Application of Polymerization Activator in the Course of Synthesis of N-Isopropylacrylamide Derivatives for Thermally Triggered Release of Naproxen Sodium
Abstract
:1. Introduction
2. Results
2.1. Nuclear Magnetic Resonance Spectroscopy
2.2. Fourier-Transform Infrared Spectroscopy
2.3. Hydrodynamic Diameter and Volume Phase Transition Temperature
2.4. Approximation of Molecular Mass of Synthesized Polymers
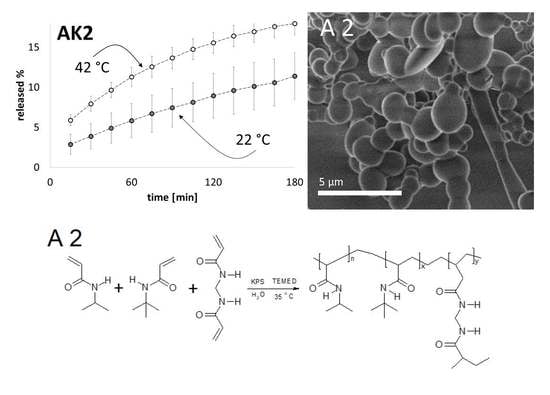
2.5. Morphology of the Polymers Measured by Scanning Electron Microscopy
2.6. Release Kinetics of Naproxen Sodium from Thermosensitive Hydrogels
3. Discussion
4. Experimental Section
4.1. Materials
4.2. Synthesis of the Particles
4.3. Nuclear Magnetic Resonance Spectroscopy
4.4. Fourier-Transform Infrared Spectroscopy
4.5. Hydrodynamic Diameter Measurements
4.6. Scanning Electron Microscopy
4.7. Preparation Hydrogels with Naproxen Sodium
4.8. Evaluation of Naproxen Sodium Release Kinetics
4.9. Evaluation of Molecular Weight via Static Light Scattering
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Buwalda, S.J.; Boere, K.W.M.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a historical perspective: From simple networks to smart materials. J. Control. Release 2014, 190, 254–273. [Google Scholar] [CrossRef] [PubMed]
- Kopecek, J. Hydrogels: From soft contact lenses and implants to self-assembled nanomaterials. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 5929–5946. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, G.; Nicoletta, F.P.; Curcio, M.; Spizzirri, U.G.; Picci, N.; Iemma, F. Enzyme immobilization on smart polymers: Catalysis on demand. React. Funct. Polym. 2014, 83, 62–69. [Google Scholar] [CrossRef]
- Pimenta, A.F.R.; Valente, A.; Pereira, J.M.C.; Pereira, J.C.F.; Filipe, H.P.; Mata, J.L.G.; Colaco, R.; Saramago, B.; Serro, A.P. Simulation of the hydrodynamic conditions of the eye to better reproduce the drug release from hydrogel contact lenses: Experiments and modeling. Drug Deliv. Transl. Res. 2016, 6, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Knipe, J.M.; Peppas, N.A. Multi-responsive hydrogels for drug delivery and tissue engineering applications. Regen. Biomater. 2014, 1, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Ashwini Kumar, G.; Bhat, A.; Lakshmi, A.P.; Reddy, K. An Overview of Stimuli-Induced Pulsatile Drug Delivery Systems. Int. J. PharmTech Res. 2010, 2, 2364–2378. [Google Scholar]
- Jin, S.; Wan, J.; Meng, L.; Huang, X.; Guo, J.; Liu, L.; Wang, C. Biodegradation and Toxicity of Protease/Redox/pH Stimuli-Responsive PEGlated PMAA Nanohydrogels for Targeting Drug delivery. ACS Appl. Mater. Interfaces 2015, 7, 19843–19852. [Google Scholar] [CrossRef] [PubMed]
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2012, 64, 49–60. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Xu, H.; Ge, L.; Zhu, J. Investigation of dual-sensitive nanogels based on chitosan and N-isopropylacrylamide and its intelligent drug delivery of 10-hydroxycamptothecine. Drug Deliv. 2015, 22, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.N.; Ekenseair, A.K.; Spicer, P.P.; Watson, B.M.; Tzouanas, S.N.; Roh, T.T.; Mikos, A.G. In vitro and in vivo evaluation of self-mineralization and biocompatibility of injectable, dual-gelling hydrogels for bone tissue engineering. J. Control. Release 2015, 205, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Acciaro, R.; Gilányi, T.; Varga, I. Preparation of Monodisperse Poly(N-isopropylacrylamide) Microgel Particles with Homogenous Cross-Link Density Distribution. Langmuir 2011, 27, 7917–7925. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Z.; Wu, D.Q.; Chu, C.C. Synthesis, characterization and controlled drug release of thermosensitive IPN–PNIPAAm hydrogels. Biomaterials 2004, 25, 3793–3805. [Google Scholar] [CrossRef] [PubMed]
- Petrusic, S.; Lewandowski, M.; Giraud, S.; Jovancic, P.; Bugarski, B.; Ostojic, S.; Koncar, V. Development and characterization of thermosensitive hydrogels based on poly(N-isopropylacrylamide) and calcium alginate. J. Appl. Polym. Sci. 2012, 124, 890–903. [Google Scholar] [CrossRef]
- Musiał, W.; Pluta, J.; Michálek, J. Thermosensitive microgels of poly-N-isopropylacrylamide for drug carriers—Practical approach to synthesis. Acta Pol. Pharm. 2015, 72, 409–422. [Google Scholar] [PubMed]
- Kim, Y.; Babu, V.R.; Rao, K.S.V.K.; Lim, J.M.; Thangadurai, T.D.; Lee, Y.I. Stimuli-Sensitive Poly(NIPA-co-APA) Hydrogels for the Controlled Release of Keterolac Tromethamine. J. Korean Chem. Soc. 2014, 58, 92–99. [Google Scholar] [CrossRef]
- Park, T.G.; Hoffman, A.S. Sodium Chloride-Induced Phase Transition in Nonionic Poly(N-isopropylacrylamide) Gel. Macromolecules 1993, 26, 5045–5048. [Google Scholar] [CrossRef]
- Musial, W.; Voncina, B.; Pluta, J.; Kokol, V. The Study of Release of Chlorhexidine from Preparations with Modified Thermosensitive Poly-N-isopropylacrylamide Microspheres. Sci. World J. 2012, 2012, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Musiał, W.; Kokol, V.; Voncina, B. The influence of increased cross-linker chain length in thermosensitive microspheres on potential sun-protection activity. Polim. Med. 2010, 40, 47–55. [Google Scholar] [PubMed]
- Wang, C.; Wang, J.; Gao, W.; Jiao, J.; Feng, H.; Liu, X.; Chen, L. One-pot preparation of thermoresponsive silica-poly(N-isopropylacrylamide) nanocomposite particles in supercritical carbon dioxide. J. Colloid Interface Sci. 2010, 343, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Huang, Z.; Bao, Y.; Weng, Z. Thermosensitive Poly(N-isopropylacrylamide-co-acrylonitrile) Hydrogels with Rapid Response. Chin. J. Chem. Eng. 2006, 14, 87–92. [Google Scholar] [CrossRef]
- Ichikawa, H.; Fukumori, Y. A novel positively thermosensitive controlled-release microcapsule with membrane of nano-sized poly(N-isopropylacrylamide) gel dispersed in ethylcellulose matrix. J. Control. Release 2000, 63, 107–119. [Google Scholar] [CrossRef]
- Gandhi, A.; Paul, A.; Sen, S.O.; Sen, K.K. Studies on thermoresponsive polymers: Phase behaviour, drug delivery and biomedical applications. Asian J. Pharm. Sci. 2015, 10, 99–107. [Google Scholar] [CrossRef]
- Gasztych, M.; Gola, A.; Kobryn, J.; Musial, W. Synthesis and Formulation of Thermosensitive Drug Carrier for Temperature Triggered Delivery of Naproxen Sodium. Molecules 2016, 21, 1473. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.R. Elaborations on the Higuchi model for drug delivery. Int. J. Pharm. 2011, 418, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Peppas, N.A. Higuchi equation: Derivation, applications, use and misuse. Int. J. Pharm. 2011, 418, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Manga, R.D.; Jha, P.K. Mathematical Models for Controlled Drug Release Through pH-Responsive Polymeric Hydrogels. J. Pharm. Sci. 2017, 106, 629–638. [Google Scholar] [CrossRef] [PubMed]






| Type of Polymer | DH at 18 °C (nm) | SD | DH at 42 °C (nm) | SD | VPTT (°C) |
|---|---|---|---|---|---|
| A1 | 577.00 | 3.25 | 256.6 | 3.86 | 28 |
| A2 | 861.47 | 17.2 | 127.23 | 1.1 | 30 |
| A3 | 971.93 | 30.79 | 226.37 | 5.8 | 28–32 |
| Type of Polymer | Average MW (kDa) | SD |
|---|---|---|
| A1 | 122.50 | 23.33 |
| A2 | 27.73 | 4.74 |
| A3 | 3430.00 | 14.14 |
| Model | Parameter | Type of Formulation | |||||||
|---|---|---|---|---|---|---|---|---|---|
| AK1 | AK2 | AK3 | REF | ||||||
| Value | SD | Value | SD | Value | SD | Value | SD | ||
| ZO | KZO (% min−1) | 6.08∙× 10−2 | 5.95∙× 10−3 | 5.68∙× 10−2 | 5.31∙× 10−3 | 6.20∙× 10−2 | 1.42∙× 10−3 | 6.08∙× 10−2 | 1.84∙× 10−2 |
| r2 | 0.96485 | 0.00992 | 0.98365 | 0.01123 | 0.94852 | 0.00450 | 0.94085 | 0.01771 | |
| FO | KFO (min−1) | 6.82∙× 10−4 | 8.19∙× 10−5 | 6.20∙× 10−4 | 7.29∙× 10−5 | 7.01∙× 10−4 | 1.84∙× 10−5 | 6.90∙× 10−4 | 2.26∙× 10−4 |
| r2 | 0.97030 | 0.00848 | 0.98626 | 0.00910 | 0.95540 | 0.00416 | 0.94805 | 0.01897 | |
| SO | KSO (min−1∙%−1) | 7.65∙× 10−6 | 1.09∙× 10−6 | 6.78∙× 10−6 | 9.59∙× 10−7 | 7.92∙× 10−6 | 2.36∙× 10−7 | 7.85∙× 10−6 | 2.76∙× 10−6 |
| r2 | 0.97526 | 0.00716 | 0.98843 | 0.00698 | 0.96177 | 0.00379 | 0.95471 | 0.01989 | |
| H | KH (min0.5) | 1.11 | 1.13∙× 10−1 | 1.02 | 1.05∙× 10−1 | 1.14 | 2.40∙× 10−2 | 1.12 | 3.31∙× 10−1 |
| r2 | 0.99723 | 0.00154 | 0.99226 | 0.00660 | 0.99335 | 0.00129 | 0.99067 | 0.00680 | |
| BF | H | H | H | H | |||||
| Model | Parameter | Type of Formulation | |||||||
|---|---|---|---|---|---|---|---|---|---|
| AK1 | AK2 | AK3 | REF | ||||||
| Value | SD | Value | SD | Value | SD | Value | SD | ||
| ZO | KZO (% min−1) | 6.73∙× 10−2 | 2.64∙× 10−3 | 7.14∙× 10−2 | 4.96∙× 10−3 | 6.12∙× 10−2 | 1.99∙× 10−3 | 5.93∙× 10−2 | 3.48∙× 10−3 |
| r2 | 0.95662 | 0.01412 | 0.95397 | 0.00820 | 0.94747 | 0.02971 | 0.96221 | 0.02017 | |
| FO | KFO (min−1) | 7.65∙× 10−4 | 2.46∙× 10−5 | 8.19∙× 10−4 | 7.18∙× 10−5 | 6.93∙× 10−4 | 1.37∙× 10−5 | 6.64∙× 10−4 | 5.17∙× 10−5 |
| r2 | 0.96358 | 0.01321 | 0.96133 | 0.00670 | 0.95426 | 0.02758 | 0.96784 | 0.01815 | |
| SO | KSO (min−1∙%−1) | 8.70∙× 10−6 | 2.18∙× 10−7 | 9.40∙× 10−6 | 9.97∙× 10−7 | 7.86∙× 10−6 | 5.45∙× 10−8 | 7.45∙× 10−6 | 7.23∙× 10−7 |
| r2 | 0.96986 | 0.01225 | 0.96803 | 0.00532 | 0.96056 | 0.02544 | 0.97300 | 0.01621 | |
| H | KH (min0.5) | 1.23 | 4.01∙× 10−2 | 1.30 | 9.48∙× 10−2 | 1.12 | 2.41∙× 10−2 | 1.08 | 7.24∙× 10−2 |
| r2 | 0.99371 | 0.00175 | 0.99455 | 0.00200 | 0.99149 | 0.00913 | 0.99638 | 0.00402 | |
| BF | H | H | H | H | |||||
| Substrate % (w/w) | Type of Component | ||||||
|---|---|---|---|---|---|---|---|
| Main Monomer | Initiator | Activator | Crosslinker | Comonomer | Solvent | ||
| Type of Polymer | A1 | NIPA | KPS | MBA | NTB | water | |
| 0.5 | 0.05 | 0.05 | 0.05 | 99.35 | |||
| A2 | NIPA | KPS | TEMED | MBA | NTB | water | |
| 0.5 | 0.05 | 0.02 | 0.05 | 0.05 | 99.33 | ||
| A3 | NIPA | KPS | TEMED | PEG-DMA | water | ||
| 0.5 | 0.05 | 0.02 | 0.05 | 99.38 | |||
| Type of Hydrogel | Component (%) | |||||
|---|---|---|---|---|---|---|
| NS | A1 | A2 | A3 | HPMC | AQ | |
| AK1 | 4 | 5 | - | - | 5 | 86 |
| AK2 | 4 | - | 5 | - | 5 | 86 |
| AK3 | 4 | - | - | 5 | 5 | 86 |
| REF | 4 | - | - | - | 5 | 91 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gasztych, M.; Kotowska, A.; Musiał, W. Application of Polymerization Activator in the Course of Synthesis of N-Isopropylacrylamide Derivatives for Thermally Triggered Release of Naproxen Sodium. Materials 2018, 11, 261. https://doi.org/10.3390/ma11020261
Gasztych M, Kotowska A, Musiał W. Application of Polymerization Activator in the Course of Synthesis of N-Isopropylacrylamide Derivatives for Thermally Triggered Release of Naproxen Sodium. Materials. 2018; 11(2):261. https://doi.org/10.3390/ma11020261
Chicago/Turabian StyleGasztych, Monika, Anna Kotowska, and Witold Musiał. 2018. "Application of Polymerization Activator in the Course of Synthesis of N-Isopropylacrylamide Derivatives for Thermally Triggered Release of Naproxen Sodium" Materials 11, no. 2: 261. https://doi.org/10.3390/ma11020261
APA StyleGasztych, M., Kotowska, A., & Musiał, W. (2018). Application of Polymerization Activator in the Course of Synthesis of N-Isopropylacrylamide Derivatives for Thermally Triggered Release of Naproxen Sodium. Materials, 11(2), 261. https://doi.org/10.3390/ma11020261