Nanostructure Design and Catalytic Performance of Mo/ZnAl-LDH in Cationic Orchid X-BL Removal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of ZnAl-LDH Carriers
2.2.1. The TP Process
2.2.2. The HS Process
2.2.3. The SG Process
2.2.4. The UC Process
2.3. Mo/ZnAl-LDH Catalysts Preparation
2.4. Characterization
2.5. Catalytic Activity Evaluation
3. Results and Discussion
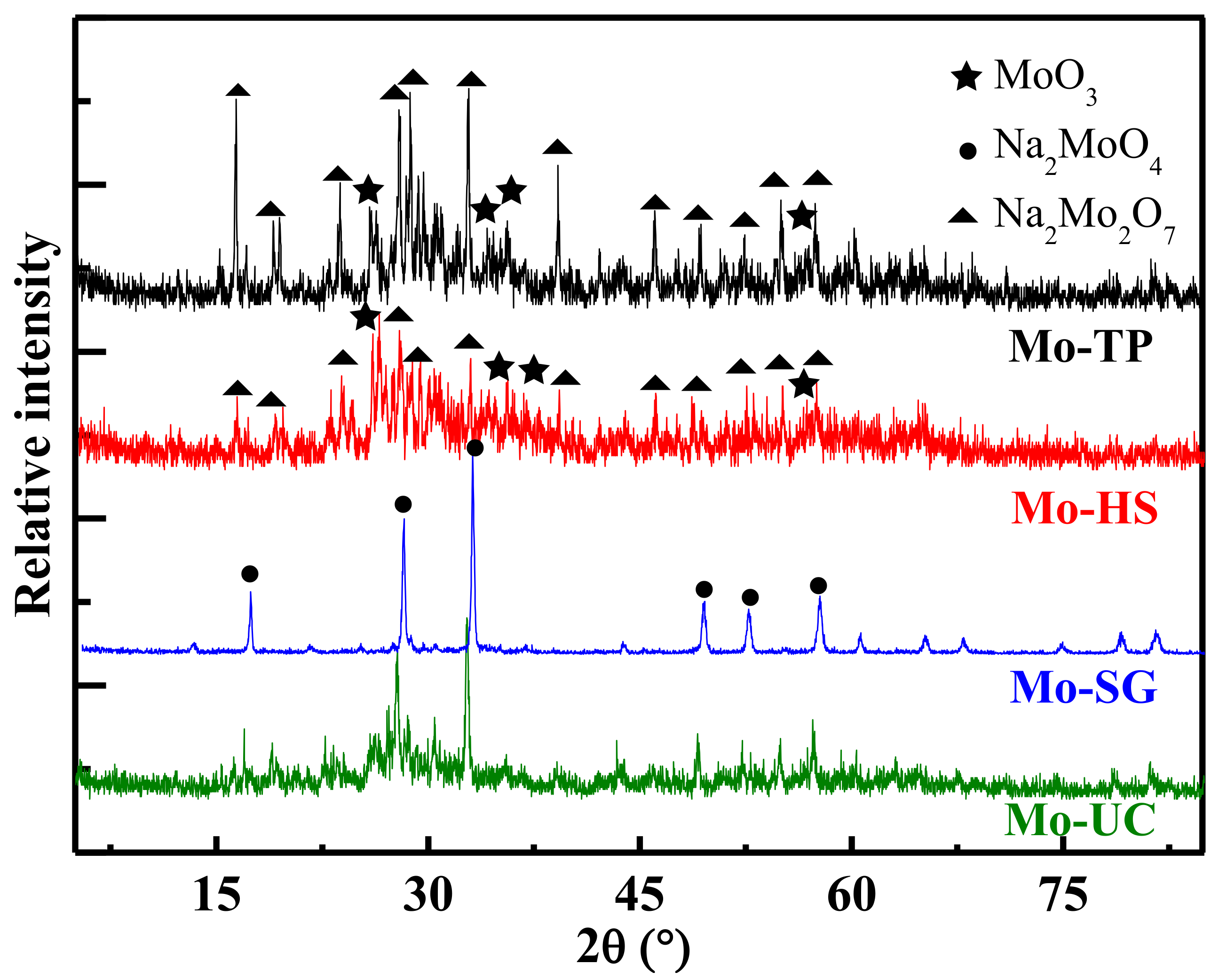
3.1. Crystal Structure of Mo/ZnAl-LDH Catalysts
3.2. TEM Characterizations of the Mo/ZnAl-LDH Catalysts
3.3. Textural Properties of Mo/ZnAl-LDH Catalysts
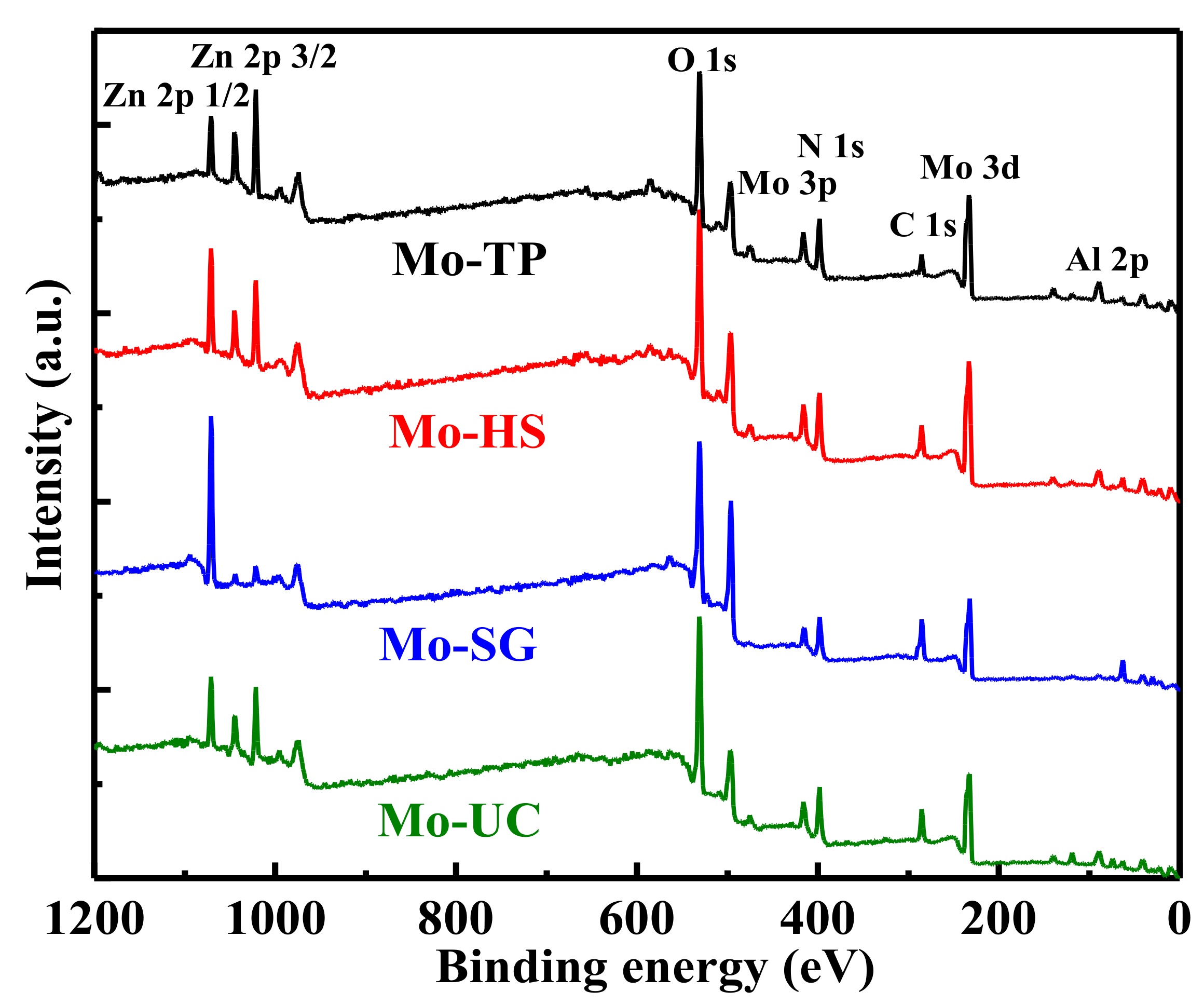
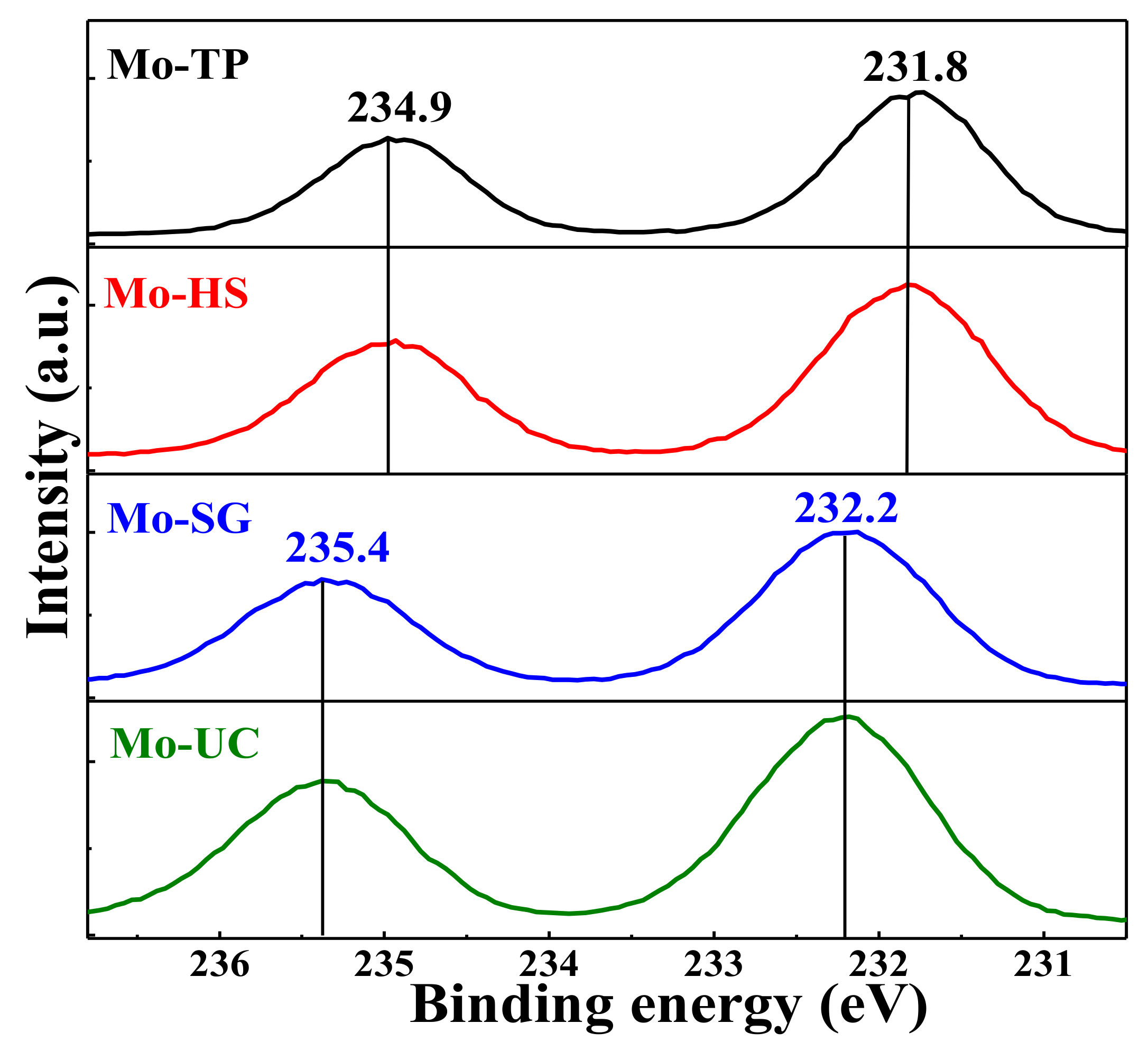
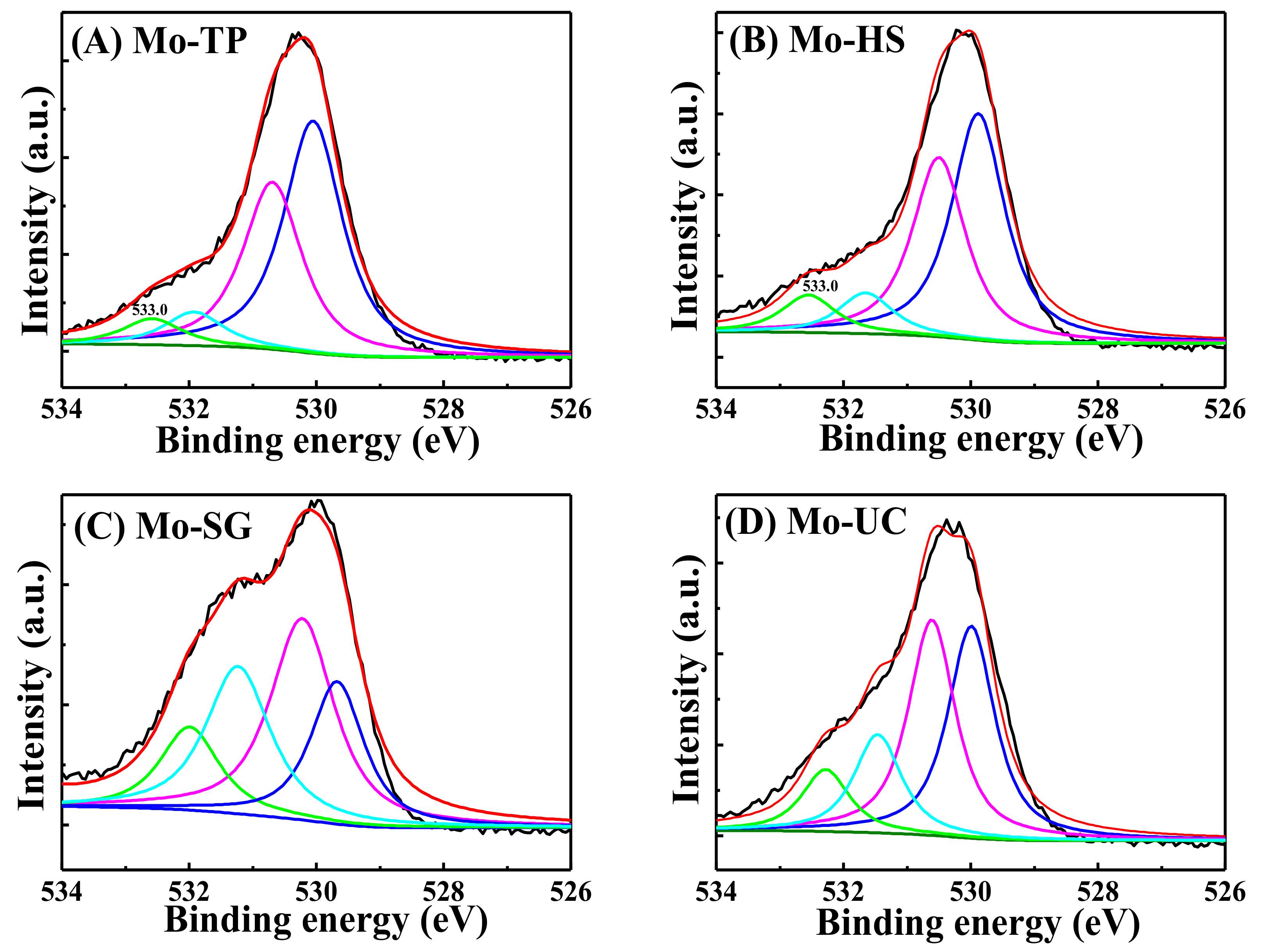
3.4. XPS Characterization of the Mo/ZnAl-LDH Catalysts
3.5. CWAO Activity of the Mo/ZnAl-LDH Catalysts
4. Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rodriguez, A.; Garcia, J.; Ovejero, G.; Mestanza, M. Wet air and catalytic wet air oxidation of several azodyes from wastewaters: the beneficial role of catalysis. Water Sci. Technol. 2009, 60, 1989–1999. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.; Song, Y.; Zeng, P.; Xiao, S.; Duan, L. Phosphorus recovery from fosfomycin pharmaceutical wastewater by wet air oxidation and phosphate crystallization. Chemosphere 2011, 84, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Shao, H.; Ge, F.; Liu, Y. Novel-structured Mo-Cu-Fe-O composite for catalytic air oxidation of dye-containing wastewater under ambient temperature and pressure. Chinese J. Catal. 2017, 38, 1719–1725. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, D. Development of Fe2O3-CeO2-TiO2/γ-Al2O3 as catalyst for catalytic wet air oxidation of methyl orange azo dye under room condition. Appl. Catal. B Environ. 2007, 72, 205–211. [Google Scholar] [CrossRef]
- Vallet, A.; Ovejero, G.; Rodríguez, A.; Peres, J.A.; García, J. Ni/MgAlO regeneration for catalytic wet air oxidation of an azo-dye in trickle-bed reaction. J. Hazard. Mater. 2013, 244, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yu, C.; Zhao, P.; Chen, G. Comparative study of supported CuOx and MnOx catalysts for the catalytic wet air oxidation of β-naphthol. Appl. Surf. Sci. 2012, 258, 9096–9102. [Google Scholar] [CrossRef]
- Xu, Y.; Li, X.; Cheng, X.; Sun, D.; Wang, X. Degradation of cationic red GTL by catalytic wet air oxidation over Mo-Zn-Al-O catalyst under room temperature and atmospheric pressure. Environ. Sci. Technol. 2012, 46, 2856–2863. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, X.Y.; Li, Y.; Ge, F.; Zhu, R.L. Quantitative structure-property relationship (QSPR) study for the degradation of dye wastewater by Mo-Zn-Al-O catalyst. J. Mol. Liq. 2016, 215, 461–466. [Google Scholar] [CrossRef]
- Wu, J.; Xia, Q.; Wang, H.; Li, Z. Catalytic performance of plasma catalysis system with nickel oxide catalysts on different supports for toluene removal: Effect of water vapor. Appl. Catal. B Environ. 2014, 156, 265–272. [Google Scholar] [CrossRef]
- Gutiérrez, O.Y.; Klimova, T. Effect of the support on the high activity of the (Ni) Mo/ZrO2-SBA-15 catalyst in the simultaneous hydrodesulfurization of DBT and 4, 6-DMDBT. J. Catal. 2011, 281, 50–62. [Google Scholar] [CrossRef]
- Yoosuk, B.; Song, C.; Kim, J.H.; Ngamcharussrivichai, C.; Prasassarakich, P. Effects of preparation conditions in hydrothermal synthesis of highly active unsupported NiMo sulfide catalysts for simultaneous hydrodesulfurization of dibenzothiophene and 4, 6-dimethyldibenzothiophene. Catal. Today 2010, 149, 52–61. [Google Scholar] [CrossRef]
- Infantes-Molina, A.; Moreno-León, C.; Pawelec, B.; Fierro, J.; Rodríguez-Castellón, E.; Jiménez-López, A. Simultaneous hydrodesulfurization and hydrodenitrogenation on MoP/SiO2 catalysts: Effect of catalyst preparation method. Appl. Catal. B Environ. 2012, 113, 87–99. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhu, R.; Parker, S.C.; Zhu, J.; He, H.; Molinari, M. Modelling the effects of surfactant loading level on the sorption of organic contaminants on organoclays. RSC Adv. 2015, 5, 47022–47030. [Google Scholar] [CrossRef]
- Zgolicz, P.D.; Stassi, J.P.; Yañez, M.J.; Scelza, O.A.; de Miguel, S.R. Influence of the support and the preparation methods on the performance in citral hydrogenation of Pt-based catalysts supported on carbon nanotubes. J. Catal. 2012, 290, 37–54. [Google Scholar] [CrossRef]
- Li, X.; Zou, X.; Qu, Z.; Zhao, Q.; Wang, L. Photocatalytic degradation of gaseous toluene over Ag-doping TiO2 nanotube powder prepared by anodization coupled with impregnation method. Chemosphere 2011, 83, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Skaf, M.; Aouad, S.; Hany, S.; Cousin, R.; Abi-Aad, E.; Aboukaïs, A. Physicochemical characterization and catalytic performance of 10% Ag/CeO2 catalysts prepared by impregnation and deposition-precipitation. J. Catal. 2014, 320, 137–146. [Google Scholar] [CrossRef]
- Qian, K.; Fang, J.; Huang, W.; He, B.; Jiang, Z.; Ma, Y.; Wei, S. Understanding the deposition-precipitation process for the preparation of supported Au catalysts. J. Mol. Catal. A Chem. 2010, 320, 97–105. [Google Scholar] [CrossRef]
- Chubar, N.; Gerda, V.; Megantari, O.; Mičušík, M.; Omastova, M.; Heister, K.; Man, P.; Fraissard, J. Applications versus properties of Mg-Al layered double hydroxides provided by their syntheses methods: Alkoxide and alkoxide-free sol-gel syntheses and hydrothermal precipitation. Chem. Eng. J. 2013, 234, 284–299. [Google Scholar] [CrossRef]
- Wang, D.; Pan, Z.; Wu, Z.; Wang, Z.; Liu, Z. Hydrothermal synthesis of MoS2 nanoflowers as highly efficient hydrogen evolution reaction catalysts. J. Power Sources 2014, 264, 229–234. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Y.; Chen, X.Y.; Ge, F.; Zhu, R.L. High catalytic activity of Mo-Zn-Al-O catalyst for dye degradation: Effect of pH in the impregnation process. Appl. Catal. B Environ. 2014, 160, 115–121. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, D. Structure and catalytic activity of Mo-Zn-Al-O catalyst for degradation of cationic red GTL under room conditions. Chem. Eng. J. 2012, 183, 332–338. [Google Scholar] [CrossRef]
- Mudher, K.S.; Keskar, M.; Krishnan, K.; Venugopal, V. Thermal and X-ray diffraction studies on Na2MoO4, Na2Mo2O7 and Na2Mo4O13. J. Alloy Compd. 2005, 396, 275–279. [Google Scholar] [CrossRef]
- Zimowska, M.; Łątka, K.; Mucha, D.; Gurgul, J.; Matachowski, L. The continuous conversion of ethanol and water mixtures into hydrogen over FexOy/MoO3 catalytic system-XPS and Mössbauer studies. J. Mol. Catal. A Chem. 2016, 423, 92–104. [Google Scholar] [CrossRef]
- Quincy, R.B.; Houalla, M.; Proctor, A.; Hercules, D.M. Distribution of molybdenum oxidation states in reduced molybdenum/titania catalysts: correlation with benzene hydrogenation activity. J. Phys. Chem. 1990, 94, 1520–1526. [Google Scholar] [CrossRef]
- Belanger, D.; Laperriere, G. Electrochromic molybdenum trioxide thin film preparation and characterization. Chem. Mater. 1990, 2, 484–486. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Zhou, T.; Lu, P.; Gao, Y.; Yu, F.; Umar, A.; Wang, Q. Synthesis and characterization of alkali metal molybdates with high catalytic activity for dye degradation. RSC Adv. 2016, 6, 54553–54563. [Google Scholar] [CrossRef]
- Yang, R.; Zhang, Z.; Umar, A.; Gao, Y.; Wang, J.; Lu, P.; Guo, Z.; Huang, L.; Zhou, T.; Wang, Q. Preparation of Ni and Fe doped molybdate-based catalyst from Ni-Fe layered double hydroxide for the catalytic wet air oxidation of dyes. Sci. Adv. Mater. 2015, 7, 1435–1442. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, R.; Gao, Y.; Zhao, Y.; Wang, J.; Huang, L.; Guo, J.; Zhou, T.; Lu, P.; Guo, Z. Novel Na2Mo4O13/α-MoO3 hybrid material as highly efficient CWAO catalyst for dye degradation at ambient conditions. Sci. Rep. 2014, 4, 6797. [Google Scholar] [CrossRef] [PubMed]
- Shokouhimehr, M.; Hong, K.; Lee, T.H.; Moon, C.W.; Hong, S.P.; Zhang, K.; Suh, J.M.; Choi, K.S.; Varma, R.S.; Jang, H.W. Magnetically retrievable nanocomposite adorned with Pd nanocatalysts: Efficient reduction of nitroaromatics in aqueous media. Green Chem. 2018, 20, 3809–3817. [Google Scholar] [CrossRef]
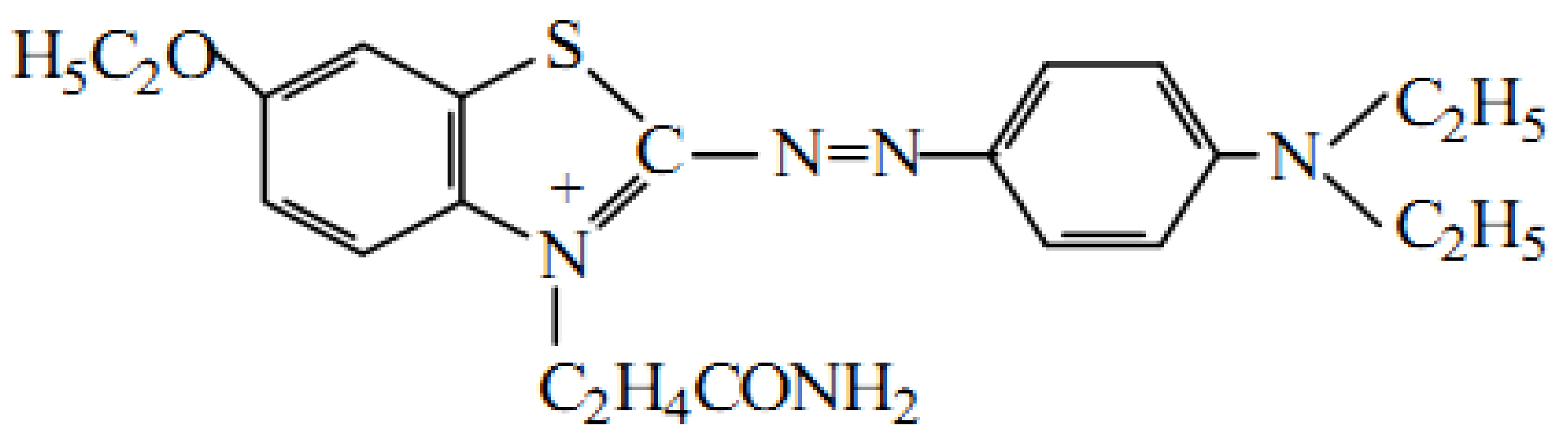

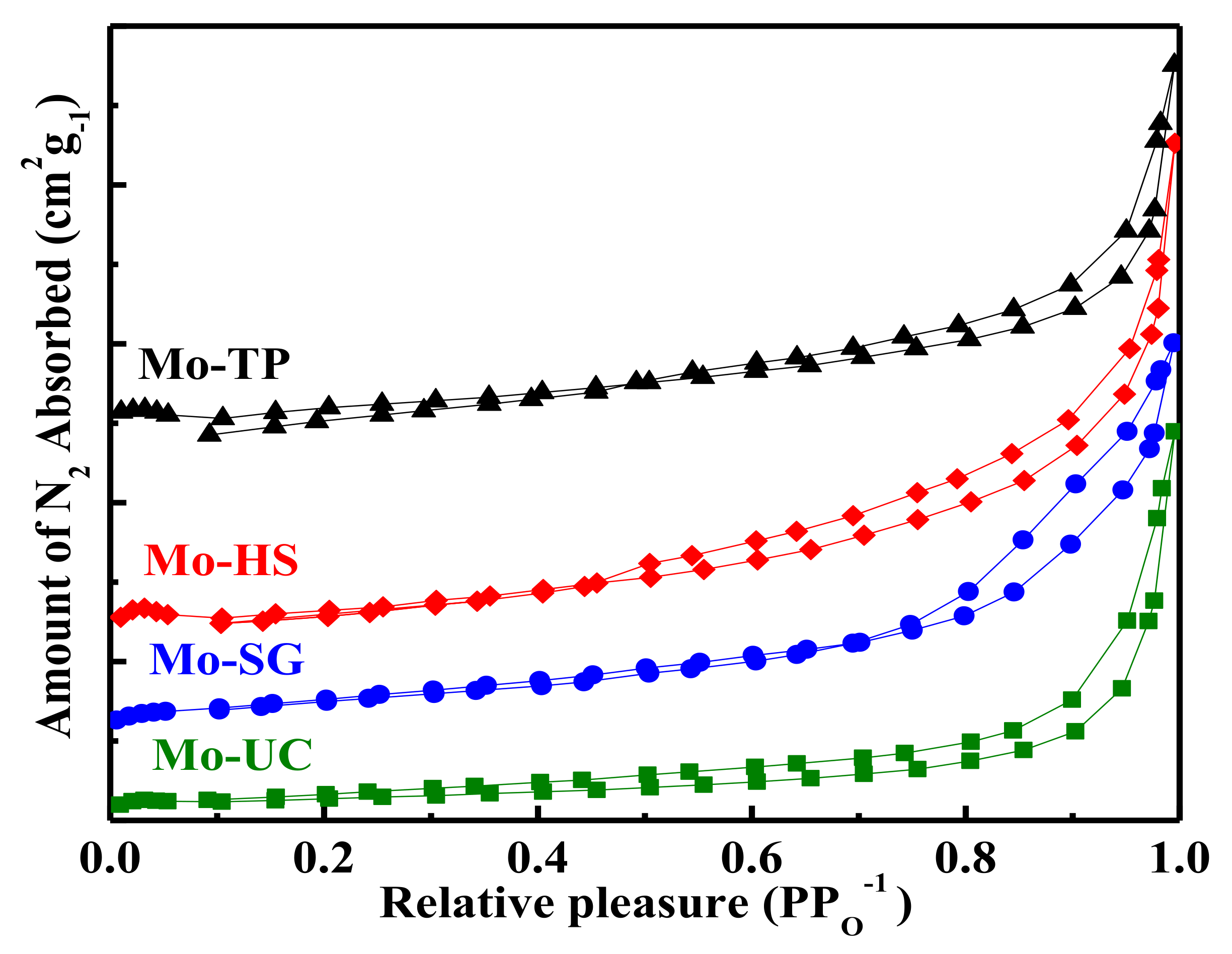

| Catalysts | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) | Zeta Potential (mV) |
|---|---|---|---|---|
| Mo-TP | 2.8 | 0.03 | 3.8 | −14.4 |
| Mo-HS | 3.9 | 0.01 | 3.8 | −18.4 |
| Mo-SG | 15.4 | 0.07 | 12.6 | −20.2 |
| Mo-UC | 5.8 | 0.04 | 2.2 | −11.2 |
| Catalysts | Mo (at %) | Zn (at %) | Al (at %) | O (at %) | Na (at %) | C (at %) |
|---|---|---|---|---|---|---|
| Mo-TP | 12.7 | 2.4 | 1.1 | 48.6 | 8.0 | 27.1 |
| Mo-HS | 15.0 | 3.5 | 0.7 | 55.8 | 7.4 | 17.7 |
| Mo-SG | 8.3 | 0.3 | 1.5 | 44.3 | 12.8 | 32.8 |
| Mo-UC | 10.3 | 2.0 | 6.7 | 23.4 | 5.4 | 52.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Liu, T.; Li, Y.; Liu, Y.; Ge, F. Nanostructure Design and Catalytic Performance of Mo/ZnAl-LDH in Cationic Orchid X-BL Removal. Materials 2018, 11, 2390. https://doi.org/10.3390/ma11122390
Xu Y, Liu T, Li Y, Liu Y, Ge F. Nanostructure Design and Catalytic Performance of Mo/ZnAl-LDH in Cationic Orchid X-BL Removal. Materials. 2018; 11(12):2390. https://doi.org/10.3390/ma11122390
Chicago/Turabian StyleXu, Yin, Tingjiao Liu, Yang Li, Yun Liu, and Fei Ge. 2018. "Nanostructure Design and Catalytic Performance of Mo/ZnAl-LDH in Cationic Orchid X-BL Removal" Materials 11, no. 12: 2390. https://doi.org/10.3390/ma11122390
APA StyleXu, Y., Liu, T., Li, Y., Liu, Y., & Ge, F. (2018). Nanostructure Design and Catalytic Performance of Mo/ZnAl-LDH in Cationic Orchid X-BL Removal. Materials, 11(12), 2390. https://doi.org/10.3390/ma11122390




