TopUp SERS Substrates with Integrated Internal Standard
Abstract
:1. Introduction
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Chemicals
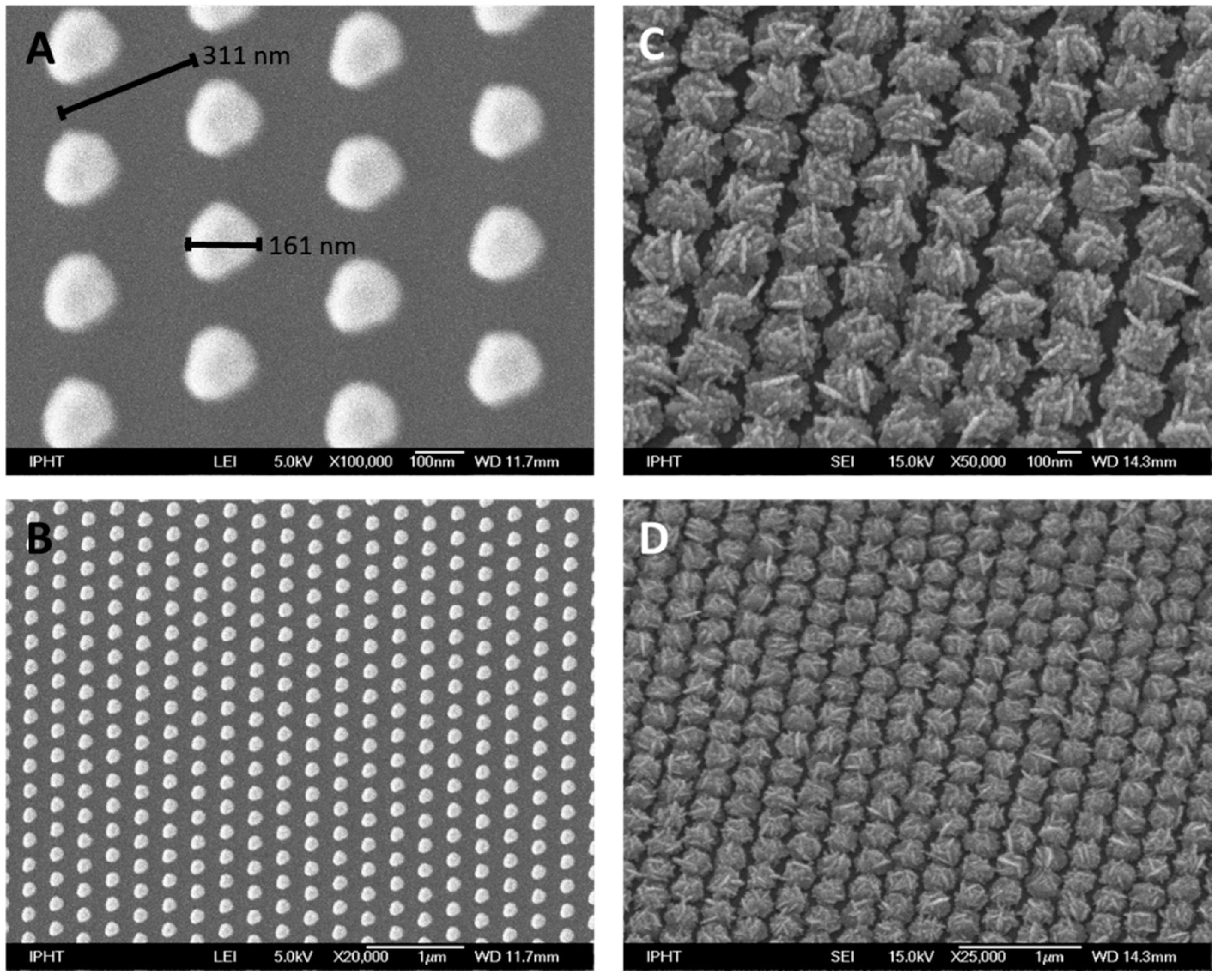
4.2. Gold Template Structure
4.3. Self-Organized Silver Structure
4.4. Instrumentation
4.5. Data Analysis
5. Patents
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ding, S.-Y.; You, E.-M.; Tian, Z.-Q.; Moskovits, M. Electromagnetic theories of surface-enhanced Raman spectroscopy. Chem. Soc. Rev. 2017, 46, 4042–4076. [Google Scholar] [CrossRef] [PubMed]
- Cialla, D.; Maerz, A.; Boehme, R.; Theil, F.; Weber, K.; Schmitt, M.; Popp, J. Surface-enhanced Raman spectroscopy (SERS): Progress and trends. Anal. Bioanal. Chem. 2012, 403, 27–54. [Google Scholar] [CrossRef] [PubMed]
- Procházka, M. Surface-Enhanced Raman Spectroscopy: Bioanalytical, Biomolecular and Medical Applications; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Yamamoto, Y.S.; Itoh, T. Why and how do the shapes of surface-enhanced Raman scattering spectra change? Recent progress from mechanistic studies. J. Raman Spectrosc. 2016, 47, 78–88. [Google Scholar] [CrossRef]
- Cialla-May, D.; Zheng, X.S.; Weber, K.; Popp, J. Recent progress in surface-enhanced Raman spectroscopy for biological and biomedical applications: from cells to clinics. Chem. Soc. Rev. 2017, 46, 3945–3961. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zong, S.; Wu, L.; Zhu, D.; Cui, Y. SERS-Activated Platforms for Immunoassay: Probes, Encoding Methods, and Applications. Chem. Rev. 2017, 117, 7910–7963. [Google Scholar] [CrossRef] [PubMed]
- Jaworska, A.; Fornasaro, S.; Sergo, V.; Bonifacio, A. Potential of Surface Enhanced Raman Spectroscopy (SERS) in Therapeutic Drug Monitoring (TDM). A critical review. Biosensors 2016, 6, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Yan, Z.; Jia, L.; Song, P.; Mei, L.; Bai, L.; Liu, Y. Gold nanoparticle decorated electrospun nanofibers: A 3D reproducible and sensitive SERS substrate. Appl. Surf. Sci. 2017, 403, 29–34. [Google Scholar] [CrossRef]
- Xiao, G.; Li, Y.; Shi, W.; Shen, L.; Chen, Q.; Huang, L. Highly sensitive, reproducible and stable SERS substrate based on reduced graphene oxide/silver nanoparticles coated weighing paper. Appl. Surf. Sci. 2017, 404, 334–341. [Google Scholar] [CrossRef]
- Bai, Y.; Yan, L.; Wang, J.; Su, L.; Chen, N.; Tan, Z. Highly reproducible and uniform SERS substrates based on Ag nanoparticles with optimized size and gap. Photonics Nanostruct. Fundam. Appl. 2017, 23, 58–63. [Google Scholar] [CrossRef]
- Kim, Y.-T.; Schilling, J.; Schweizer, S.L.; Sauer, G.; Wehrspohn, R.B. Au coated PS nanopillars as a highly ordered and reproducible SERS substrate. Photonics Nanostruct. Fundam. Appl. 2017, 25, 65–71. [Google Scholar] [CrossRef]
- Jiang, S.; Guo, J.; Zhang, C.; Li, C.; Wang, M.; Li, Z.; Gao, S.; Chen, P.; Si, H.; Xu, S. A sensitive, uniform, reproducible and stable SERS substrate has been presented based on MoS2@Ag nanoparticles@pyramidal silicon. RSC Adv. 2017, 7, 5764–5773. [Google Scholar] [CrossRef]
- Chamuah, N.; Vaidya, G.P.; Joseph, A.M.; Nath, P. Diagonally aligned squared metal nano-pillar with increased hotspot density as a highly reproducible SERS substrate. Plasmonics 2017, 12, 1353–1358. [Google Scholar] [CrossRef]
- Liu, X.; Osada, M.; Kitamura, K.; Nagata, T.; Si, D. Ferroelectric-assisted gold nanoparticles array for centimeter-scale highly reproducible SERS substrates. Sci. Rep. 2017, 7, 3630. [Google Scholar] [CrossRef] [PubMed]
- Jahn, I.J.; Zukovskaja, O.; Zheng, X.S.; Weber, K.; Bocklitz, T.W.; Cialla-May, D.; Popp, J. Surface-enhanced Raman spectroscopy and microfluidic platforms: challenges, solutions and potential applications. Analyst 2017, 142, 1022–1047. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-L.; Li, B.-W.; Wang, Y.-Q. Surface-enhanced Raman scattering microfluidic sensor. RSC Adv. 2013, 3, 13015–13026. [Google Scholar] [CrossRef]
- Tycova, A.; Prikryl, J.; Foret, F. Recent strategies toward microfluidic-based surface-enhanced Raman spectroscopy. Electrophoresis 2017, 38, 1977–1987. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.E.J.; Sirimuthu, N.M.S. Quantitative surface-enhanced Raman spectroscopy. Chem. Soc. Rev. 2008, 37, 1012–1024. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.E.J.; Fido, L.A.; Sirimuthu, N.M.S.; Speers, S.J.; Peters, K.L.; Cosbey, S.H. Screening tablets for DOB using Surface-Enhanced Raman Spectroscopy*. J. Forensic Sci. 2007, 52, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.E.J.; Mackle, J.N.; Sirimuthu, N.M.S. Quantitative surface-enhanced Raman spectroscopy of dipicolinic acid-towards rapid anthrax endospore detection. Analyst 2005, 130, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Perera, P.N.; Deb, S.K.; Jo Davisson, V.; Ben-Amotz, D. Multiplexed concentration quantification using isotopic surface-enhanced resonance Raman scattering. J. Raman Spectrosc. 2010, 41, 752–757. [Google Scholar] [CrossRef]
- Zhou, Y.; Ding, R.; Joshi, P.; Zhang, P. Quantitative surface-enhanced Raman measurements with embedded internal reference. Anal. Chim. Acta 2015, 874, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Ingram, W.M.; Han, C.; Zhang, Q.; Zhao, Y. Optimization of Ag-Coated polystyrene nanosphere substrates for quantitative Surface-Enhanced Raman Spectroscopy analysis. J. Phys. Chem. C 2015, 119, 27639–27648. [Google Scholar] [CrossRef]
- Peksa, V.; Jahn, M.; Štolcová, L.; Schulz, V.; Proška, J.; Procházka, M.; Weber, K.; Cialla-May, D.; Popp, J. Quantitative SERS Analysis of Azorubine (E 122) in Sweet Drinks. Anal. Chem. 2015, 87, 2840–2844. [Google Scholar] [CrossRef] [PubMed]
- Patze, S.; Huebner, U.; Weber, K.; Cialla-May, D.; Popp, J. TopUp plasmonic arrays for Surface-Enhanced Raman Spectroscopy. Adv. Mater. Interfaces 2016, 3, 1600549. [Google Scholar] [CrossRef]
- Uchinokura, K.; Sekine, T.; Matsuura, E. Raman scattering by silicon. Solid State Commun. 1972, 11, 47–49. [Google Scholar] [CrossRef]
- Kosemura, D.; Che Mohd Yusoff, S.N.B.; Ogura, A. Electrical field analysis of metal-surface plasmon resonance using a biaxially strained Si substrate. J. Raman Spectrosc. 2014, 45, 414–417. [Google Scholar] [CrossRef]
- Hashiguchi, H.; Takei, M.; Kosemura, D.; Ogura, A. Stress evaluation in thin strained-Si film by polarized Raman spectroscopy using localized surface plasmon resonance. Appl. Phys. Lett. 2012, 101, 172101. [Google Scholar] [CrossRef]
- Weinstein, B.A.; Cardona, M. Two-phonon Raman spectra of Si and GaP. Solid State Commun. 1972, 10, 961–965. [Google Scholar] [CrossRef]
- Versteegh, J.F.M.; van der Aa, N.G.F.M.; Dijkman, E. Geneesmiddelen in Drinkwater en Drinkwaterbronnen [Pharmaceuticals in Drinking Water and Resources for Drinking Water], RIVM Rapport 703719016, Rijksinstituut voor Volksgezondheid en Milieu RIVM, Bilthoven—The Netherlands. 2007, 53. Available online: https://www.rivm.nl/bibliotheek/rapporten/703719016.pdf (accessed on 22 February 2018).
- Cunliffe, D. Australian Guidelines for Water Recycling: Managing Health and Environmental Risks (Phase 2)—Augmentation of Drinking Water Supplies, Canberra. 2008. Available online: https://www.nhmrc.gov.au/_files_nhmrc/publications/attachments/eh56_water_recycling_guidelines_augmentation_drinking_supplies_22.pdf (accessed on 22 February 2018).
- Tausch, C. Arzneimittelwirkstoffe und Weitere Polare Spurenstoffe in Roh- und Trinkwasser Bayerisches Landesamt für Umwelt Bayerisches Landesamt für Gesundheit und Lebensmittelsicherheit, Augsburg. 2010. Available online: https://www.lgl.bayern.de/lebensmittel/warengruppen/wc_59_trinkwasser/ue_2009_wasser_arzneimittel.htm (accessed on 22 February 2018).
- Mosier-Boss, P.A.; Sorensen, K.C.; George, R.D.; Sims, P.C.; O'Braztsova, A. SERS substrates fabricated using ceramic filters for the detection of bacteria: Eliminating the citrate interference. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 180, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Kubinyi, M.; Keresztury, G. Infrared and Raman spectroscopic study of molecular interactions in quinhydrone crystals. Spectrochim. Acta A Mol. Biomol. Spectrosc. 1989, 45, 421–429. [Google Scholar] [CrossRef]
- Patze, S.; Huebner, U.; Liebold, F.; Weber, K.; Cialla-May, D.; Popp, J. SERS as an analytical tool in environmental science: The detection of sulfamethoxazole in the nanomolar range by applying a microfluidic cartridge setup. Anal. Chim. Acta 2017, 949, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Schlücker, S. Surface-Enhanced Raman Spectroscopy: Concepts and chemical applications. Angew. Chem. Int. Ed. Engl. 2014, 53, 4756–4795. [Google Scholar] [CrossRef] [PubMed]
- Morton, S.M.; Silverstein, D.W.; Jensen, L. Theoretical studies of plasmonics using electronic structure methods. Chem. Rev. 2011, 111, 3962–3994. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.H.; Lee, S. SERS and DFT investigation of the adsorption behavior of 4-mercaptobenzoic acid on silver colloids. Colloid Surf. A Physicochem. Eng. Asp. 2015, 474, 29–35. [Google Scholar] [CrossRef]
- Nash, A.P.; Ye, D. Silver coated nickel nanotip arrays for low concentration surface enhanced Raman scattering. J. Appl. Phys. 2015, 118, 073106. [Google Scholar] [CrossRef]
- Huebner, U.; Falkner, M.; Zeitner, U.D.; Banasch, M.; Dietrich, K.; Kley, E.-B. Multi-Stencil Character Projection e-Beam Lithography: A Fast and Flexible Way for High Quality Optical Metamaterials. In Proceedings of the 30th European Mask and Lithography Conference, Dresden, Germany, 17 October 2014. [Google Scholar] [CrossRef]
- Kley, E.-B.; Schmidt, H.; Zeitner, U.; Banasch, M.; Schnabel, B. Enhanced e-beam pattern writing for nano-optics based on character projection. In Proceedings of the 28th European Mask and Lithography Conference (EMLC 2012), Dresden, Germany, 17 April 2012. [Google Scholar] [CrossRef]
- Zeitner, U.D.; Harzendorf, T.; Fuchs, F.; Banasch, M.; Schmidt, H.; Kley, E.-B. Efficient fabrication of complex nano-optical structures by E-beam lithography based on character projection. In Proceedings of the SPIE MOEMS-MEMS, San Francisco, CA, USA, 7 March 2014. [Google Scholar] [CrossRef]
- Ryan, C.G.; Clayton, E.; Griffin, W.L.; Sie, S.H.; Cousens, D.R. SNIP, a statistics-sensitive background treatment for the quantitative analysis of PIXE spectra in geoscience applications. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 1988, 34, 396–402. [Google Scholar] [CrossRef]
- Curran, J.; Bolstad, W. Bolstad: Bolstad Functions. R Package Version 0.2-34. 2017. Available online: https://cran.r-project.org/web/packages/Bolstad/index.html (accessed on 22 February 2018).




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patze, S.; Huebner, U.; Weber, K.; Cialla-May, D.; Popp, J. TopUp SERS Substrates with Integrated Internal Standard. Materials 2018, 11, 325. https://doi.org/10.3390/ma11020325
Patze S, Huebner U, Weber K, Cialla-May D, Popp J. TopUp SERS Substrates with Integrated Internal Standard. Materials. 2018; 11(2):325. https://doi.org/10.3390/ma11020325
Chicago/Turabian StylePatze, Sophie, Uwe Huebner, Karina Weber, Dana Cialla-May, and Juergen Popp. 2018. "TopUp SERS Substrates with Integrated Internal Standard" Materials 11, no. 2: 325. https://doi.org/10.3390/ma11020325
APA StylePatze, S., Huebner, U., Weber, K., Cialla-May, D., & Popp, J. (2018). TopUp SERS Substrates with Integrated Internal Standard. Materials, 11(2), 325. https://doi.org/10.3390/ma11020325





