeric foams for oil spills remediation. J. Environ. Manag. 2018, 206, 872–889. [Google Scholar] [CrossRef] [PubMed]
- Adebajo, M.O.; Frost, R.L.; Kloprogge, J.T.; Carmody, O.; Kokot, S. Porous Materials for Oil Spill Cleanup: A Review of Synthesis and Absorbing Properties. J. Porous Mater. 2003, 10, 159–170. [Google Scholar] [CrossRef]
- Liu, L.; Lei, J.; Li, L.; Zhang, R.; Mi, N.; Chen, H.; Huang, D.; Li, N. A facile method to fabricate the superhydrophobic magnetic sponge for oil-water separation. Mater. Lett. 2017, 195, 66–70. [Google Scholar] [CrossRef]
- Hou, K.; Jin, Y.; Chen, J.; Wen, X.; Xu, S.; Cheng, J.; Pi, P. Fabrication of superhydrophobic melamine sponges by thiol-ene click chemistry for oil removal. Mater. Lett. 2017, 202, 99–102. [Google Scholar] [CrossRef]
- Fan, Y.; He, Y.; Luo, P.; Chen, X.; Yu, Z.; Li, M. Facile way in building superhydrophobic zirconium surface for controllable water-oil separation. Mater. Lett. 2017, 188, 115–118. [Google Scholar] [CrossRef]
- Calcagnile, P.; Fragouli, D.; Bayer, I.S.; Anyfantis, G.C.; Martiradonna, L.; Cozzoli, P.D.; Cingolani, R.; Athanassiou, A. Magnetically Driven Floating Foams for the Removal of Oil Contaminants from Water. ACS Nano 2012, 6, 5413–5419. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Li, W.; Ma, C.; Wu, Q.; Xu, Z.; Liu, S. Synthesis of honeycomb-like carbon foam from larch sawdust as efficient absorbents for oil spills cleanup and recovery. Materials 2018, 11, 1106. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.; Athanassiou, A.; Fragouli, D. Effect of the porous structure of polymer foams on the remediation of oil spills. J. Phys. D Appl. Phys. 2016, 49, 145601–145608. [Google Scholar] [CrossRef]
- Tao, M.; Xue, L.; Liu, F.; Jiang, L. An intelligent superwetting PVDF membrane showing switchable transport performance for oil/water separation. Adv. Mater. 2014, 26, 2943–2948. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, C.; Zhang, Y.; Wang, R.; Zha, F.; She, H. Robust superhydrophobic attapulgite coated polyurethane sponge for efficient immiscible oil/water mixture and emulsion separation. J. Mater. Chem. A 2016, 4, 15546–15553. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Y. Oil/water mixtures and emulsions separation of stearic acid-functionalized sponge fabricated via a facile one-step coating method. Sep. Purif. Technol. 2017, 181, 183–191. [Google Scholar] [CrossRef]
- Wang, C.F.; Lin, S.J. Robust Superhydrophobic/superoleophilic sponge for effective continuous absorption and expulsion of oil pollutants from Water. ACS Appl. Mater. Interfaces 2013, 5, 8861–8864. [Google Scholar] [CrossRef] [PubMed]
- Paul, U.; Fragouli, D.; Bayer, I.; Athanassiou, A. Functionalized Cellulose Networks for Efficient Oil Removal from Oil–Water Emulsions. Polymers 2016, 8, 52. [Google Scholar] [CrossRef]
- Nikkhah, A.A.; Zilouei, H.; Asadinezhad, A.; Keshavarz, A. Removal of oil from water using polyurethane foam modified with nanoclay. Chem. Eng. J. 2015, 262, 278–285. [Google Scholar] [CrossRef]
- Barroso-Solares, S.; Pinto, J.; Nanni, G.; Fragouli, D.; Athanassiou, A. Enhanced oil removal from water in oil stable emulsions using electrospun nanocomposite fiber mats. RSC Adv. 2018, 8, 7641–7650. [Google Scholar] [CrossRef]
- Barroso-Solares, S.; Zahedi, M.G.; Pinto, J.; Nanni, G.; Fragouli, D.; Athanassiou, A. Oil removal from water–oil emulsions using magnetic nanocomposite fibrous mats. RSC Adv. 2016, 6, 71100–71107. [Google Scholar] [CrossRef]
- Li, X.; Wang, M.; Wang, C.; Cheng, C.; Wang, X. Facile immobilization of Ag nanocluster on nanofibrous membrane for oil/water separation. ACS Appl. Mater. Interfaces 2014, 6, 15272–15282. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Si, Y.; Tang, X.; Zhu, Z.; Ding, B.; Liu, L.; Zheng, G.; Luo, W.; Yu, J. Gravity driven separation of emulsified oil–water mixtures utilizing in situ polymerized superhydrophobic and superoleophilic nanofibrous membranes. J. Mater. Chem. A 2013, 1, 14071–14074. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, C.; Liu, H.; Huang, Q.; Hao, J.; Fu, H. Poly(vinylidene fluoride-hexafluoropropylene) porous membrane with controllable structure and applications in efficient oil/water separation. Materials 2018, 11, 443. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhan, J.; Pan, H.; Wang, W.; Tang, G.; Song, L.; Hu, Y. Effect of Fully Biobased Coatings Constructed via Layer-by-Layer Assembly of Chitosan and Lignosulfonate on the Thermal, Flame Retardant, and Mechanical Properties of Flexible Polyurethane Foam. ACS Sustain. Chem. Eng. 2016, 4, 1431–1438. [Google Scholar] [CrossRef]
- Linul, E.; Linul, P.; Valean, C.; Marsavina, L.; Silaghi-Perju, D. Manufacturing and Compressive Mechanical Behavior of Reinforced Polyurethane Flexible (PUF) Foams. IOP Conf. Ser. Mater. Sci. Eng. 2018, 416, 012053. [Google Scholar] [CrossRef]
- Pinto, J.; Heredia-Guerrero, J.A.; Athanassiou, A.; Fragouli, D. Reusable nanocomposite-coated polyurethane foams for the remediation of oil spills. Int. J. Environ. Sci. Technol. 2017. [Google Scholar] [CrossRef]
- Hejna, A.; Kirpluks, M.; Kosmela, P.; Cabulis, U.; Haponiuk, J.; Piszczyk, Ł. The influence of crude glycerol and castor oil-based polyol on the structure and performance of rigid polyurethane-polyisocyanurate foams. Ind. Crops Prod. 2017, 95, 113–125. [Google Scholar] [CrossRef]
- Asefnejad, A.; Khorasani, M.T.; Behnamghader, A.; Farsadzadeh, B.; Bonakdar, S. Manufacturing of biodegradable polyurethane scaffolds based on polycaprolactone using a phase separation method: physical properties and in vitro assay. Int. J. Nanomed. 2011, 6, 2375–2384. [Google Scholar] [CrossRef] [PubMed]




| Foam | Pore Size (µm) | AWCA (°) | Cwater (g/g) | Coil (g/g) |
|---|---|---|---|---|
| PU-1 | 1741 | 98.4 ± 11.0 | 28.94 ± 0.14 | 23.25 ± 0.10 |
| PU-2 | 817 | 122.8 ± 7.6 | 31.01 ± 2.95 | 29.62 ± 0.24 |
| PU-3 | 435 | 133.3 ± 4.4 | 15.64 ± 3.18 | 30.50 ± 0.01 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barroso-Solares, S.; Pinto, J.; Fragouli, D.; Athanassiou, A. Facile Oil Removal from Water-in-Oil Stable Emulsions Using PU Foams. Materials 2018, 11, 2382. https://doi.org/10.3390/ma11122382
Barroso-Solares S, Pinto J, Fragouli D, Athanassiou A. Facile Oil Removal from Water-in-Oil Stable Emulsions Using PU Foams. Materials. 2018; 11(12):2382. https://doi.org/10.3390/ma11122382
Chicago/Turabian StyleBarroso-Solares, Suset, Javier Pinto, Despina Fragouli, and Athanassia Athanassiou. 2018. "Facile Oil Removal from Water-in-Oil Stable Emulsions Using PU Foams" Materials 11, no. 12: 2382. https://doi.org/10.3390/ma11122382
APA StyleBarroso-Solares, S., Pinto, J., Fragouli, D., & Athanassiou, A. (2018). Facile Oil Removal from Water-in-Oil Stable Emulsions Using PU Foams. Materials, 11(12), 2382. https://doi.org/10.3390/ma11122382








