Synthesis and Properties of Magnetic Aryl-Imidazolium Ionic Liquids with Dual Brønsted/Lewis Acidity
Abstract
:1. Introduction
2. Experiments
2.1. Chemicals
2.2. General Procedure for Synthesis of Target FeCl4 Anion Salts (5a–5d)
2.3. Characterization
2.4. Acidity Measurement
3. Results and Discussion
3.1. Synthesis and Characterization of B-L MILs
3.2. Solubility of B-L MILs
3.3. Thermal Stability of B-L MILs
3.4. Melting Point Properties of B-L MILs
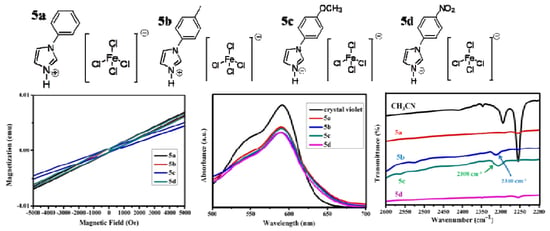
3.5. Magnetic Properties of B-L MILs
3.6. Brønsted Acidity Properties of B-L MILs
3.7. Lewis Acidity Properties of B-L MILs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wasserscheid, P.; Welton, T. Ionic Liquids in Synthesis; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar]
- Welton, T. Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem. Rev. 1999, 99, 2071–2084. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Qin, L.; Mu, T.; Xue, Z.; Gao, G. Are ionic liquids chemically stable. Chem. Rev. 2017, 117, 7113–7131. [Google Scholar] [CrossRef] [PubMed]
- Prodius, D.; Mudring, A.-V. Rare earth metal-containing ionic liquids. Coord. Chem. Rev. 2018, 363, 1–16. [Google Scholar] [CrossRef]
- Itoh, T. Ionic liquids as tool to improve enzymatic organic synthesis. Chem. Rev. 2017, 117, 10567–10607. [Google Scholar] [CrossRef] [PubMed]
- Vafaeezadeh, M.; Alinezhad, H. Brønsted acidic ionic liquids: Green catalysts for essential organic reactions. J. Mol. Liq. 2016, 218, 95–105. [Google Scholar] [CrossRef]
- Huang, H.-C.; Huang, C.-W.; Hsieh, C.-T.; Teng, H. Electric double layer capacitors of high volumetric energy based on ionic liquids and hierarchical-pore carbon. J. Mater. Chem. A 2014, 2, 14963–14972. [Google Scholar] [CrossRef]
- Huang, H.-C.; Yen, Y.-C.; Chang, J.-C.; Su, C.-W.; Chang, P.-Y.; Sun, I.-W.; Hsieh, C.-T.; Lee, Y.-L.; Teng, H. An ether bridge between cations to extend the applicability of ionic liquids in electric double layer capacitors. J. Mater. Chem. A 2016, 4, 19160–19169. [Google Scholar] [CrossRef]
- Cui, G.; Li, Y.; Liu, J.; Wang, H.; Li, Z.; Wang, J. Tuning environmental friendly chelate-based ionic liquids for highly efficient and reversible SO2 chemisorption. ACS Sustain. Chem. Eng. 2018, 6, 15292–15300. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.; Dong, K.; Zhang, Y.; Shen, Y.; Lv, X. Supported absorption of CO2 by tetrabutylphosphonium amino acid ionic liquids. Chem. Eur. J. 2006, 12, 4021–4026. [Google Scholar] [CrossRef] [PubMed]
- Egorova, K.S.; Gordeev, E.G.; Ananikov, V.P. Biological activity of ionic liquids and their application in pharmaceutics and medicine. Chem. Rev. 2017, 117, 7132–7189. [Google Scholar] [CrossRef]
- Smiglak, M.; Pringle, J.M.; Lu, X.; Han, L.; Zhang, S.; Gao, H.; MacFarlane, D.R.; Rogers, R.D. Ionic liquids for energy, materials, and medicine. Chem. Commun. 2014, 50, 9228–9250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, J.A.; Zhang, C.; Devasurendra, A.M.; Tillekeratne, L.M.V.; Anderson, J.L.; Kirchhoff, J.R. Conductive polymeric ionic liquids for electroanalysis and solid-phase microextraction. Anal. Chim. Acta 2016, 910, 45–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devasurendra, A.M.; Zhang, C.; Young, J.A.; Tillekeratne, L.M.V.; Anderson, J.L.; Kirchhoff, J.R. Electropolymerized pyrrole-based conductive polymeric ionic liquids and their application for solid-phase microextraction. ACS Appl. Mater. Interfaces 2017, 9, 24955–24963. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Huang, P.; Zhang, C.; Hu, H.; Bao, C.; Gao, G.; He, R.; Cui, D. Dual phase-controlled synthesis of uniform lanthanide-doped NaGdF4 upconversion nanocrystals via an OA/ionic liquid two-phase system for in vivo dual-modality imaging. Adv. Funct. Mater. 2011, 21, 4470–4477. [Google Scholar] [CrossRef]
- Izumi, R.; Yao, Y.; Tsuda, T.; Torimoto, T.; Kuwabata, S. Oxygen reduction electrocatalysts sophisticated by using Pt nanoparticle-dispersed ionic liquids with electropolymerizable additives. J. Mater. Chem. A 2018, 6, 11853–11862. [Google Scholar] [CrossRef]
- Talebi, M.; Patil, R.A.; Armstrong, D.W. Physicochemical properties of branched-chain dicationic ionic liquids. J. Mol. Liq. 2018, 256, 247–255. [Google Scholar] [CrossRef]
- Payagala, T.; Huang, J.; Breitbach, Z.S.; Sharma, P.S.; Armstrong, D.W. Unsymmetrical dicationic ionic liquids: Manipulation of physicochemical properties using specific structural architectures. Chem. Mater. 2007, 19, 5848–5850. [Google Scholar] [CrossRef]
- Zhang, H.; Li, M.; Yang, B. Design, synthesis, and analysis of thermophysical properties for imidazolium-based geminal dicationic ionic liquids. J. Phys. Chem. C 2018, 122, 2467–2474. [Google Scholar] [CrossRef]
- Moumene, T.; Belarbi, E.H.; Haddad, B.; Villemin, D.; Abbas, O.; Khelifa, B.; Bresson, S. Study of imidazolium dicationic ionic liquids by Raman and FTIR spectroscopies: The effect of the nature of the anion. J. Mol. Struct. 2015, 1083, 179–186. [Google Scholar] [CrossRef]
- Gindri, I.M.; Siddiqui, D.A.; Bhardwaj, P.; Rodriguez, L.C.; Palmer, K.L.; Frizzo, C.P.; Martins, M.A.P.; Rodrigues, D.C. Dicationic imidazolium-based ionic liquids: A new strategy for non-toxic and antimicrobial materials. RSC Adv. 2014, 4, 62594–62602. [Google Scholar] [CrossRef]
- Clark, K.D.; Emaus, M.N.; Varona, M.; Bowers, A.N.; Anderson, J.L. Ionic liquids: Solvents and sorbents in sample preparation. J. Sep. Sci. 2018, 41, 209–235. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.D.; Anderson, J.L. Ionic liquids as tunable materials in (bio) analytical chemistry. Anal. Bioanal. Chem. 2018, 410, 4565–4566. [Google Scholar]
- Li, M.; DeRooy, S.L.; Bwambok, D.K.; El-Zahab, B.; DiTusa, J.F.; Warner, I.M. Magnetic chiral ionic liquids derived from amino acids. Chem. Commun. 2009, 6922–6924. [Google Scholar] [CrossRef] [PubMed]
- Branco, A.; Branco, L.C.; Pina, F. Electrochromic and magnetic ionic liquids. Chem. Commun. 2011, 47, 2300–2302. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.; Butts, C.P.; Eastoe, J.; Hernandez, E.P.; Machadob, F.L.; de Oliveira, R.J. Dication magnetic ionic liquids with tuneable heteroanions. Chem. Commun. 2013, 49, 2765–2767. [Google Scholar] [CrossRef]
- Clark, K.D.; Nacham, O.; Purslow, J.A.; Pierson, S.A.; Anderson, J.L. Magnetic ionic liquids in analytical chemistry: A review. Anal. Chim. Acta 2016, 934, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.C.; Hogg, J.M.; Swadzba-Kwasny, M. Lewis acidic ionic liquids. Top. Curr. Chem. 2017, 375, 78–117. [Google Scholar] [CrossRef] [PubMed]
- Amarasekara, A.S. Acidic ionic liquids. Chem. Rev. 2016, 116, 6133–6183. [Google Scholar] [CrossRef] [PubMed]
- Skoda-Földes, R. The use of supported acidic ionic liquids in organic synthesis. Molecules 2014, 19, 8840–8884. [Google Scholar] [CrossRef] [PubMed]
- Santos, E.; Albo, J.; Irabien, A. Magnetic ionic liquids: Synthesis, properties and applications. RSC Adv. 2014, 4, 40008–40018. [Google Scholar] [CrossRef]
- Sharma, R.; Mahajan, R.K. Influence of various additives on the physicochemical properties of imidazolium based ionic liquids: A comprehensive review. RSC Adv. 2014, 4, 748–774. [Google Scholar] [CrossRef]
- Ahrens, S.; Peritz, A.; Strassner, T. Tunable aryl alkyl ionic liquids (TAAILs): The next generation of ionic liquids. Angew. Chem. Int. Ed. 2009, 48, 7908–7910. [Google Scholar] [CrossRef] [PubMed]
- Stolte, S.; Schulz, T.; Cho, C.-W.; Arning, J.; Strassner, T. Synthesis, toxicity, and biodegradation of tunable aryl alkyl ionic liquids (TAAILs). ACS Sustain. Chem. Eng. 2013, 1, 410–418. [Google Scholar] [CrossRef]
- Khalili, B.; Rimaz, M. An investigation on the physicochemical properties of the nanostructured [(4-X)PMAT][N(CN)2] ion pairs as energetic and tunable aryl alkyl amino tetrazolium based ionic liquids. J. Mol. Struct. 2017, 1137, 530–542. [Google Scholar] [CrossRef]
- Özdemir, M.C.; Özgün, B. Tunable aryl alkyl ionic liquids (TAAILs) based on 1-aryl-3,5-dimethyl-1H-pyrazoles. J. Mol. Liq. 2017, 248, 314–321. [Google Scholar] [CrossRef]
- Schroeter, F.; Lerch, S.; Kaliner, M.; Strassner, T. Cobalt-catalyzed hydroarylations and hydroaminations of alkenes in tunable aryl alkyl ionic liquids. Org. Lett. 2018, 20, 6215–6219. [Google Scholar] [CrossRef]
- Lage-Estebanez, I.; del Olmo, L.; López, R.; de la Vega, J.M.G. Molecular modeling and physicochemical properties of 1-alkyl-3-methylimidazolium-FeX4 and -Fe2X7 (X = Cl and Br) magnetic ionic liquids. J. Mol. Liq. 2018, 256, 175–182. [Google Scholar] [CrossRef]
- Yoshida, Y.; Saito, G. Influence of structural variations in 1-alkyl-3-methylimidazolium cation and tetrahalogenoferrate (III) anion on the physical properties of the paramagnetic ionic liquids. J. Mater. Chem. 2006, 16, 1254–1262. [Google Scholar] [CrossRef]
- Hayashi, S.; Hamaguchi, H. Discovery of a magnetic ionic liquid [bmim]FeCl4. Chem. Lett. 2004, 33, 1590–1591. [Google Scholar] [CrossRef]
- Sesto, R.E.D.; McCleskey, T.M.; Burrell, A.K.; Baker, G.A.; Thompson, J.D.; Scott, B.L.; Wilkes, J.S.; Williams, P. Structure and magnetic behavior of transition metal based ionic liquids. Chem. Commun. 2008, 447–449. [Google Scholar] [CrossRef]
- Bica, K.; Gaertner, P. An iron-containing ionic liquid as recyclable catalyst for aryl grignard cross-coupling of alkyl halides. Org. Lett. 2006, 8, 733–735. [Google Scholar] [CrossRef] [PubMed]
- Sayyahi, S.; Azin, A.; Saghanezhad, S.J. Synthesis and characterization of a novel paramagnetic functionalized ionic liquid as a highly efficient catalyst in one-pot synthesis of 1-amidoalkyl-2-naphtols. J. Mol. Liq. 2014, 198, 30–36. [Google Scholar] [CrossRef]
- Liu, H.; Zhao, R.; Song, X.; Liu, F.; Yu, S.; Liu, S.; Ge, X. Lewis acidic ionic liquid [Bmim]FeCl4 as a high efficient catalyst for methanolysis of poly (lactic acid). Catal. Lett. 2017, 147, 2298–2305. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Rafiee, F. Acidic Bronsted ionic liquids. Org. Prep. Proced. Int. 2010, 42, 285–362. [Google Scholar] [CrossRef]
- Solomons, G.; Fryhle, C.; Snyder, S. Organic Chemistry; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Liu, S.; Obuchi, A.; Oi-Uchisawa, J.; Nanbab, T.; Kushiyama, S. Synergistic catalysis of carbon black oxidation by Pt with MoO3 or V2O5. Appl. Catal. B 2001, 30, 259–265. [Google Scholar] [CrossRef]
- Tran, P.H.; Nguyen, H.T.; Hansen, P.E.; Le, T.N. An efficient and green method for regio- and chemo-selective Friedel–Crafts acylations using a deep eutectic solvent ([CholineCl][ZnCl2]3). RSC Adv. 2016, 6, 37031–37038. [Google Scholar] [CrossRef]
- Lunagariya, J.; Dhar, A.; Vekariya, R.L. Efficient esterification of n-butanol with acetic acid catalyzed by the Bronsted acidic ionic liquids: Influence of acidity. RSC Adv. 2017, 7, 5412–5420. [Google Scholar] [CrossRef]
- Chen, X.; Guo, H.; Abdeltawab, A.A.; Guan, Y.; Al-Deyab, S.S.; Yu, G.; Yu, L. Brønsted−lewis acidic ionic liquids and application in oxidative desulfurization of diesel fuel. Energy Fuels 2015, 29, 2998–3003. [Google Scholar] [CrossRef]
- Liu, S.; Wang, Z.; Li, K.; Li, L.; Yu, S.; Liu, F.; Song, Z. Brønsted-lewis acidic ionic liquid for the “one-pot” synthesis of biodiesel from waste oil. J. Renew. Sustain. Energy 2013, 5, 023111–023116. [Google Scholar] [CrossRef]
- Thomazeau, C.; Olivier-Bourbigou, H.; Magna, L.; Luts, S.; Gilbert, B. Determination of an acidic scale in room temperature ionic liquids. J. Am. Chem. Soc. 2003, 125, 5264–5265. [Google Scholar] [CrossRef]
- Yang, J.; Zhou, H.; Lu, X.; Yuan, Y. Brønsted acidic ionic liquid as an efficient and recyclable promoter for hydroesterification of olefins catalyzed by a triphenylphosphine-palladium complex. Catal. Commun. 2010, 11, 1200–1204. [Google Scholar] [CrossRef]
- Mihichuk, L.M.; Driver, G.W.; Johnson, K.E. Brønsted acidity and the medium: Fundamentals with a focus on ionic liquids. ChemPhysChem 2011, 12, 1622–1632. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-L.; Kou, Y. Determination of the lewis acidity of ionic liquids by means of an IR spectroscopic probe. Chem. Commun. 2004, 226–227. [Google Scholar] [CrossRef] [PubMed]
- Kouwer, P.H.J.; Swager, T.M. Synthesis and mesomorphic properties of rigid-core ionic liquid crystals. J. Am. Chem. Soc. 2007, 129, 14042–14052. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Pan, P. A spectrophotometric study of Fe(II)-chloride complexes in aqueous solutions from 10 to 100 °C. Can. J. Chem. 2001, 79, 131–144. [Google Scholar] [CrossRef]
- Hayashi, S.; Saha, S.; Hamaguchi, H. A new class of magnetic fluids: Bmim[FeCl4] and nbmim[FeCl4] ionic liquids. IEEE Trans. Magn. 2006, 42, 12–14. [Google Scholar] [CrossRef]
- Sitze, M.S.; Schreiter, E.R.; Patterson, E.V.; Freeman, R.G. Ionic liquids based on FeCl3 and FeCl2. Raman scattering and ab initio calculations. Inorg. Chem. 2001, 40, 2298–2304. [Google Scholar] [CrossRef] [PubMed]
- Housecroft, C.E.; Sharpe, A.G. Inorganic Chemistry; Pearson: New York, NY, USA, 2012. [Google Scholar]
- Crosthwaite, J.M.; Muldoon, M.J.; Dixon, J.K.; Anderson, J.L.; Brennecke, J.F. Phase transition and decomposition temperatures, heat capacities and viscosities of pyridinium ionic liquids. J. Chem. Thermodyn. 2005, 37, 559–568. [Google Scholar] [CrossRef]
- Anderson, J.L.; Armstrong, D.W. High-stability ionic liquids. A new class of stationary phases for gas chromatography. Anal. Chem. 2003, 75, 4851–4858. [Google Scholar] [CrossRef]
- Maton, C.; DeVos, N.; Stevens, C.V. Ionic liquid thermal stabilities: Decomposition mechanisms and analysis tools. Chem. Soc. Rev. 2013, 42, 5963–5977. [Google Scholar] [CrossRef]
- Baranyai, K.J.; Deacon, G.B.; MacFarlane, D.R.; Pringle, J.M.; Scott, J.L. Thermal degradation of ionic liquids at elevated temperatures. Aust. J. Chem. 2004, 57, 145–147. [Google Scholar] [CrossRef]
- Schulz, T.; Ahrens, S.; Meyer, D.; Allolio, C.; Peritz, A.; Strassner, T. Electronic effects of para-substitution on the melting points of TAAILs. Chem. Asian J. 2011, 6, 863–867. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.; Strassner, T. 1,2,4-Triazole-based tunable aryl/alkyl ionic liquids. J. Org. Chem. 2011, 76, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Kahn, O. Molecular Magnetism; Wiley-VCH: New York, NY, USA, 1993. [Google Scholar]
- Chang, J.-C.; Ho, W.-Y.; Sun, I.-W.; Chou, Y.-K.; Hsieh, H.-H.; Wu, T.-Y.; Liang, S.-S. Synthesis and properties of new (μ-oxo)bis[trichloroferrate(III)] dianion salts. Polyhedron 2010, 29, 2976–2984. [Google Scholar] [CrossRef]
- Chang, J.-C.; Ho, W.-Y.; Sun, I.-W.; Chou, Y.-K.; Hsieh, H.-H.; Wu, T.-Y. Synthesis and properties of new tetrachlorocobaltate (II) and tetrachloromanganate (II) anion salts with dicationic counterions. Polyhedron 2011, 30, 497–507. [Google Scholar] [CrossRef]
- Criado, J.J.; Jimenez-Sanchez, A.; Cano, F.H.; Saez-Puche, R.; Rodriguez-Fernandez, E. Preparation and characterization of tetrachlorocobaltates(II) of α,ω-alkylenediammonium. Magnetic and thermal properties. Crystal structure of [NH3(CH2)5NH3]CoCl4. Acta Crystallogr. B 1999, 55, 947–952. [Google Scholar] [CrossRef]
- Krieger, B.M.; Lee, H.Y.; Emge, T.J.; Wishart, J.F.; Castner, E.W., Jr. Ionic liquids and solids with paramagnetic anions. Phys. Chem. Chem. Phys. 2010, 12, 8919–8925. [Google Scholar] [CrossRef]










| Compound | Td (°C) a | Td (°C) b | Td (°C) c |
|---|---|---|---|
| 4a | 106 | 153 | 176 |
| 4b | 129 | 157 | 170 |
| 4c | 127 | 165 | 181 |
| 4d | 157 | 182 | 195 |
| 5a | 205 | 224 | 250 |
| 5b | 207 | 228 | 255 |
| 5c | 192 | 219 | 246 |
| 5d | 213 | 228 | 253 |
| Compound | DMSO | H2O | ACN | MeOH | EtOH | Acetone | EA | DCM | Et2O | Hex |
|---|---|---|---|---|---|---|---|---|---|---|
| ε a | 46.7 | 80.1 | 37.5 | 32.7 | 24.5 | 20.7 | 6.02 | 8.93 | 4.33 | 1.88 |
| 5a | + | + | + | + | + | + | + | + | ± | − |
| 5b | + | + | + | + | + | + | + | ± | ± | − |
| 5c | + | + | + | + | + | + | + | + | ± | − |
| 5d | + | + | + | + | + | + | + | ± | ± | − |
| Compound | DSC (°C) | MP-S3 (°C) a |
|---|---|---|
| 5a | 90.2 | 88.5–90.4 |
| 5b | 68.6 | 64.5–67.8 |
| 5c | 83.4 | 83.2–84.1 |
| 5d | 83.7 | 82.3–83.4 |
| Compound | χMT (emu K mole−1) | μeff | Θ (K) g | Reference |
|---|---|---|---|---|
| 5a | 4.85 | 6.23 | −16.28 | This work |
| 5b | 5.02 | 6.34 | −13.14 | This work |
| 5c | 4.01 | 5.66 | −10.08 | This work |
| 5d | 4.87 | 6.24 | −7.34 | This work |
| [Bmim][FeCl4] a | 4.11 | 5.73 | nr h | [40] |
| [Nbmim][FeCl4] b | 4.38 | 5.91 | nr h | [58] |
| [N1444][FeCl4] c | 4.42 | 5.95 | nr h | [71] |
| [Pyrr14][FeCl4] d | 4.47 | 5.98 | nr h | [71] |
| [C10mim][FeCl4] e | 4.01 | 5.66 | −3.30 | [41] |
| [Emim][FeCl4] f | 4.03 | 5.67 | −2.50 | [39] |
| Compound | Amax | [I] | [IH+] | Ho |
|---|---|---|---|---|
| Blank | 1.0874 | 100 | 0 | - |
| 5a | 0.8541 | 78.57 | 21.43 | 1.36 |
| 5b | 0.7934 | 73.12 | 26.88 | 1.23 |
| 5c | 0.8388 | 77.19 | 22.81 | 1.33 |
| 5d | 0.7899 | 72.59 | 27.05 | 1.23 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, J.-C.; Yang, C.-H.; Sun, I.-W.; Ho, W.-Y.; Wu, T.-Y. Synthesis and Properties of Magnetic Aryl-Imidazolium Ionic Liquids with Dual Brønsted/Lewis Acidity. Materials 2018, 11, 2539. https://doi.org/10.3390/ma11122539
Chang J-C, Yang C-H, Sun I-W, Ho W-Y, Wu T-Y. Synthesis and Properties of Magnetic Aryl-Imidazolium Ionic Liquids with Dual Brønsted/Lewis Acidity. Materials. 2018; 11(12):2539. https://doi.org/10.3390/ma11122539
Chicago/Turabian StyleChang, Jui-Cheng, Che-Hsuan Yang, I-Wen Sun, Wen-Yueh Ho, and Tzi-Yi Wu. 2018. "Synthesis and Properties of Magnetic Aryl-Imidazolium Ionic Liquids with Dual Brønsted/Lewis Acidity" Materials 11, no. 12: 2539. https://doi.org/10.3390/ma11122539
APA StyleChang, J.-C., Yang, C.-H., Sun, I.-W., Ho, W.-Y., & Wu, T.-Y. (2018). Synthesis and Properties of Magnetic Aryl-Imidazolium Ionic Liquids with Dual Brønsted/Lewis Acidity. Materials, 11(12), 2539. https://doi.org/10.3390/ma11122539