The Influence of PSA Pre-Anodization of AA2024 on PEO Coating Formation: Composition, Microstructure, Corrosion, and Wear Behaviors
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Surface Treatment
2.3. Characterization Methods
3. Results and Discussion
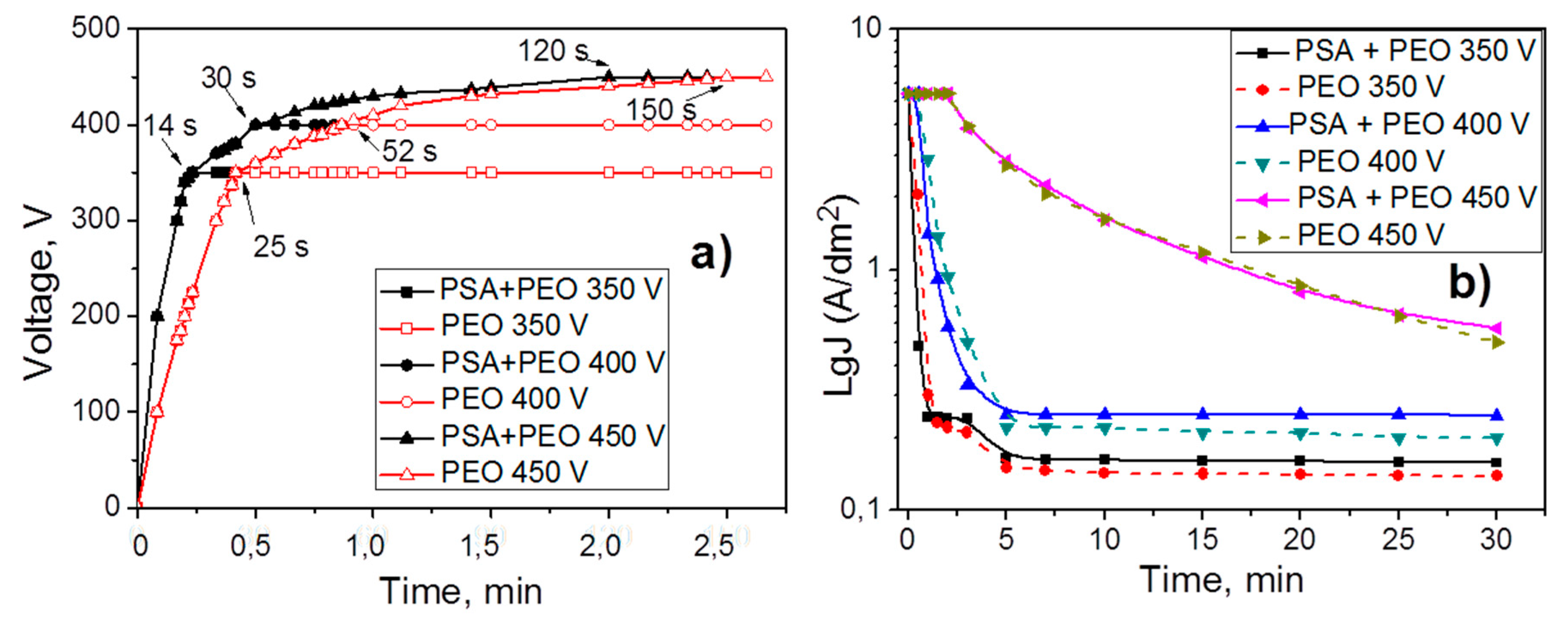
3.1. Voltage Evolution and Current Density Variation
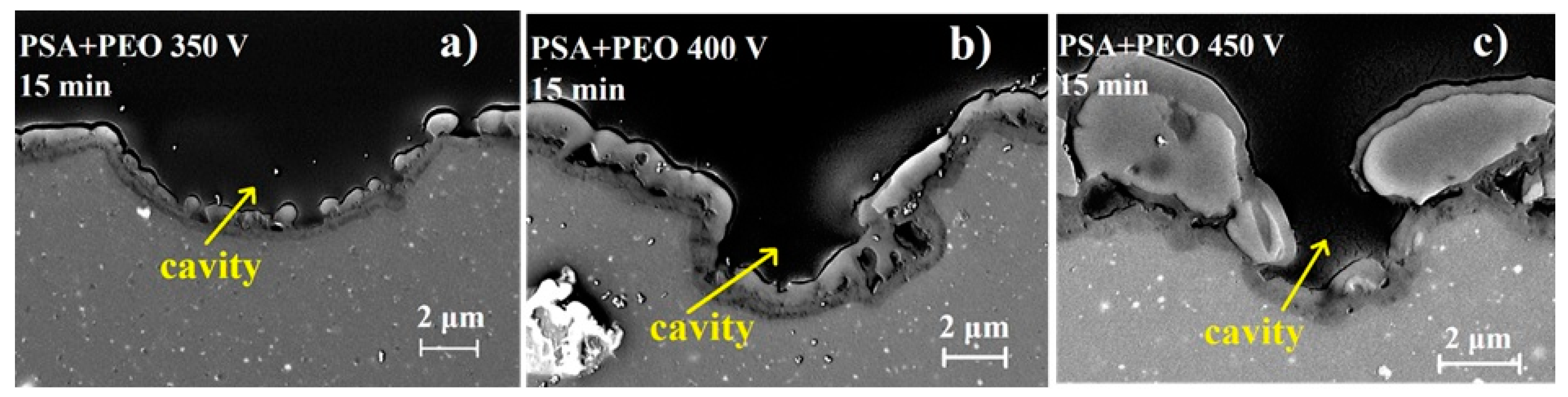
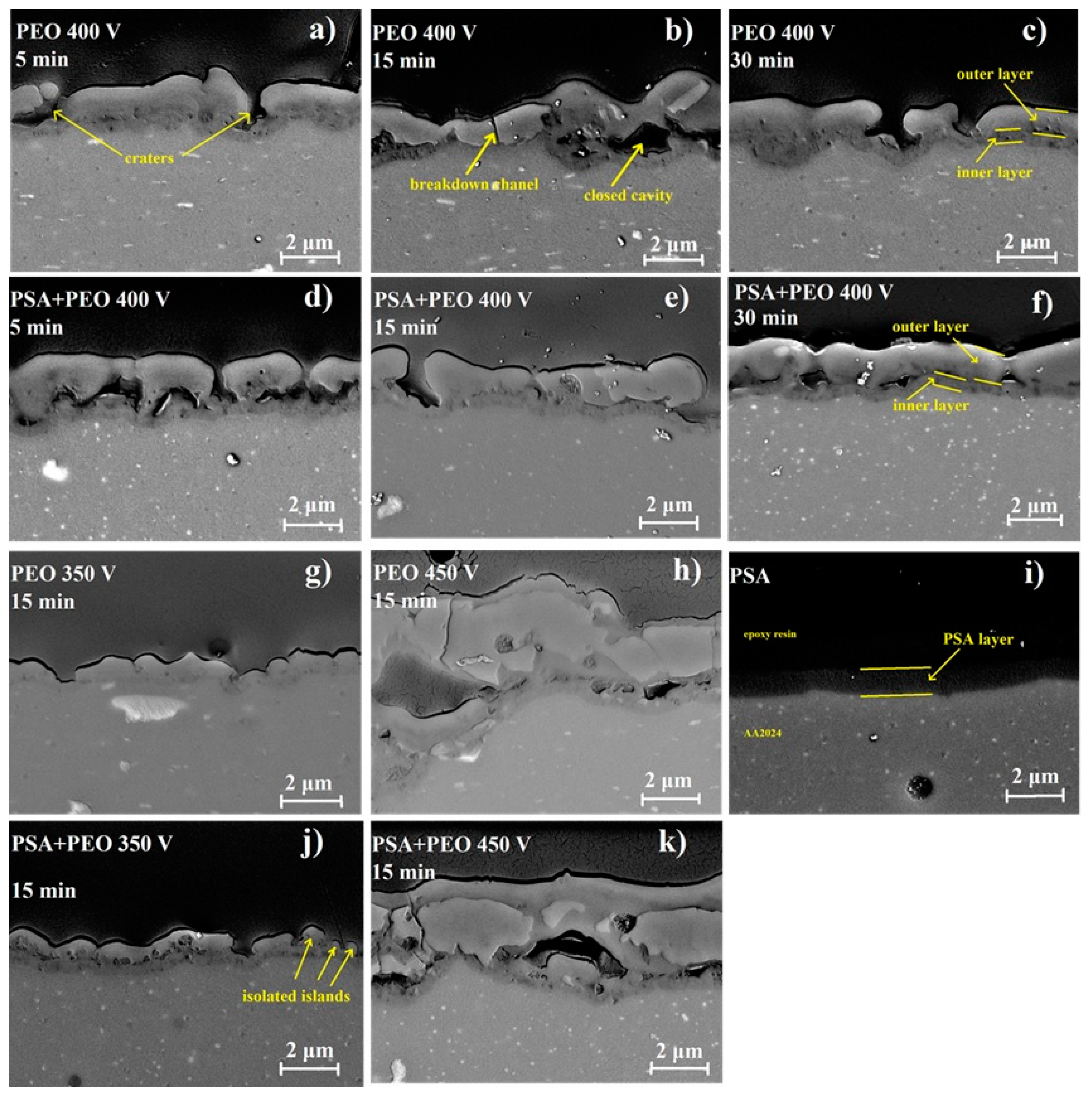
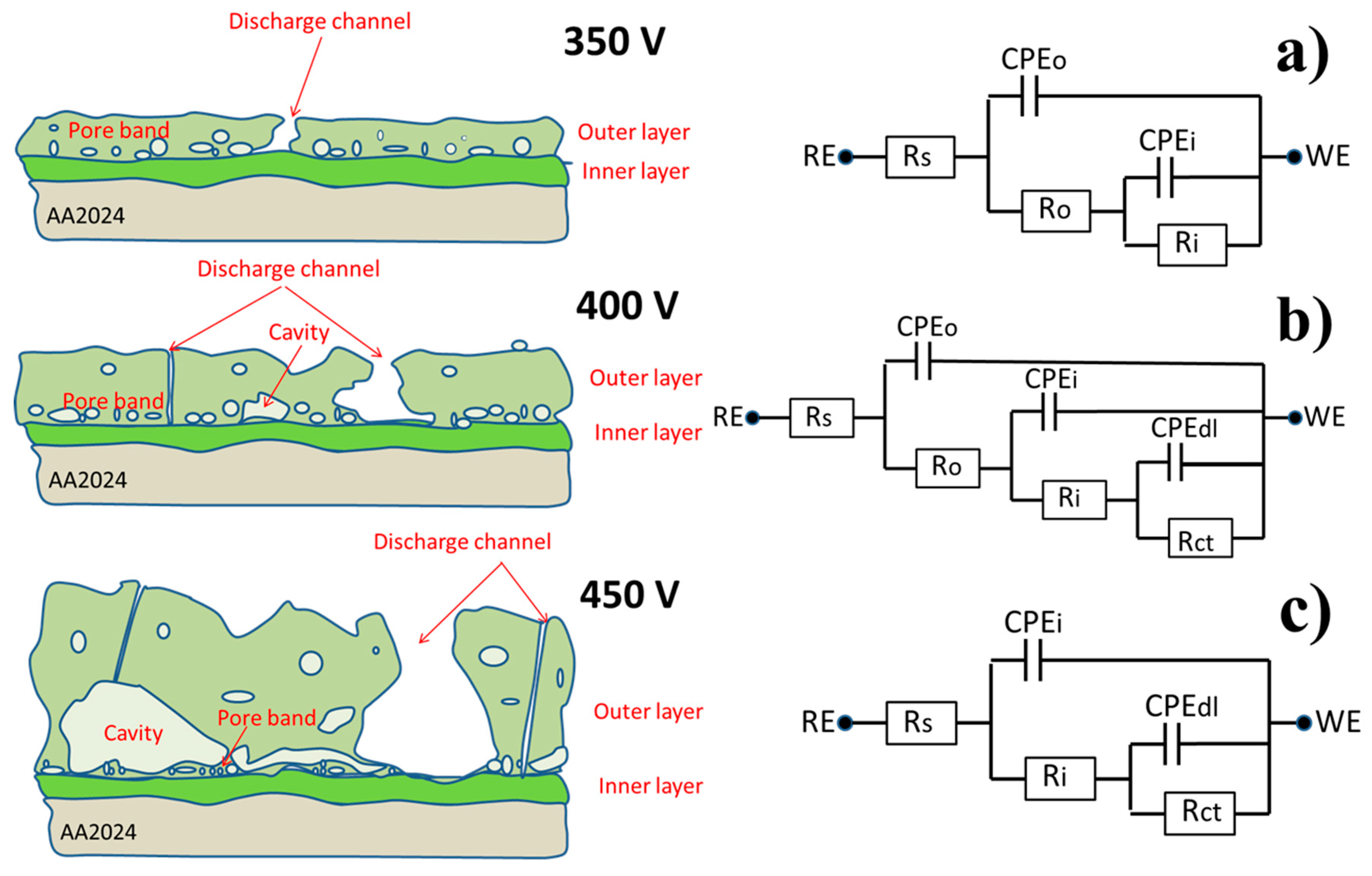
3.2. Surface and Cross-Sectional Morphologies
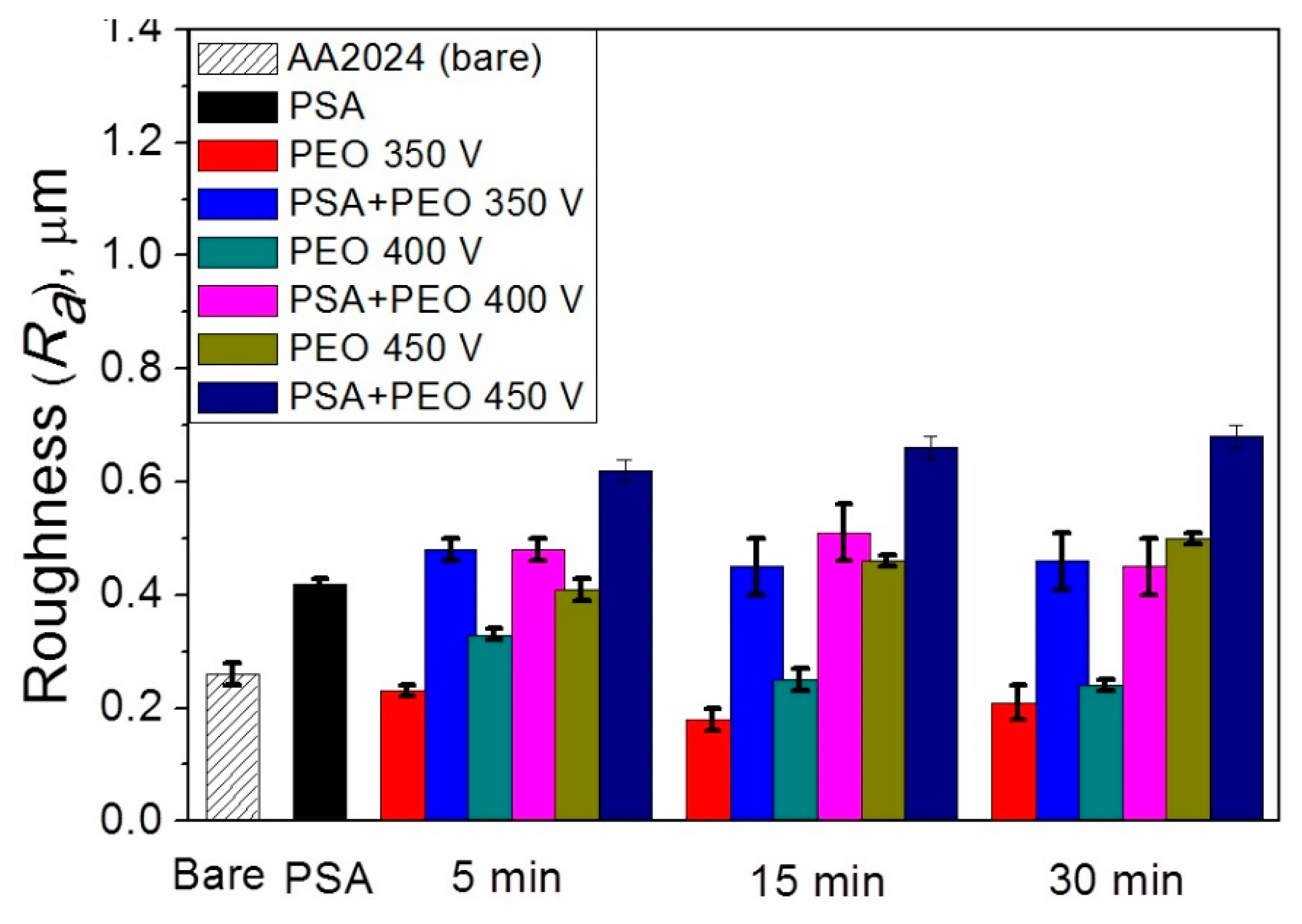
3.3. Roughness of the PEO Coating
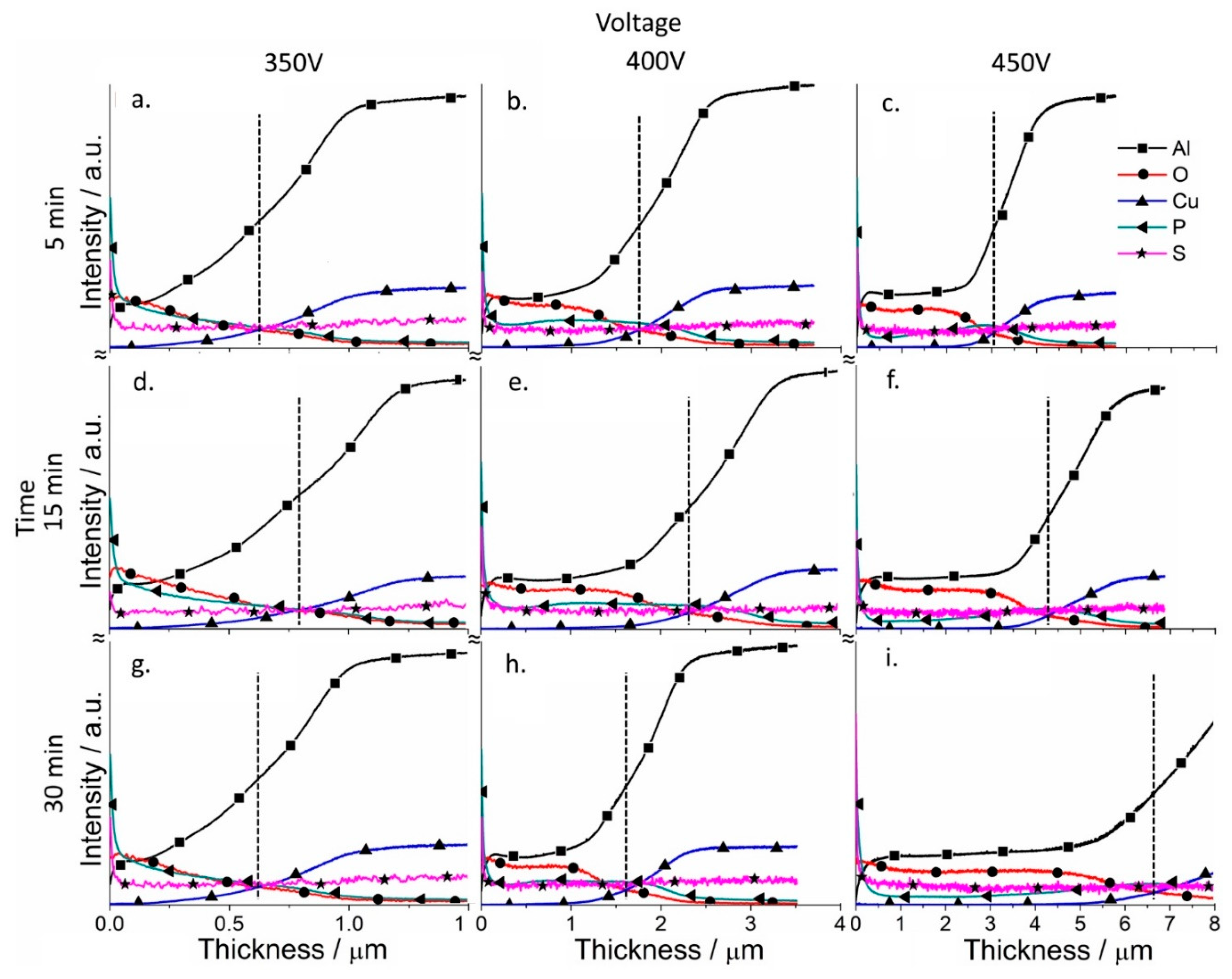
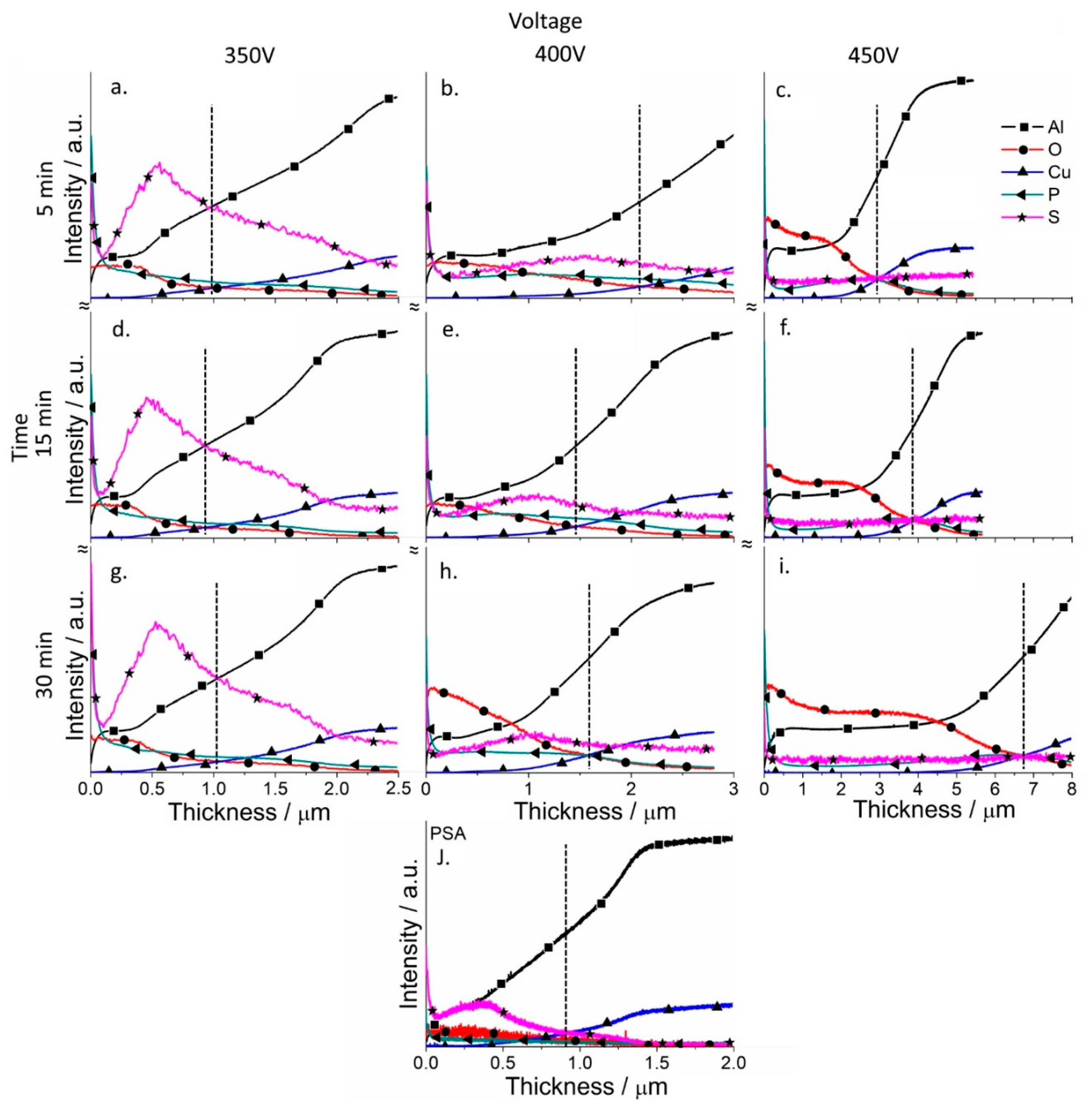
3.4. Chemical and Phase Composition of the Coatings
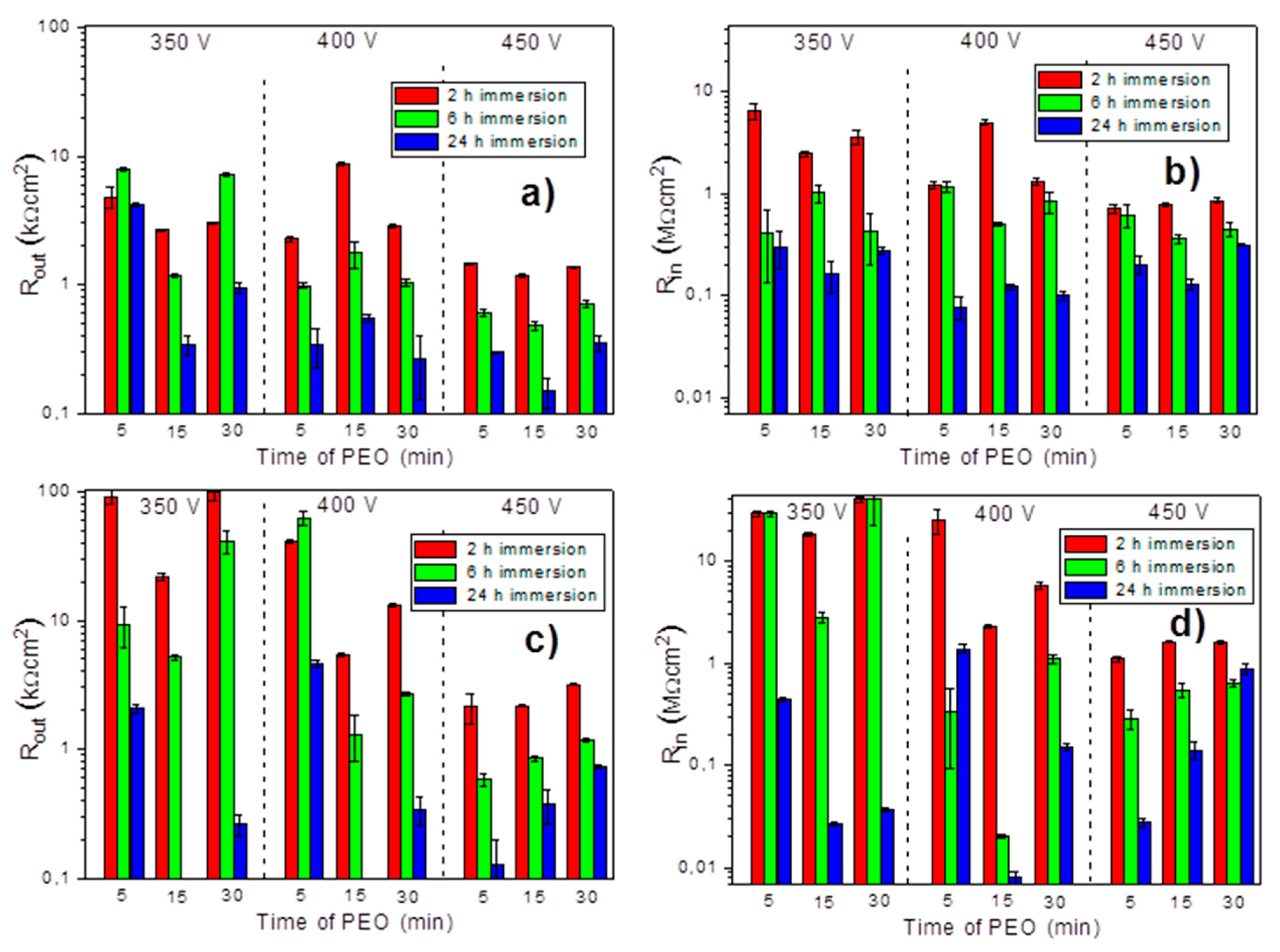
3.5. Corrosion Protection Performance of the PEO Coatings
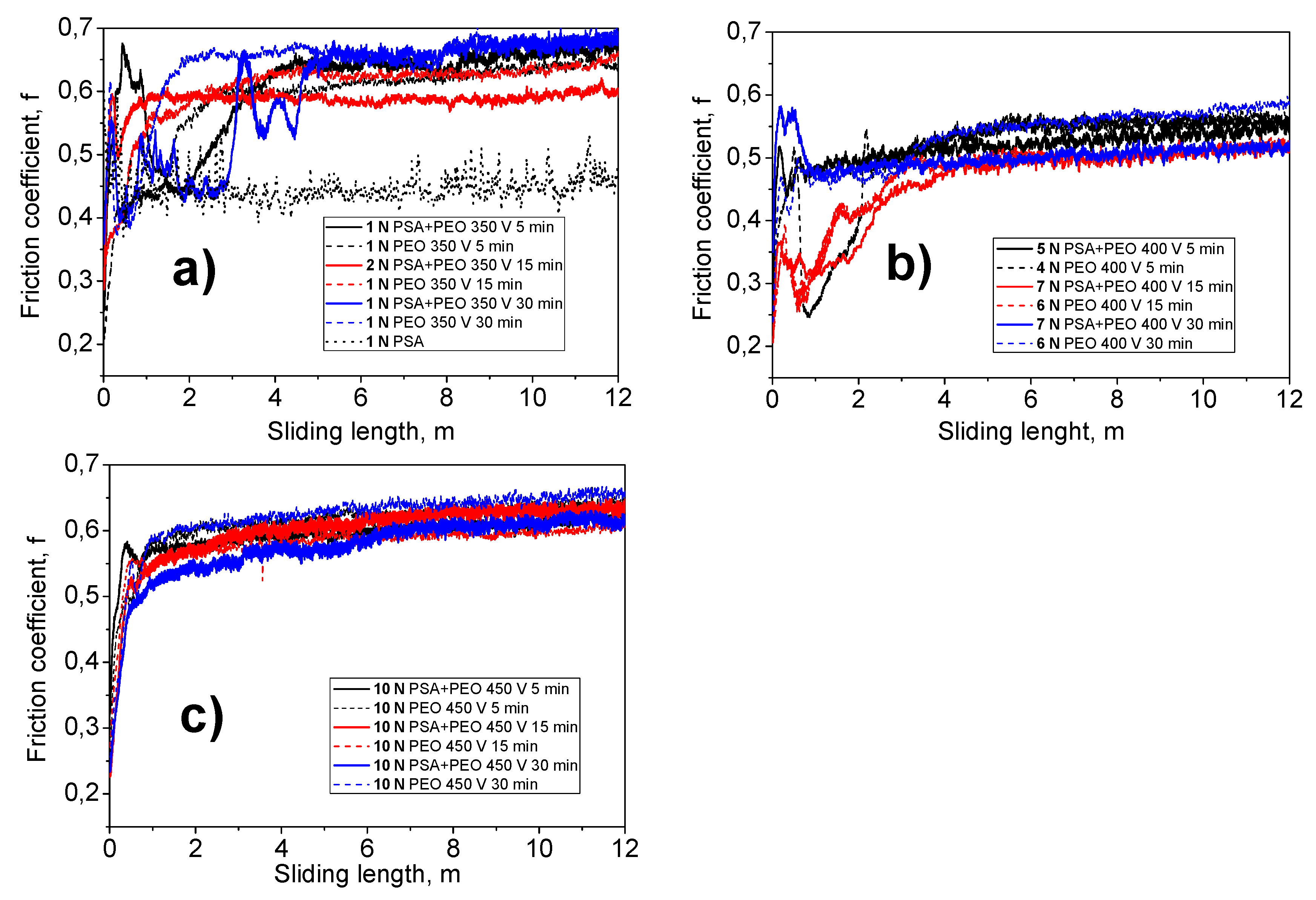
3.6. Wear Behavior of the Coatings
4. Conclusions
- (1)
- PEO coatings can be formed on preliminary PSA anodized AA2024 alloy, opening the possibility of local reinforcement for future industrial applications.
- (2)
- Preliminary formation of the PSA layer on the surface of aluminum alloy AA2024 facilitates further PEO processes and promotes the formation of PEO-coatings with lower crystallinity.
- (3)
- Under the CC mode, the PSA layer survives under the final voltage of 350 V, whilst at 400 V, there is an intermediate stage; and under 450 V, the PSA layer is fully converted after 5 min of the treatment.
- (4)
- It was shown that during the “sparking” mode (at 400 V) of PEO formation, the PEO coatings formed on the samples with a PSA layer were more wear resistant than the same PEO coatings on bare AA2024.
Author Contributions
Funding
Conflicts of Interest
References
- Dursun, T.; Soutis, C. Recent developments in advanced aircraft aluminium alloy. Mater. Des. 2014, 56, 862–871. [Google Scholar] [CrossRef]
- Starke, E.A., Jr.; Staley, J.T. Application of modern aluminum alloys to aircraft. Prog. Aerosp. Sci. 1996, 32, 131–172. [Google Scholar] [CrossRef]
- Shi, X.; Dalal, N.S. Generation of Hydroxyl Radical by Chromate in Biologically Relevant Systems: Role of Cr(V) Complexes versus Tetraperoxochromate(V). Environ. Health Perspect. 1994, 102, 231–236. [Google Scholar] [PubMed]
- Osborne, J.H. Observation on chromate conversion coatings from a sol-gel perspective. Prog. Org. Coat. 2001, 41, 280–286. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Serra, R.; Montemor, M.F.; Yasakau, K.A.; Salvado, I.M.M.; Ferreira, M.G.S. Nanostructured sol-gel coatings doped with cerium nitrate as pre-treatments for AA2024-T3: Corrosion protection performance. Electrochim. Acta 2005, 51, 208–217. [Google Scholar] [CrossRef]
- Van Put, M.A.; Abrahami, S.T.; Elisseeva, O.; de Kok, J.M.M.; Mol, J.M.C.; Terryn, H. Potentiodynamic anodizing of aluminum alloys in Cr(VI)-free electrolytes. Surf. Interface Anal. 2016, 48, 946–952. [Google Scholar] [CrossRef]
- Curioni, M.; Skeldon, P.; Koroleva, E.; Thompson, G.T.; Ferguson, J. Role of Tartaric Acid on the Anodizing and Corrosion Behavior of AA2024 T3 Aluminum Alloy. J. Electrochem. Soc. 2009, 156, C147–C153. [Google Scholar] [CrossRef]
- Trevino, M.; Garza-Montes-de-Oca, N.F.; Perez, A.; Hernandez-Rodriguez, M.A.L.; Juarez, A.; Colas, R. Wear of aluminium alloy coated by plasma electrolytic oxidation. Surf. Coat. Technol. 2012, 206, 2213–2219. [Google Scholar] [CrossRef]
- Curran, A.; Clyne, T.W. Thermo-physical properties of plasma electrolytic oxide coatings on aluminium. Surf. Coat. Technol. 2005, 199, 168–179. [Google Scholar] [CrossRef]
- Mertsalo, I.P.; Yavors’kyi, V.T.; Klapkiv, M.D.; Mardarevych, R.S. Wear resistance of anodic-spark coatings on aluminum alloys. Mater. Sci. 2003, 39, 136–139. [Google Scholar] [CrossRef]
- Matykina, E.; Arrabal, R.; Skeldon, P.; Thompson, G.E. Investigation of the growth processes of coatings formed by AC plasma electrolytic oxidation of aluminium. Surf. Coat. Technol. 2009, 54, 6767–6778. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Nie, X.; Leyland, A.; Matthews, A.; Dowey, S.J. Plasma electrolysis for surface engineering. Surf. Coat. Technol. 1999, 122, 73–93. [Google Scholar] [CrossRef]
- Yerokhin, A.L.; Shatrov, A.; Samsonov, V.; Shashkov, P.; Pilkington, A.; Leyland, A.; Matthews, A. Oxide ceramic coatings on aluminium alloys produced by a pulsed bipolar plasma electrolytic oxidation process. Surf. Coat. Technol. 2005, 199, 150–157. [Google Scholar] [CrossRef]
- Lugovskoy, A.; Zinigrad, M.; Kossenko, A.; Kazanski, B. Production of ceramic layers on aluminum alloys by plasma electrolytic oxidation in alkaline silicate electrolytes. Appl. Surf. Sci. 2013, 264, 743–747. [Google Scholar] [CrossRef]
- Rakoch, A.G.; Gladkova, A.A.; Linn, Z.; Strekalina, D.M. The evidence of cathodic micro-discharges during plasma electrolytic oxidation of light metallic alloys and micro-discharge intetsity depending on pH of the electrolyte. Surf. Coat. Technol. 2015, 269, 138–144. [Google Scholar] [CrossRef]
- Snizhko, L.O.; Yerokhin, A.L.; Pilkington, A.; Guverina, N.L.; Misnyankin, D.O.; Leyland, A.; Matthews, A. Anodic processes in plasma electrolytic oxidation of aluminium in alkaline solutions. Electrochim. Acta 2004, 49, 2085–2095. [Google Scholar] [CrossRef]
- Xue, W.; Wu, X.; Li, X.; Tian, H. Anti-corrosion film on 2024/SiC aluminum matrix composite fabricated by microarc oxidation in silicate electrolyte. J. Alloys Comp. 2006, 425, 302–306. [Google Scholar] [CrossRef]
- Javidi, M.; Fadaee, H. Plasma electrolytic oxidation of 2024-T3 aluminum alloy and investigation on microstructure and wear behavior. Appl. Surf. Sci. 2013, 286, 212–219. [Google Scholar] [CrossRef]
- Velterop, L. Phosphoric sulphuric acid anodizing: An alternative for chromic acid anodizing in aerospace applications? ATB Metall. 2003, 34, 284–289. [Google Scholar]
- Serdechnova, M.; Mohedano, M.; Bouali, A.; Höche, D.; Kuznetsov, B.; Karpushenkov, S.; Blawert, C.; Zheludkevich, M.L. Role of Phase Composition of PEO Coatings on AA2024 for In-Situ LDH Growth. Coatings 2017, 7, 190. [Google Scholar] [CrossRef]
- Serdechnova, M.; Mohedano, M.; Kuznetsov, B.; Mendis, C.L.; Starykevich, M.; Karpushenkov, S.; Tedim, J.; Ferreira, M.G.S.; Blawert, C.; Zheludkevich, M.L. PEO Coatings with Active Protection Based on In-Situ Formed LDH-Nanoconteiners. J. Electrochem. Soc. 2017, 164, C36–C45. [Google Scholar] [CrossRef]
- Matykina, E.; Arrabal, R.; Pardo, A.; Mohedano, M.; Mingo, B.; Rodriguez, I.; Gonzalez, J. Energy-efficient PEO process of aluminum alloys. Mater. Lett. 2014, 127, 13–16. [Google Scholar] [CrossRef]
- Dunleavy, S.C.; Curran, J.A.; Clyne, T.W. Time dependent statistics of plasma discharge parameters during bulk AC plasma electrolytic oxidation of aluminium. Appl. Surf. Sci. 2013, 268, 397–409. [Google Scholar] [CrossRef]
- Hussein, R.O.; Nie, X.; Northwood, D.O. Influence of process parameters on electrolytic plasma discharging behavior and aluminum oxide coating microstructure. Surf. Coat. Technol. 2010, 205, 1659–1667. [Google Scholar] [CrossRef]
- Walsh, F.C.; Low, C.T.J.; Wood, R.J.K.; Stevens, K.T.; Archer, J.; Poeton, A.R.; Ryder, A. Plasma electrolytic oxidation (PEO) for production of anodized coatings on lightweight metal (Al, Mg, Ti) alloys. Trans. Inst. Met. Ginishing 2009, 87, 122–135. [Google Scholar] [CrossRef]
- Xue, W.; Deng, Z.; Chen, R.; Zhang, T.; Ma, H. Microstructure and properties of ceramic coatings produced on AA2024 aluminum alloy by microarc oxidation. J. Mater. Sci. 2001, 36, 2615–2619. [Google Scholar] [CrossRef]
- Mohedano, M.; Serdechnova, M.; Starykevich, M.; Karpushenkov, S.; Bouali, A.C.; Ferreira, M.G.S.; Zheludkevich, M.L. Active protective PEO coatings on AA2024: Role of voltage on in-situ LDH growth. Mater. Des. 2017, 120, 36–46. [Google Scholar] [CrossRef]
- Srinivasan, P.B.; Liang, J.; Blawert, C.; Störmer, M.; Dietzel, W. Effect of current density on the microstructure and corrosion behavior of plasma electrolytic oxidation treated AM50 magnesium alloy. Appl. Surf. Sci. 2009, 255, 4212–4218. [Google Scholar] [CrossRef]
- Xu, J.; Liu, F.; Luo, J.; Zhao, L. Effects of anodic voltages on microstructure and properties of plasma electrolytic oxidation coatings on biomedical NiTi alloy. J. Mater. Sci. Technol. 2013, 29, 22–28. [Google Scholar] [CrossRef]
- Zhu, W.; Fang, Y.; Zheng, H.; Tan, G.; Cheng, H.; Ning, C. Effect of applied voltage on phase components of composite coatings preapared by micro-arc oxidation. Thin Solid Films 2013, 544, 79–82. [Google Scholar] [CrossRef]



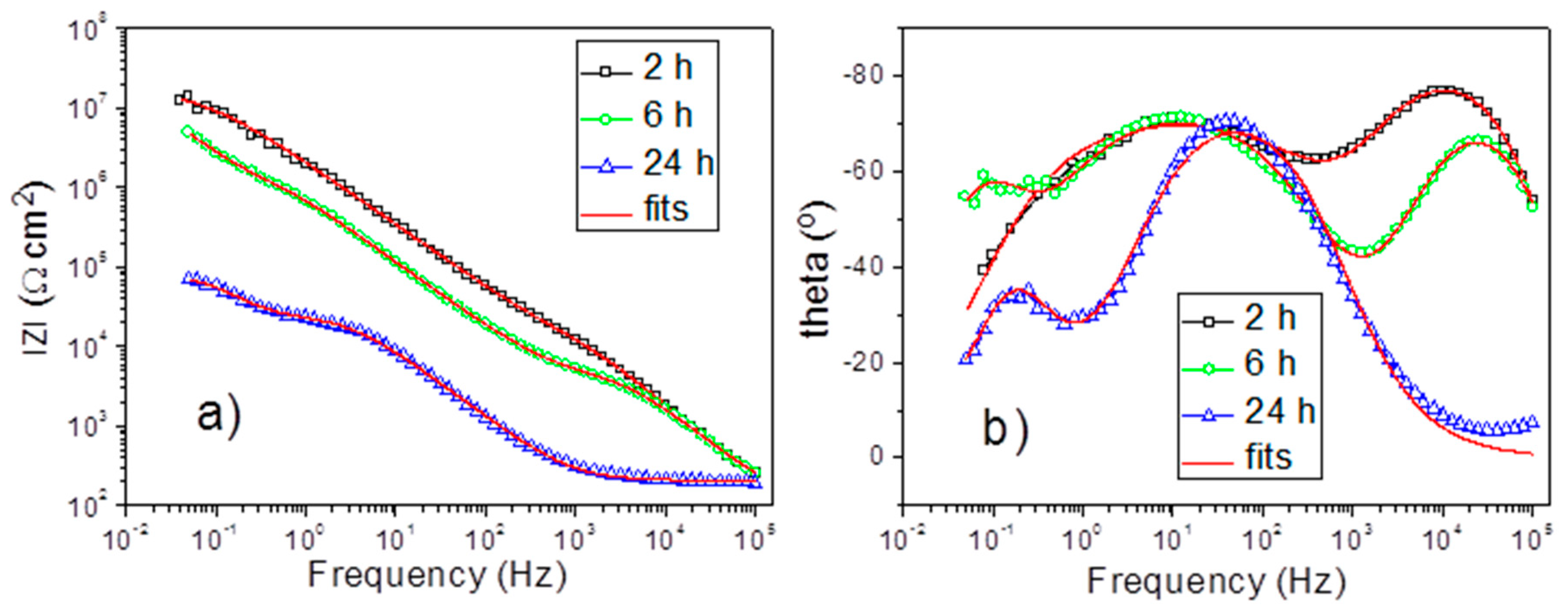
| Immersion Time (h) | 2 | 6 | 24 |
|---|---|---|---|
| Equivalent Circuit | A | B | C |
| Rsolut (Ω cm2) | 132.2 ± 6.6 | 117.3 ± 8.9 | 206.0 ± 1.7 |
| Rout (kΩ cm2) | 21.94 ± 1.66 | 5.197 ± 0.227 | - |
| Qout (nS cm−2) | 14.63 ± 1.34 | 23.07 ± 3.497 | - |
| nout | 0.951 ± 0.008 | 0.916 ± 0.313 | - |
| Rin (MΩ cm2) | 18.09 ± 0.80 | 2.80 ± 0.29 | 27.05 ± 0.58 |
| Qin (nS cm−2) | 95.62 ± 2.47 | 260.7 ± 10.3 | 2955.0 ± 920.2 |
| nin | 0.756 ± 0.004 | 0.813 ± 0.006 | 0.864 ± 0.004 |
| Rct (MΩ cm2) | - | 10.10 ± 1.89 | 0.053 ± 0.003 |
| Qdl (μS cm−2) | - | 0.350 ± 0.024 | 29300 ± 920 |
| ndl | - | 0.9997 ± 0.0741 | 1.000 ± 0.029 |
| Goodness | 0.534 × 10−3 | 0.518 × 10−3 | 2.484 × 10−3 |
| Specimen | Voltage, V | Time, min | Force, N max | Diameter Wear Ball, μm (mean) | Track Width, μm (mean) | Friction Coefficient | Static Friction Partner Wear Rate WK, mm3 N−1 m−1 | ||
|---|---|---|---|---|---|---|---|---|---|
| min | max | average | |||||||
| PSA | - | - | 1 (not) | 490.4 ± 61.5 | 508.8 ± 11.3 | 0.370 | 0.600 | 0.448 | 7.8773 × 10−5 |
| PEO | 350 | 5 | 1 | 378.8 ± 12.9 | 348.8 ± 15.2 | 0.208 | 0.661 | 0.587 | 2.7867 × 10−5 |
| PSA + PEO | 350 | 5 | 1 | 412.2 ± 7.8 | 389.4 ± 9.3 | 0.240 | 0.682 | 0.611 | 3.9339 × 10−5 |
| PEO | 350 | 15 | 1 | 397.8 ± 16.4 | 337.0 ± 12.3 | 0.242 | 0.665 | 0.605 | 3.3912 × 10−5 |
| PSA + PEO | 350 | 15 | 2 | 447.8 ± 14.1 | 435.0 ± 31.4 | 0.267 | 0.621 | 0.586 | 2.7264 × 10−5 |
| PEO | 350 | 30 | 1 | 421.0 ± 8.6 | 378.8 ± 16.4 | 0.305 | 0.704 | 0.642 | 4.2895 × 10−5 |
| PSA + PEO | 350 | 30 | 1 | 414.2 ± 4.9 | 420.6 ± 16.0 | 0.286 | 0.702 | 0.601 | 4.0108 × 10−5 |
| PEO | 400 | 5 | 4 | 500.2 ± 21.6 | 457.8 ± 15.0 | 0.212 | 0.575 | 0.511 | 2.1354 × 10−5 |
| PSA + PEO | 400 | 5 | 5 | 535.8 ± 8.7 | 532.0 ± 23.0 | 0.236 | 0.565 | 0.518 | 2.2392 × 10−5 |
| PEO | 400 | 15 | 6 | 485.6 ± 16.6 | 602.0 ± 31.0 | 0.189 | 0.534 | 0.473 | 1.2597 × 10−5 |
| PSA + PEO | 400 | 15 | 7 | 491.4 ± 2.2 | 459.4 ± 10.7 | 0.179 | 0.528 | 0.462 | 1.1345 × 10−5 |
| PEO | 400 | 30 | 6 | 537.2 ± 7.6 | 524.6 ± 14.5 | 0.212 | 0.598 | 0.534 | 1.8944 × 10−5 |
| PSA + PEO | 400 | 30 | 7 | 512.0 ± 2.4 | 484.4 ± 8.8 | 0.276 | 0.582 | 0.502 | 1.3417 × 10−5 |
| PEO | 450 | 5 | 10 | 747.4 ± 6.9 | 823.2 ± 9.2 | 0.227 | 0.653 | 0.611 | 4.2676 × 10−5 |
| PSA + PEO | 450 | 5 | 10 | 700.8 ± 5.4 | 736.0 ± 9.4 | 0.278 | 0.665 | 0.593 | 3.2877 × 10−5 |
| PEO | 450 | 15 | 10 | 708.0 ± 13.9 | 767.6 ± 8.9 | 0.248 | 0.618 | 0.580 | 3.4416 × 10−5 |
| PSA + PEO | 450 | 15 | 10 | 761.4 ± 7.4 | 851.8 ± 13.3 | 0.214 | 0.649 | 0.600 | 4.5967 × 10−5 |
| PEO | 450 | 30 | 10 | 774.4 ± 13.0 | 855.8 ± 17.0 | 0.243 | 0.671 | 0.620 | 4.9194 × 10−5 |
| PSA + PEO | 450 | 30 | 10 | 748.2 ± 7.6 | 819.0 ± 9.0 | 0.219 | 0.630 | 0.575 | 4.2898 × 10−5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serdechnova, M.; Karpushenkov, S.A.; Karpushenkava, L.S.; Starykevich, M.; Ferreira, M.G.S.; Hack, T.; Iuzviuk, M.H.; Zobkalo, I.A.; Blawert, C.; Zheludkevich, M.L. The Influence of PSA Pre-Anodization of AA2024 on PEO Coating Formation: Composition, Microstructure, Corrosion, and Wear Behaviors. Materials 2018, 11, 2428. https://doi.org/10.3390/ma11122428
Serdechnova M, Karpushenkov SA, Karpushenkava LS, Starykevich M, Ferreira MGS, Hack T, Iuzviuk MH, Zobkalo IA, Blawert C, Zheludkevich ML. The Influence of PSA Pre-Anodization of AA2024 on PEO Coating Formation: Composition, Microstructure, Corrosion, and Wear Behaviors. Materials. 2018; 11(12):2428. https://doi.org/10.3390/ma11122428
Chicago/Turabian StyleSerdechnova, Maria, Sergey A. Karpushenkov, Larisa S. Karpushenkava, Maksim Starykevich, Mario G. S. Ferreira, Theodor Hack, Mariia H. Iuzviuk, Igor A. Zobkalo, Carsten Blawert, and Mikhail L. Zheludkevich. 2018. "The Influence of PSA Pre-Anodization of AA2024 on PEO Coating Formation: Composition, Microstructure, Corrosion, and Wear Behaviors" Materials 11, no. 12: 2428. https://doi.org/10.3390/ma11122428
APA StyleSerdechnova, M., Karpushenkov, S. A., Karpushenkava, L. S., Starykevich, M., Ferreira, M. G. S., Hack, T., Iuzviuk, M. H., Zobkalo, I. A., Blawert, C., & Zheludkevich, M. L. (2018). The Influence of PSA Pre-Anodization of AA2024 on PEO Coating Formation: Composition, Microstructure, Corrosion, and Wear Behaviors. Materials, 11(12), 2428. https://doi.org/10.3390/ma11122428