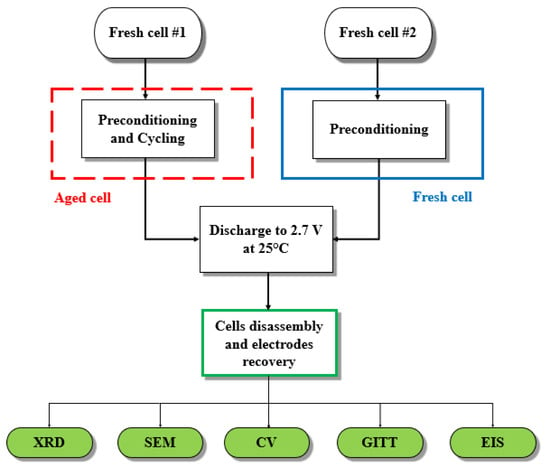
3.2.1. Ex-Situ X-ray Diffraction
Figure 3 illustrates the XRD patterns of the uncycled and cycled NMC electrodes in their lithiated and delithiated states. The characteristic peaks are corresponding to the trigonal (or rhombohedral)
-
(R-3m) structure as reported in [
28,
29]. The separation of the doublets peaks (006)/(102) and (108)/(110) reveals highly ordered layered structures with a good hexagonal ordering for both electrodes. After long-term cycling, the diffraction peaks of the cycled electrode are reduced compared to those of the uncycled electrode. A new peak is observed in
Figure 3a around 20
angle which translates for the formation of an amorphous phase. This might indicate for side reactions between the electrodes and the electrolyte and further for the degradation of their crystal structure. Particularly, the amorphous phase is believed to present a low conductivity and hinder the
insertion/extraction process [
30].
Experimental XRD patterns were recorded and the crystal structures of the electrodes matching their peaks were determined from Rietveld refinements. Changes in the lattice parameters are generally tracked for understanding modifications induced in the electrodes crystal structure after cycling. Corresponding
a and
c lattice parameters and unit cells volumes are listed in
Table 2. Lattice parameters
a and
c are related to the average metal-metal intra slab distance and to the average metal-metal inter slab distance, respectively. Considering the rhombohedral crystal structure of the electrodes for which the volume of a unit cell equals to one third of that of an hexagonal unit cell, the maximum
concentration
in the electrodes is computed as follows with Equation (
1):
where V is the volume corresponding to one hexagonal unit cell and
is the Avogadro constant (
mol
).
Table 2 summarises the lattice parameters calculated from Rietveld refinements and the calculated maximum
concentrations in the lithiated and delithiated electrodes.
Difference in the structural parameters is seen between the lithiated and delithiated states of both uncycled and cycled electrodes. This indicates as expected the dependence of the unit cell parameters on the lithium content in the electrodes. Increase in the lattice parameter
a and decrease in the lattice parameter
c are observed to occur with the
insertion process in both electrodes types. The unit cell volume evaluated for the lithiated electrodes are seen to be greater than that in the delithiated electrodes. Consequently, maximum
concentrations in the lithiated electrodes are then well lower than in the delithiated electrodes. These observations are in agreement with the literature [
31]. Further, uncycled and cycled electrodes are seen to also differ from their unit cell parameters. Cycling is observed to induce shrinkage in the unit cells volume and the lattice parameter
c of the lithiated electrode in comparison to the initially uncycled electrode. This reflects the loss of atoms from the crystal structure of the lithiated electrodes. Shifts in the peaks positions towards higher
angles is observed after cycling for the lithiated electrodes, while characteristic peaks of the delithiated electrodes are noticed to move with ageing towards lower
angles. In practice, shifts towards lower angles translate for vacancies in the crystal structure of the electrodes while shifts to higher
are associated to the existence of ions with an ionic radius lower than
.
Results from the analysis of the crystal structure parameters with ageing and the lithiation states of the NMC electrodes are presented in
Table 3.
It can be observed that the
ratio for both uncycled and cycled NMC electrodes is greater than the 4.899, which demonstrates their crystalline layered structure [
32]. The
intensity ratio is inversely proportional to the degree of cation mixing between
and
in the lithium layer [
33]. Cation mixing is a structural disorder where a partial occupation of
on the crystallographic 3b sites of a
and conversely a partial occupation of
in 3a sites of
in transition metal layers may occur considering the close ionic radii of
(0.76 Å) and
(0.69 Å). In practice, this phenomenon is known for deteriorating the electrochemical performance of the layered oxides materials by decreasing their discharge capacity and hindering the diffusion pathway of
[
34,
35]. A higher concentration of cation mixing would lead to materials that are less ordered with a greater
diffusion barrier [
36].
intensity ratios greater than 1.2 indicate little cation mixing for both uncycled and cycled electrodes [
37]. The
ratio (or so called R-factor) informs about the hexagonal ordering of the crystal structure of the electrodes. In practice, a small ratio translates into a good separation in the transition metal and the
respective planes [
38,
39] yielding to a good hexagonal ordering [
40]. With a higher R factor after cycling, it is found that the hexagonal ordering of the initially delithiated electrodes is affected by ageing.
3.2.2. Scanning Electron Microscopy
Figure 4 illustrates the SEM images of the uncycled (a,c) and cycled (b,d) NMC electrodes at different magnifications. The morphology of the active material particles of the electrodes were examined from powders in their delithiated state. Both uncycled and cycled NMC active material particles are seen to present spherical morphology. A slightly increased brightness is shown by the SEM images of the cycled compared to the uncycled electrodes. This would suggest a relative higher amount of non-electronic conductive material in the cycled electrodes.
The determination of the radius of the primary particles and the secondary particles (or also designated by “agglomerates”) were conducted from the determination of the particle size distribution of the SEM images in
Figure 4. Particle size distributions associated to both electrodes types are displayed in
Figure 5. After cycling, particle size distribution is seen to shift towards lower particle radii. The radius of the secondary particles in the uncycled electrodes is identified to be equal to 3.42
m. After cycling, the agglomerates are observed to have slightly decomposed into smaller particles of 3.07
m radius. This might possibly indicate for the starting of chemical ex-foliation/corrosion and particle cracking taking place in the cycled electrode samples due to cycling. The distribution of the cycled particles radii is also seen to be broader than that of the uncycled electrode, which suggests for more heterogeneous particle size distribution in cycled electrode in comparison to uncycled electrode.