Influence of Building Direction on the Oxidation Behavior of Inconel 718 Alloy Fabricated by Additive Manufacture of Electron Beam Melting
Abstract
:1. Introduction
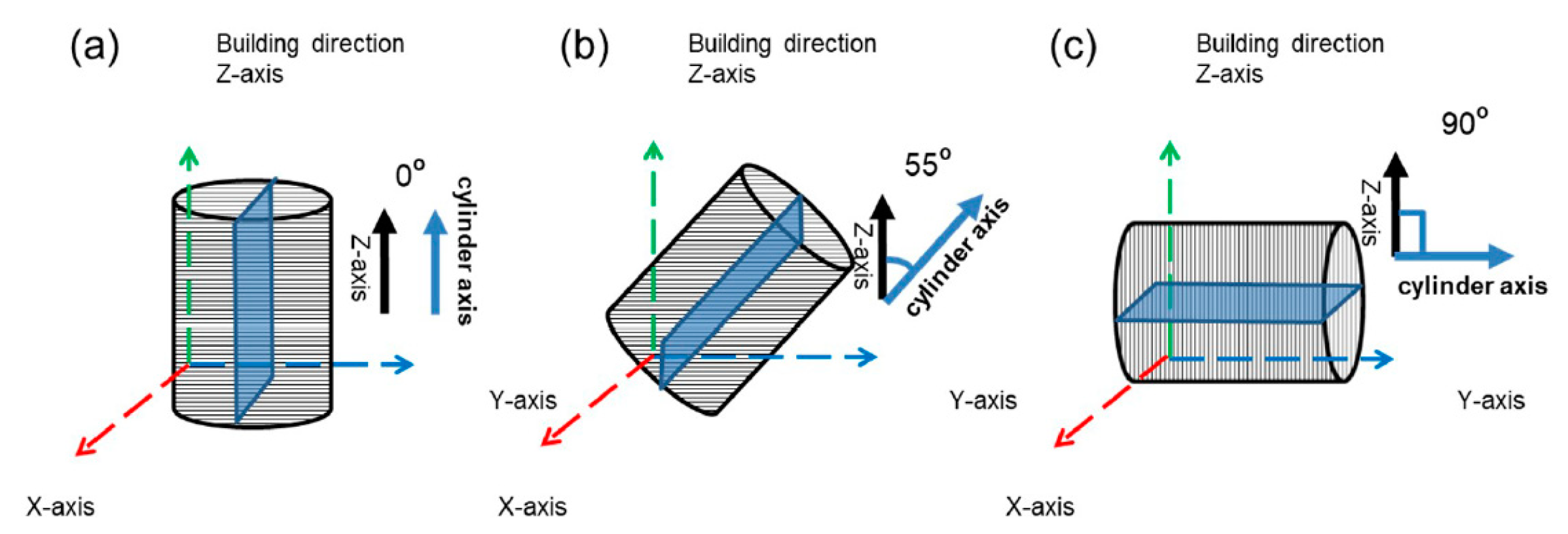
2. Materials and Experiments
2.1. Materials
2.2. Isothermal Oxidation Test
2.3. Microstructure Characterization
3. Results
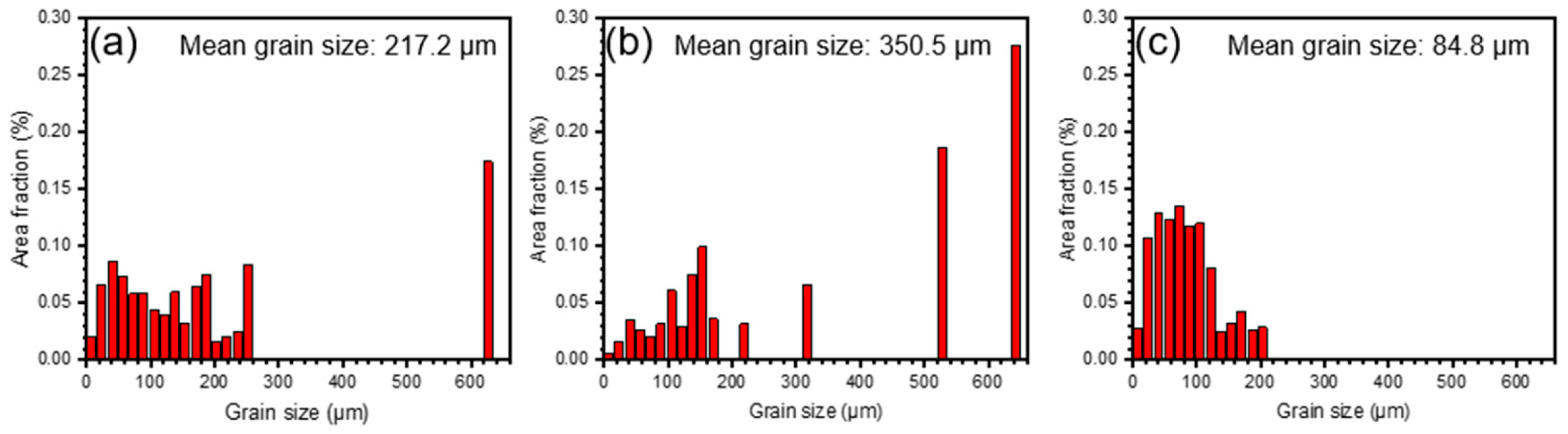
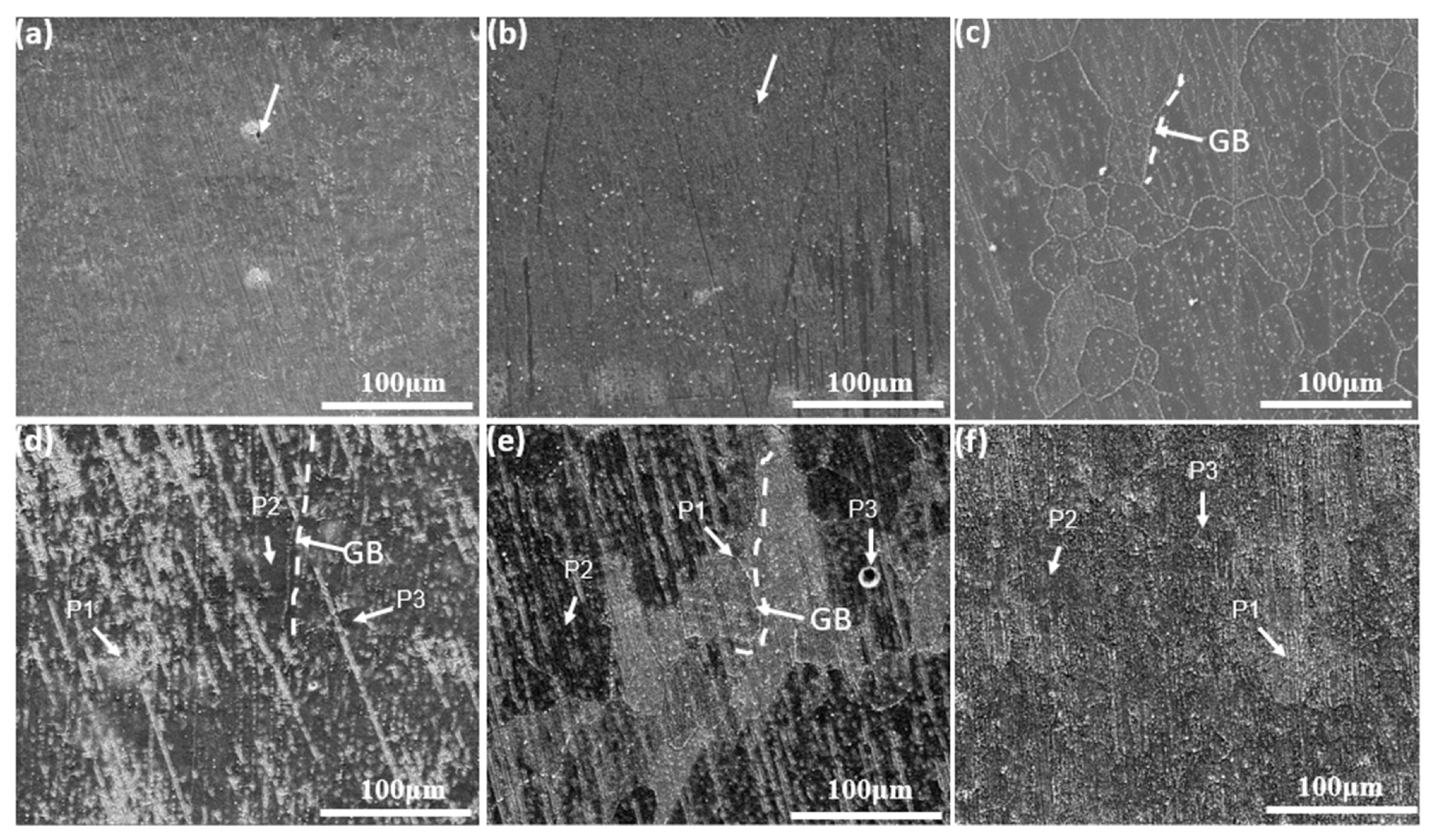
3.1. Alloy Characteristics
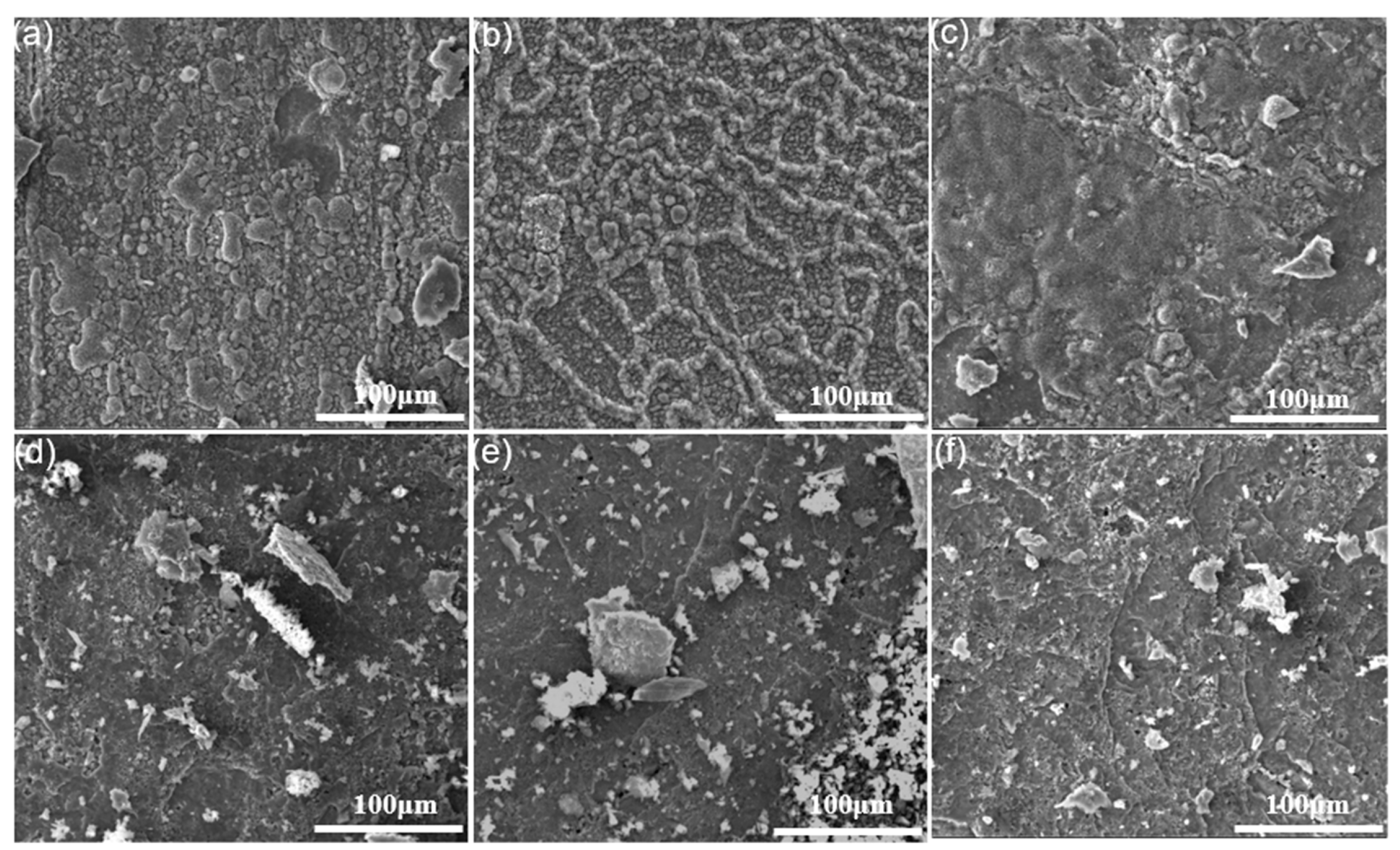
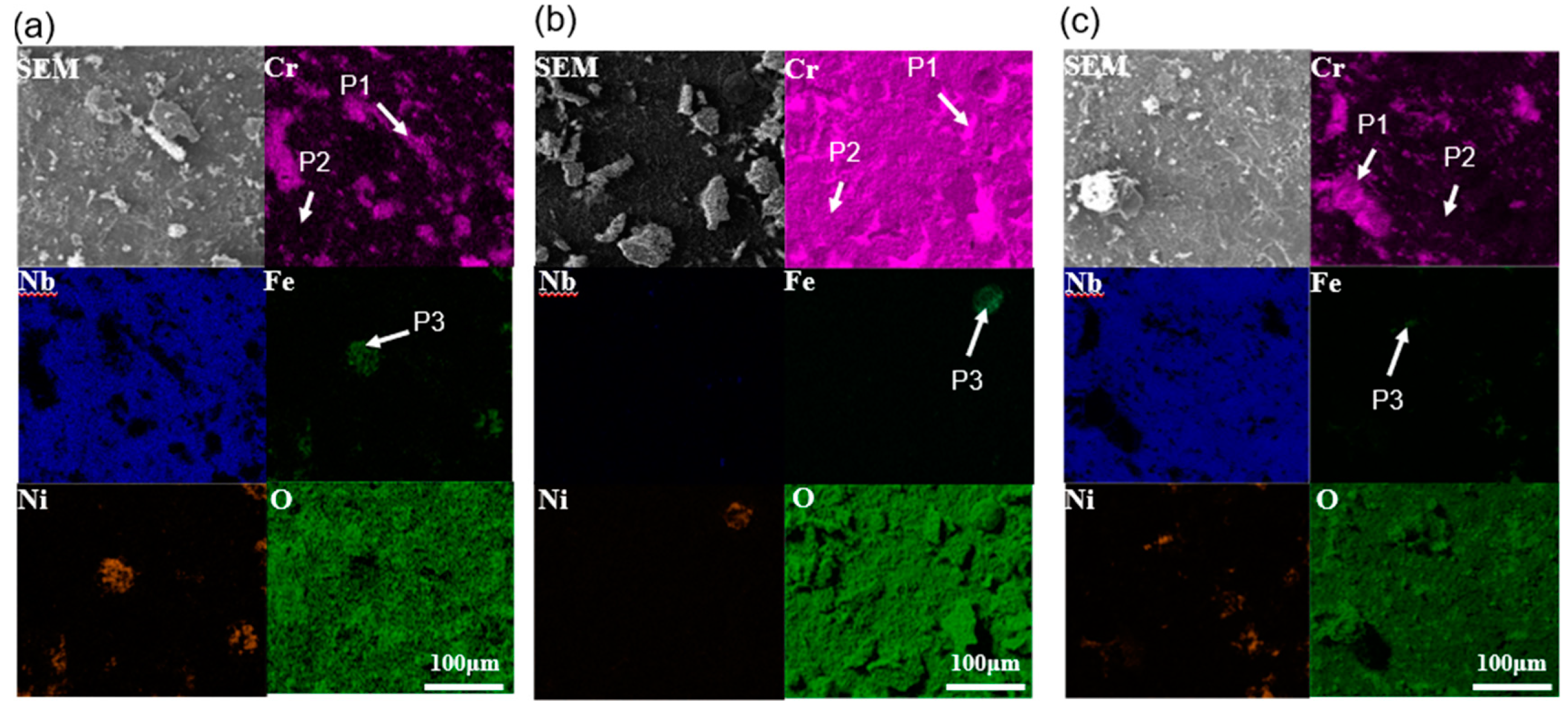
3.2. Characterization of Oxide Scales
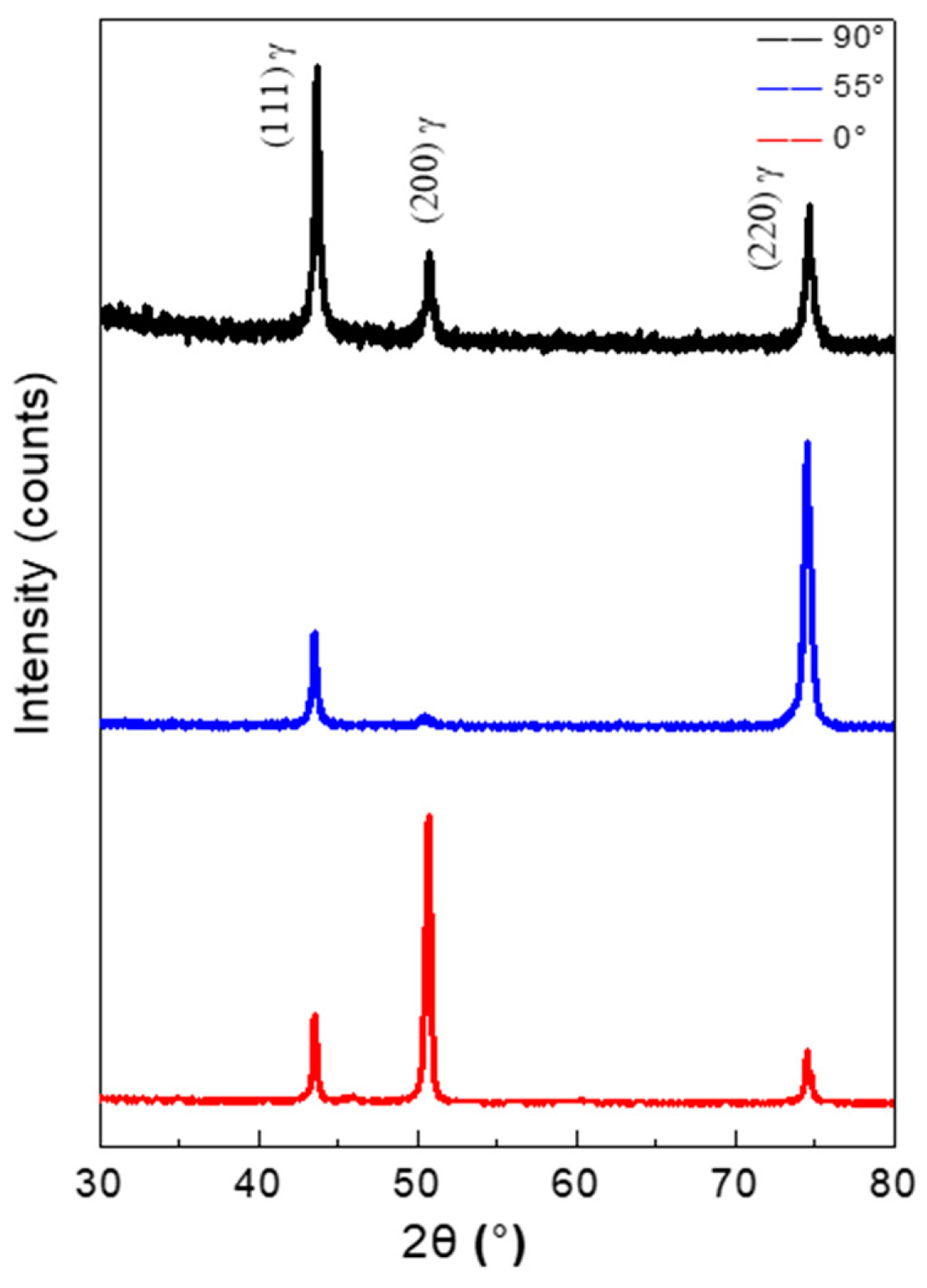
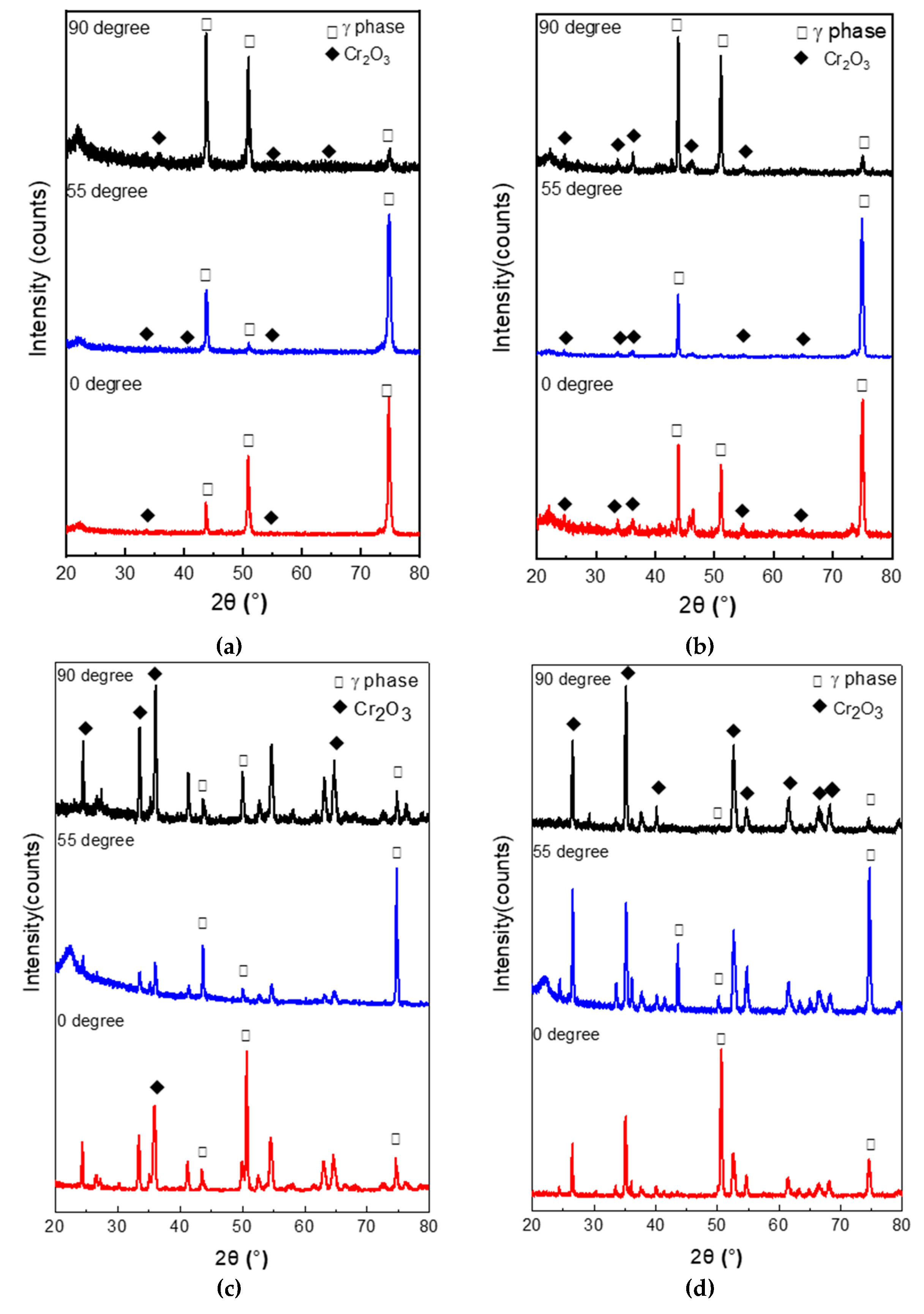
3.3. Phase Analysis of Oxide Scales
3.4. Cross-Section Profiles
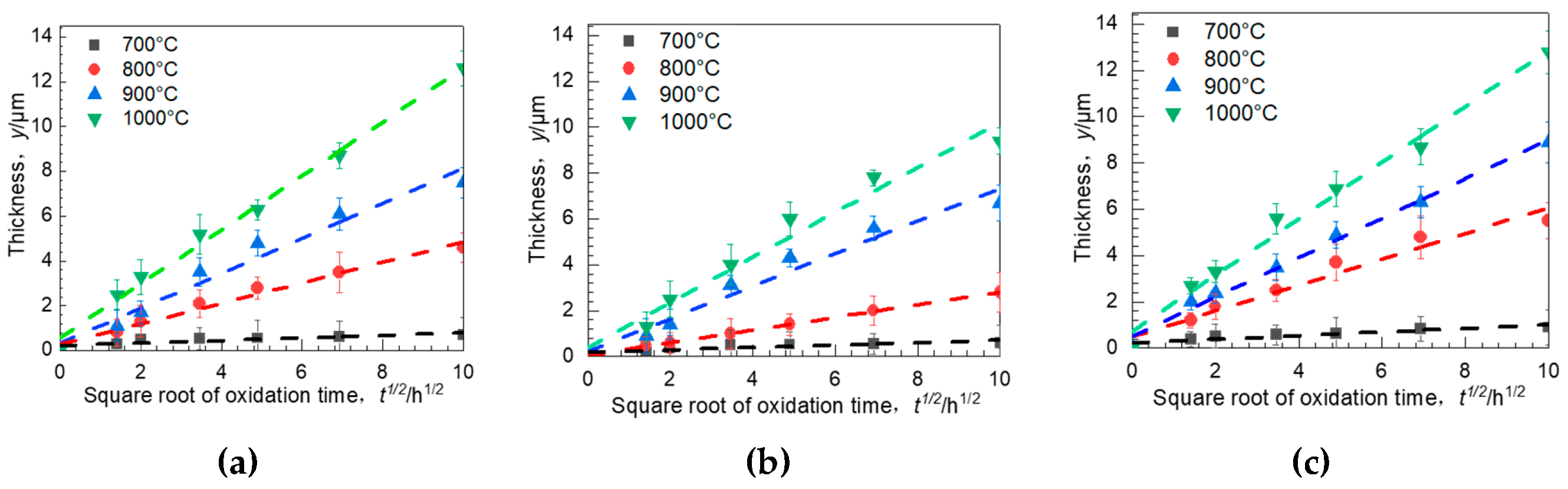
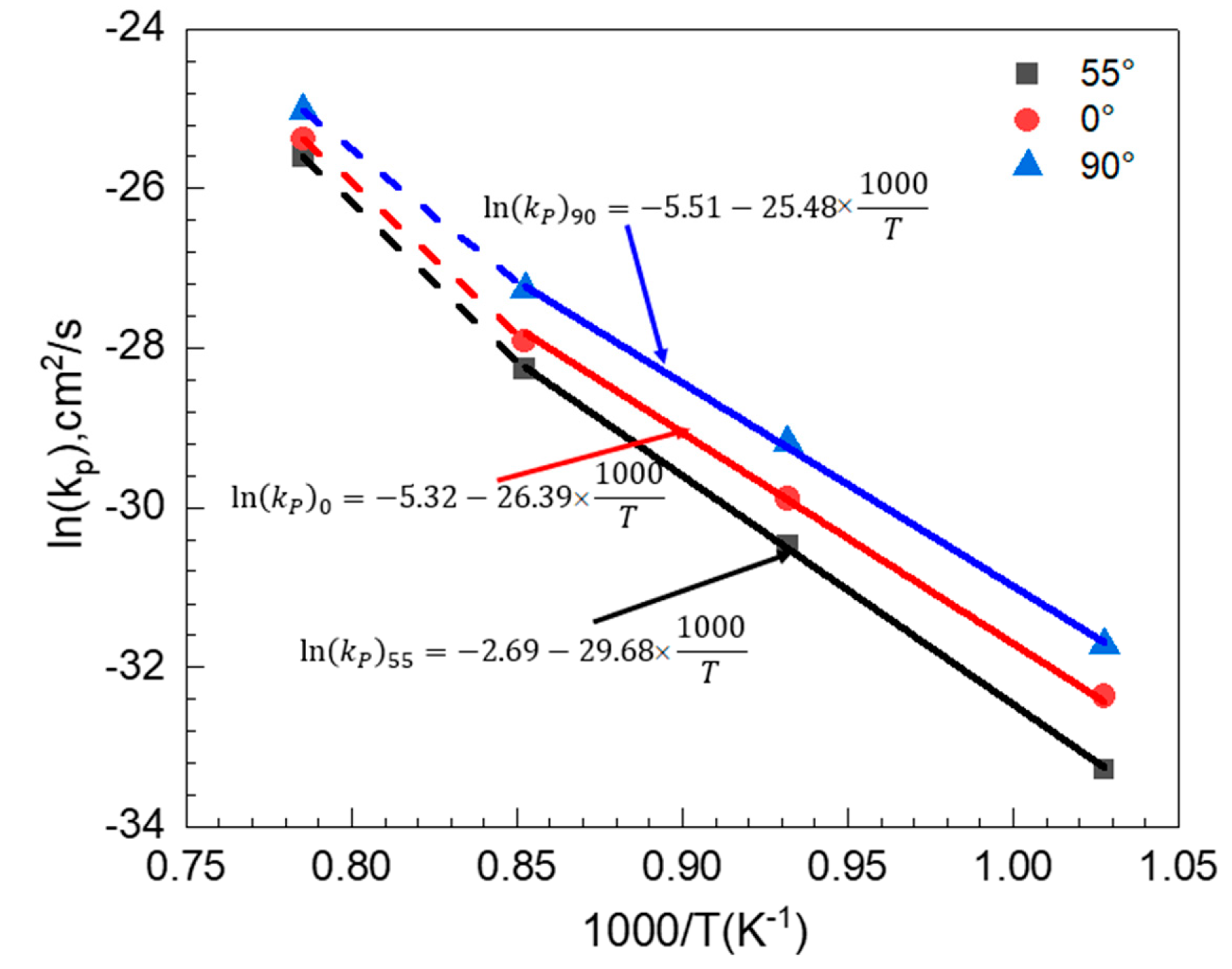
3.5. Oxidation Kinetics
4. Discussion
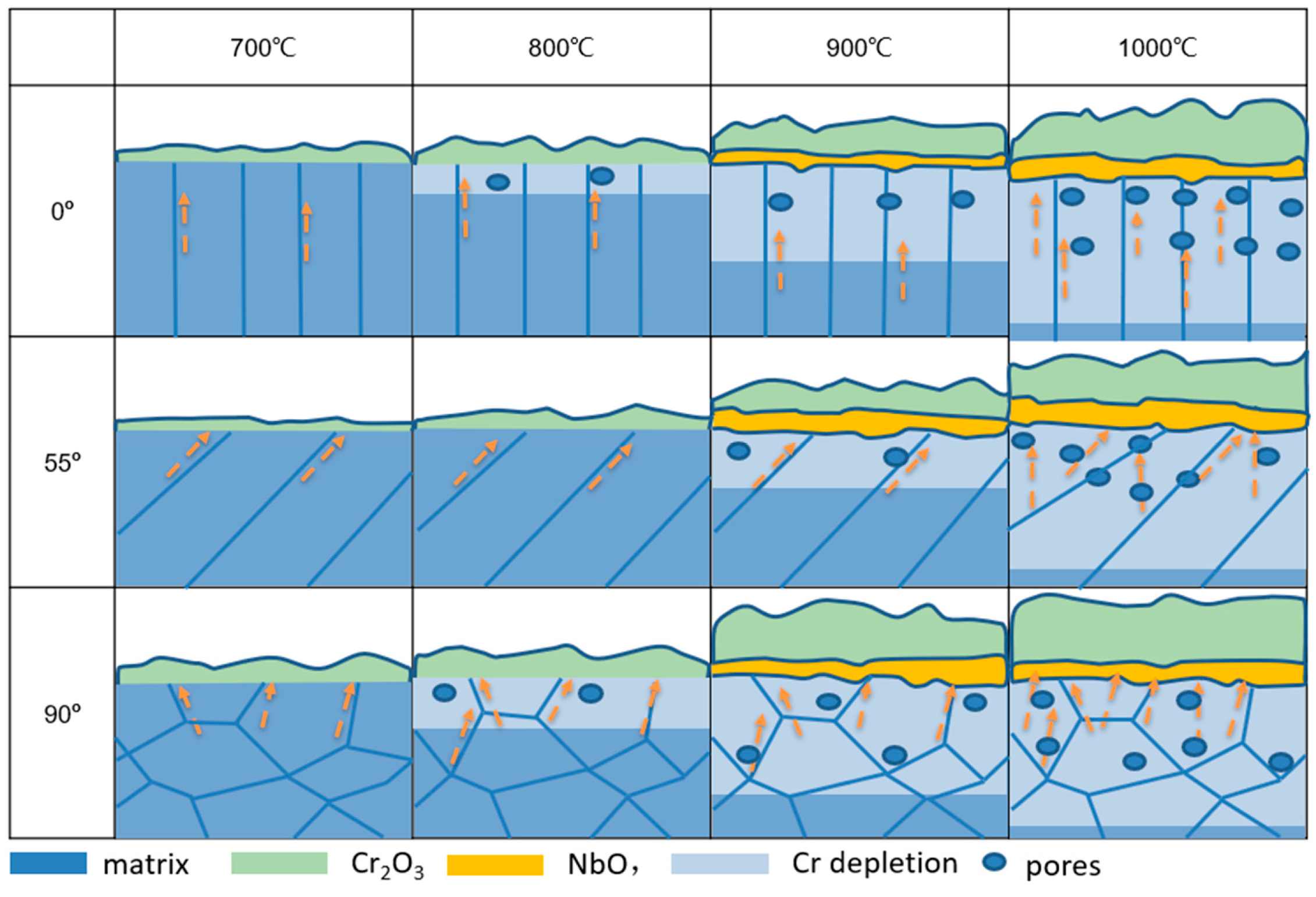
4.1. Oxidation Behavior of EBM-In718 Alloys
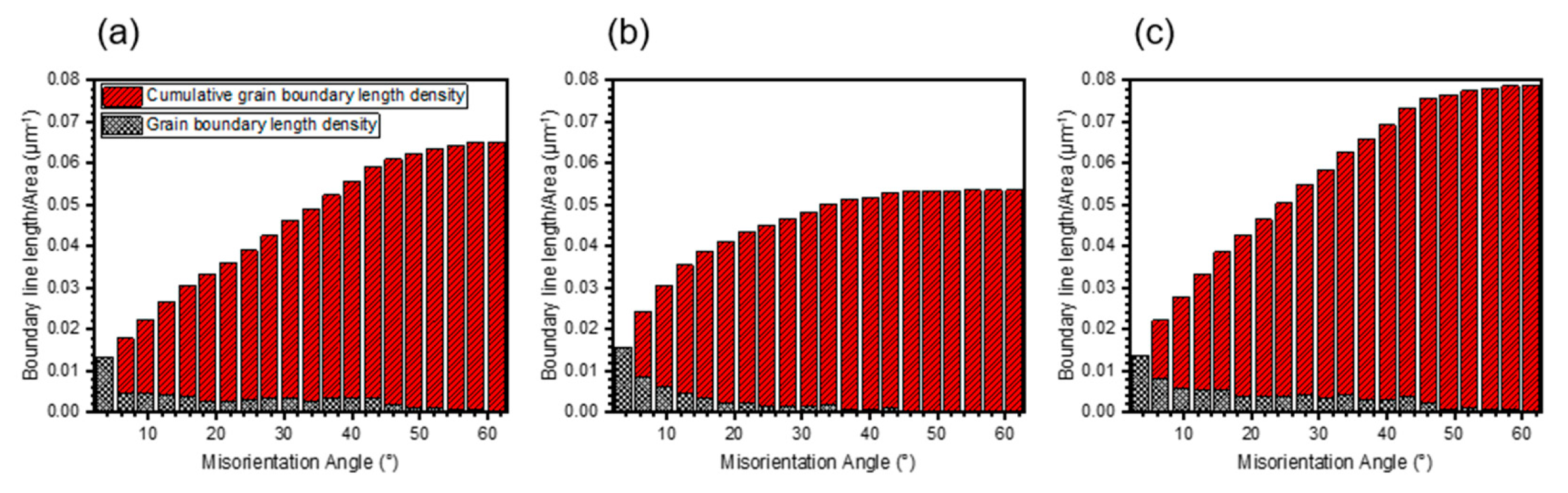
4.2. Effect of Grain Boundary Density on Scale Growth
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Decker, R.F. Strengthening Mechanisms in Nickel-Base Superalloys. In Proceedings of the Steel Strengthening Mechanisms Symposium, Zurich, Switzerland, 5–6 May 1969; pp. 1–23. [Google Scholar]
- Patterson, R.J.; Cox, A.R.; van Reuth, E.C. Rapid Solidification Rate Processing and Application to Turbine Engine Materials. JOM 2014, 32, 34–39. [Google Scholar] [CrossRef]
- Acharya, R.; Bansal, R.; Gambone, J.J.; Kaplan, M.A.; Fuchs, G.E.; Rudawski, N.G.; Das, S. Additive Manufacturing and Characterization of René 80 Superalloy Processed Through Scanning Laser Epitaxy for Turbine Engine Hot-Section Component Repair. Adv. Eng. Mater. 2015, 17, 942–950. [Google Scholar] [CrossRef]
- Christian, S.; Zimmermann, M.; HansJürgen, C. Effect of precipitation condition, prestrain and temperature on the fatigue behaviour of wrought nickel-based superalloys in the vhcf range. Acta Mater. 2011, 59, 5288–5304. [Google Scholar]
- Paa-Rai, C.; Norachan, J.; Wangyao, P.; Polsilapa, S.; Lothongkum, G. Effect of Long-Term Aging on Microstructural Restoration in Cast Nickel Base Superalloy, GTD-111. Mater. Sci. Forum 2017, 891, 426–432. [Google Scholar] [CrossRef]
- Rezaie, A.; Vahdat, S.E. Study of Effects of Temperature and Pressure in HIP Process on Mechanical Properties of Nickel-based Superalloys. Mater. Today 2017, 4, 152–156. [Google Scholar] [CrossRef]
- Yu, B.; Li, Y.; Nie, Y.; Mei, H. High temperature oxidation behavior of a novel cobalt-nickel-base superalloy. J. Alloys Compd. 2018, 765, 1148–1157. [Google Scholar] [CrossRef]
- Li, Y.; Ye, X.; Li, J.; Zhang, Y.; Koizumi, Y.; Chiba, A. Influence of cobalt addition on microstructure and hot workability of IN713C superalloy. Mater. Des. 2017, 122, 340–346. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Niu, W.; Cao, X.Y.; Liu, Z. Effect of standard heat treatment on the microstructure and mechanical properties of selective laser melting manufactured Inconel 718 superalloy. Mater. Sci. Eng. A 2015, 644, 32–40. [Google Scholar] [CrossRef]
- Helmer, H.E.; Körner, C.; Singer, R.F. Additive manufacturing of nickel-based superalloy Inconel 718 by selective electron beam melting: Processing window and microstructure. J. Mater. Res. 2014, 29, 1987–1996. [Google Scholar] [CrossRef]
- Mumtaz, K.A.; Hopkinson, N.; Stapleton, D.; Todd, I.; Derguti, F.; Vora, P. Benchmarking metal powder bed Additive Manufacturing processes (SLM and EBM) to build flat overhanging geometries without supports. In Proceedings of the Solid Freeform Fabrication Symposium, Austin, TX, USA, 6–8 August 2012. [Google Scholar]
- Guillen, G.; Marisela, S. Fundamental studies of Cobalt-base superalloy biomedical prototypes fabricated by electron beam melting: microstructures and microstructural architecture. Ph.D. Thesis, The University of Texas at El Paso, El Paso, TX, USA, December 2011. [Google Scholar]
- Gong, X.; Li, Y.; Nie, Y.; Huang, Z.; Liu, F.; Huang, L.; Jiang, L.; Mei, H. Corrosion behaviour of CoCrMo alloy fabricated by electron beam melting. Corros. Sci. 2018, 139, 68–75. [Google Scholar] [CrossRef]
- Bian, H.; Li, Y.; Wei, D.; Cui, Y.; Wang, F.; Sun, S.; Yamanaka, K.; Koizumi, Y.; Chiba, A. Precipitation behavior of a novel cobalt-based superalloy subjected to prior plastic deformations. Mater. Des. 2016, 112, 1–10. [Google Scholar] [CrossRef]
- Strondl, A.; Palm, M.; Gnauk, J.; Frommeyer, G. Microstructure and mechanical properties of nickel based superalloy IN718 produced by rapid prototyping with electron beam melting (EBM). Mater. Sci. Technol. 2011, 27, 876–883. [Google Scholar] [CrossRef]
- Hrabe, N.; Gnäupel-Herold, T.; Quinn, T. Fatigue properties of a titanium alloy (Ti-6Al-4V) fabricated via electron beam melting (EBM): Effects of internal defects and residual stress. Int. J. Fatigue 2016, 94, 202–210. [Google Scholar] [CrossRef]
- Gong, X.; Cui, Y.; Wei, D.; Liu, B.; Liu, R.; Nie, Y.; Li, Y. Building Direction Dependence of Corrosion Resistance Property of Ti-6Al-4V Alloy Fabricated by Electron Beam Melting. Corros. Sci. 2017, 127, 101–109. [Google Scholar] [CrossRef]
- Qi, H.B.; Yan, Y.N.; Lin, F.; He, W.; Zhang, R.J. Direct metal part forming of 316L stainless steel powder by electron beam selective melting. Proc. Inst. Mech. Eng. B-J. Eng. Manuf. 2006, 220, 1845–1853. [Google Scholar] [CrossRef]
- Zhong, Y.; Rännar, L.E.; Liu, L.; Koptyug, A.; Wikman, S.; Olsen, J.; Cui, D.; Shen, Z. Additive manufacturing of 316L stainless steel by electron beam melting for nuclear fusion applications. J. Nucl. Mater. 2017, 486, 234–245. [Google Scholar] [CrossRef]
- Hinojos, A.; Mireles, J.; Reichardt, A.; Frigola, P.; Hosemann, P.; Murr, L.E.; Wicker, R.B. Joining of Inconel 718 and 316 Stainless Steel using electron beam melting additive manufacturing technology. Mater. Des. 2016, 94, 17–27. [Google Scholar] [CrossRef] [Green Version]
- Mahale, T.R. Electron beam melting of advanced materials and structures. Ph.D. Thesis, North Carolina State University, Raleigh, NC, USA, December 2009. [Google Scholar]
- Liu, Y.J.; Li, S.J.; Wang, H.L.; Hou, W.T.; Hao, Y.L.; Yang, R.; Sercombe, T.B.; Zhang, L.C. Microstructure, defects and mechanical behavior of beta-type titanium porous structures manufactured by electron beam melting and selective laser melting. Acta Mater. 2016, 113, 56–67. [Google Scholar] [CrossRef]
- Strondl, A.; Fischer, R.; Frommeyer, G.; Schneider, A. Investigations of MX and γ′/γ″ precipitates in the nickel-based superalloy 718 produced by electron beam melting. Mater. Sci. Eng. A 2008, 480, 138–147. [Google Scholar] [CrossRef]
- Sun, S.H.; Koizumi, Y.; Saito, T.; Yamanaka, K.; Li, Y.-P.; Cui, Y.; Chiba, A. Electron beam additive manufacturing of Inconel 718 alloy rods: Impact of build direction on microstructure and high-temperature tensile properties. Addit. Manuf. 2018, 23, 457–470. [Google Scholar] [CrossRef]
- Mrowec, S.; Stokłosa, A. Calculations of parabolic rate constants for metal oxidation. Oxid. Met. 1974, 8, 379–391. [Google Scholar] [CrossRef]
- Tunthawiroon, P.; Li, Y.; Chiba, A. Influences of alloyed Si on the corrosion resistance of Co-Cr-Mo alloy to molten Al by iso-thermal oxidation in air. Corros. Sci. 2015, 100, 428–434. [Google Scholar] [CrossRef]
- Ter-Ovanessian, B.; Poquillon, D.; Cloué, J.M.; Andrieu, E. Influence of local mechanical loading paths on the oxidation assisted crack initiation of alloy 718. Mater. Sci. Eng. A 2012, 533, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Connolley, T.; Reed, P.A.S.; Starink, M.J. Short crack initiation and growth at 600 °C in notched specimens of Inconel718. Mater. Sci. Eng. A 2003, 340, 139–154. [Google Scholar] [CrossRef]
- Greene, G.A.; Finfrock, C.C. Oxidation of Inconel 718 in Air at High Temperatures. Oxid. Met. 2001, 55, 505–521. [Google Scholar] [CrossRef]
- Berthier, C.; Lenglet, M.; Lameille, J.M. SEM-EDS and XPS Studies of the High Temperature Oxidation Behaviour of Inconel 718. Microchim. Acta 2000, 132, 337–343. [Google Scholar]
- Cruchley, S.; Evans, H.; Taylor, M. An overview of the oxidation of Ni-based superalloys for turbine disc applications: surface condition, applied load and mechanical performance. High. Temp. Technol. 2016, 33, 465–475. [Google Scholar] [CrossRef]
- Horita, T.; Kishimoto, H.; Yamaji, K.; Xiong, Y.; Brito, M.E.; Yokokawa, H.; Baba, Y.; Ogasawara, K.; Kameda, H.; Matsuzaki, Y. Diffusion of oxygen in the scales of Fe-Cr alloy interconnects and oxide coating layer for solid oxide fuel cells. Solid State Ion. 2008, 179, 2216–2221. [Google Scholar] [CrossRef]
- Li, Y.; Tunthawiroon, P.; Tang, N.; Bian, H.; Wang, F.; Sun, S.; Chen, Y.; Omura, K.; Koizumi, Y.; Chiba, A. Effects of Al, Ti, and Zr doping on oxide film formation in Co-29Cr-6Mo alloy used as mould material for Al die-casting. Corros. Sci. 2014, 84, 147–158. [Google Scholar] [CrossRef]





| Fe | Cr | Mo | Nb | Ti | Co | Al | C | N | Ni |
|---|---|---|---|---|---|---|---|---|---|
| 17.8 | 19.4 | 2.97 | 4.88 | 0.84 | 0.10 | 0.48 | 0.0355 | 0.0077 | Bal. |
| Condition | Alloy | P1 | P2 | P3 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ni | Cr | Fe | Nb | O | Ni | Cr | Fe | Nb | O | Ni | Cr | Fe | Nb | O | ||
| 700 °C, 100 h | 0° | 19.6 | 27.3 | 3.2 | 1.8 | 48.1 | 12.8 | 13.4 | 8.8 | 38.3 | 26.7 | 23.3 | 13.5 | 15.2 | 1.6 | 46.4 |
| 55° | 21.9 | 25.8 | 9.6 | 2.1 | 40.6 | 14.7 | 10.2 | 9.4 | 40.6 | 25.1 | 31.3 | 6.5 | 23.3 | 1.8 | 37.1 | |
| 90° | 14.8 | 33.3 | 2.6 | 0.4 | 48.9 | 10.9 | 15.1 | 8.4 | 35.4 | 30.2 | 22.5 | 16.3 | 11.1 | 1.1 | 49 | |
| 1000 °C, 100 h | 0° | 13.4 | 32.5 | 5.3 | 1.3 | 47.5 | 9.5 | 9.3 | 6.1 | 45.3 | 29.8 | 27.5 | 7.6 | 19.2 | 1.1 | 44.6 |
| 55° | 15.7 | 28.8 | 7.5 | 1.6 | 46.4 | 10.7 | 8.6 | 6.5 | 51.6 | 22.6 | 33.1 | 7.1 | 24.6 | 1.5 | 33.7 | |
| 90° | 9.2 | 37.6 | 3.8 | 0.9 | 48.5 | 8.8 | 10.1 | 5.5 | 39.2 | 36.4 | 25.4 | 10.2 | 18.3 | 0.7 | 45.4 | |
| Parabolic Constant | 700 °C | 800 °C | 900 °C | 1000 °C | Q kJ/mol |
|---|---|---|---|---|---|
| 0° | 8.79 × 10−15 (0.9014) | 8.89 × 10−14 (0.9986) | 9.07 × 10−13 (0.9706) | 2.01 × 10−12 (0.9645) | 219.39 |
| 55° | 3.88 × 10−15 (0.9786) | 6.54 × 10−14 (0.9801) | 7.04 × 10−13 (0.9687) | 1.34 × 10−12 (0.9927) | 246.78 |
| 90° | 1.68 × 10−14 (0.9543) | 2.11 × 10−13 (0.9559) | 1.45 × 10−12 (0.9914) | 3.11 × 10−12 (0.9879) | 211.84 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Gong, X.; Ye, X.; Teng, J.; Nie, Y.; Li, Y.; Lei, Q. Influence of Building Direction on the Oxidation Behavior of Inconel 718 Alloy Fabricated by Additive Manufacture of Electron Beam Melting. Materials 2018, 11, 2549. https://doi.org/10.3390/ma11122549
Li L, Gong X, Ye X, Teng J, Nie Y, Li Y, Lei Q. Influence of Building Direction on the Oxidation Behavior of Inconel 718 Alloy Fabricated by Additive Manufacture of Electron Beam Melting. Materials. 2018; 11(12):2549. https://doi.org/10.3390/ma11122549
Chicago/Turabian StyleLi, Lv, Xiaojuan Gong, Xianjue Ye, Jianwei Teng, Yan Nie, Yunping Li, and Qian Lei. 2018. "Influence of Building Direction on the Oxidation Behavior of Inconel 718 Alloy Fabricated by Additive Manufacture of Electron Beam Melting" Materials 11, no. 12: 2549. https://doi.org/10.3390/ma11122549






