Modelling and Optimization of Polycaprolactone Ultrafine-Fibres Electrospinning Process Using Response Surface Methodology
Abstract
:1. Introduction
2. Results
2.1. Experimental Runs
2.2. Fibre Diameter
2.3. Elastic Modulus
2.4. Parameter Optimization and Confirmation Test
3. Discussion
4. Materials and Methods
4.1. Electrospinning
4.2. Scanning Electron Microscopy
4.3. Mechanical Testing
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| RSM | Response Surface Methodology |
| PCL | Polycaprolactone |
| CCD | Central Composite Design |
| FDA | Food and Drug Administration |
| RPM | Revolutions per minute |
Appendix A






References
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Ikada, Y. Challenges in tissue engineering. J. R. Soc. Interface 2006, 3, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Z.; Czernuszka, J.T. Development of biodegradable scaffolds for tissue engineering: A perspective on emerging technology. Mater. Sci. Technol. 2007, 23, 379–391. [Google Scholar] [CrossRef]
- Li, W.-J.; Cooper, J.A., Jr.; Mauck, R.L.; Tuan, R.S. Fabrication and characterization of six electrospun poly(α-hydroxy ester)-based fibrous scaffolds for tissue engineering applications. Acta Biomater. 2006, 2, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.M.; Mauck, R.L. The effect of nanofiber alignment on the maturation of engineered meniscus constructs. Biomaterials 2007, 28, 1967–1977. [Google Scholar] [CrossRef] [PubMed]
- Nerurkar, N.L.; Sen, S.; Huang, A.H.; Elliott, D.M.; Mauck, R.L. Engineered disc-like angle-ply structures for intervertebral disc replacement. Spine 2010, 35, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, H.; Roy, A.K.; Zaporojan, V.; Vacanti, C.A.; Ueda, M.; Bonassar, L.J. Biomechanical and biochemical characterization of composite tissue-engineered intervertebral discs. Biomaterials 2006, 27, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Reneker, D.H.; Yarin, A.L.; Fong, H.; Koombhongse, S. Bending instability of electrically charged liquid jets of polymer solutions in electrospinning. J. Appl. Phys. 2000, 104, 4531–4547. [Google Scholar] [CrossRef]
- Kim, H.W.; Kim, H.E.; Knowles, J.C. Production and potential of bioactive glass nanofibers as a next-generation biomaterial. Adv. Funct. Mater. 2006, 16, 1529–1535. [Google Scholar] [CrossRef]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospinning of polymeric nanofibers for tissue engineering applications: A review. Tissue Eng. 2006, 12, 1197–1211. [Google Scholar] [CrossRef] [PubMed]
- Reneker, D.H.; Chun, I. Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology 1996, 7, 216–223. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Electrospinning of nanofibers: Reinventing the wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Baek, J.; Sovani, S.; Choi, W.; Jin, S.; Grogan, S.P.; D’Lima, D.D. Meniscal tissue engineering using aligned collagen fibrous scaffolds: Comparison of different human cell sources. Tissue Eng. Part A 2018, 24, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Ferreira, A.M.; Callaghan, J.T.; Miller, C.A.; Atkinson, J.; Freeman, C.; Hatton, P.V. Multilayer nanoscale encapsulation of biofunctional peptides to enhance bone tissue regeneration in vivo. Adv. Healthc. Mater. 2017, 6, 1601182. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Li, W.; Lv, X.; Lei, Z.; Bian, Y.; Deng, H.; Wang, H.; Li, J.; Li, X. Biomimetic lbl structured nanofibrous matrices assembled by chitosan/collagen for promoting wound healing. Biomaterials 2015, 53, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Kyle, W.B.; Mohammad, Z.A.; Dennis, D.; Weixin, Z.; James, J.Y.; Anthony, A. Hybrid printing of mechanically and biologically improved constructs for cartilage tissue engineering applications. Biofabrication 2013, 5, 15001. [Google Scholar] [CrossRef]
- Steinberg, D.M.; Bursztyn, D. Response surface methodology in biotechnology. Qual. Eng. 2010, 22, 78–87. [Google Scholar] [CrossRef]
- Standards. Medical Devices—Iso 13485 and iso 9001; BSI Standards Ltd.: London, UK, 2005. [Google Scholar]
- Byrne, D.M.; Taguchi, S. Taguchi Approach to Parameter Design; ASQ: Milwaukee, WI, USA, 1986; pp. 168–177. [Google Scholar]
- Montgomery, D.C. Design and Analysis of Experiments; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Myers, R.H.; Montgomery, D.C.; Geoffrey Vining, G.; Borror, C.M.; Kowalski, S.M. Response surface methodology: A retrospective and literature survey. J. Qual. Technol. 2004, 36, 53–78. [Google Scholar] [CrossRef]
- Myers, R.H. Response surface methodology—Current status and future directions. J. Qual. Technol. 1999, 31, 30–44. [Google Scholar] [CrossRef]
- Benyounis, K.Y.; Olabi, A.G. Optimization of different welding processes using statistical and numerical approaches—A reference guide. Adv. Eng. Softw. 2008, 39, 483–496. [Google Scholar] [CrossRef] [Green Version]
- Maleki, H.; Gharehaghaji, A.A.; Criscenti, G.; Moroni, L.; Dijkstra, P.J. The influence of process parameters on the properties of electrospun plla yarns studied by the response surface methodology. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Honary, S.; Ebrahimi, P.; Hadianamrei, R. Optimization of particle size and encapsulation efficiency of vancomycin nanoparticles by response surface methodology. Pharm. Dev. Technol. 2014, 19, 987–998. [Google Scholar] [CrossRef] [PubMed]
- Jou, Y.T.; Lin, W.T.; Lee, W.C.; Yeh, T.M. Integrating the taguchi method and response surface methodology for process parameter optimization of the injection molding. Appl. Math. Inf. Sci. 2014, 8, 1277–1285. [Google Scholar] [CrossRef]
- Doshi, J.; Reneker, D.H. Electrospinning process and applications of electrospun fibers. J. Electrostat. 1995, 35, 151–160. [Google Scholar] [CrossRef]
- Khan, S.P.; Bhasin, K.; Newaz, G.M. Optimizing Process Variables to Control Fiber Diameter of Electrospun Polycaprolactone Nanofiber Using Factorial Design. Mrs Proc. 2011, 1316, 65–73. [Google Scholar] [CrossRef]
- Liao, C.-C.; Wang, C.-C.; Chen, C.-Y. Stretching-induced crystallinity and orientation of polylactic acid nanofibers with improved mechanical properties using an electrically charged rotating viscoelastic jet. Polymer 2011, 52, 4303–4318. [Google Scholar] [CrossRef]
- Haghi, A.K. Optimization of Electrospinning Process via Response Surface Methodology. 2013; 25–60. [Google Scholar]
- Koepsell, L.; Remund, T.; Bao, J.; Neufeld, D.; Fong, H.; Deng, Y. Tissue engineering of annulus fibrosus using electrospun fibrous scaffolds with aligned polycaprolactone fibers. J. Biomed. Mater. Res. Part A 2011, 99A, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Shim, W.G.; Lee, I.H. Modeling and optimization of electrospun polyvinylacetate (pvac) nanofibers by response surface methodology (rsm). J. Nanosci. Nanotechnol. 2011, 11, 1359–1363. [Google Scholar] [CrossRef] [PubMed]
- Karim, S.A.; Sulong, A.B.; Azhari, C.H.; Lee, T.H.; Hwei, N.M. Optimization of electrospinning parameters using response surface methods to enhance fiber diameter, mechanical properties and orientation of nanofibers. J. Appl. Sci. Res. 2012, 8, 2510–2517. [Google Scholar]
- Agarwal, P.; Mishra, P.K.; Srivastava, P. Statistical optimization of the electrospinning process for chitosan/polylactide nanofabrication using response surface methodology. J. Mater. Sci. 2012, 47, 4262–4269. [Google Scholar] [CrossRef]
- Doustgani, A.; Vasheghani-Farahani, E.; Soleimani, M.; Hashemi-Najafabadi, S. Process optimization of electrospun polycaprolactone and nanohydroxyapatite composite nanofibers using response surface methodology. J. Nanosci. Nanotechnol. 2013, 13, 4708–4714. [Google Scholar] [CrossRef] [PubMed]
- Gönen, S.Ö.; Taygun, M.E.; Küçükbayrak, S. Effects of electrospinning parameters on gelatin/poly(ϵ-caprolactone) nanofiber diameter. Chem. Eng. Technol. 2015, 38, 844–850. [Google Scholar] [CrossRef]
- Azimi, B.; Nourpanah, P.; Rabiee, M.; Arbab, S.; Cascone, M.G.; Baldassare, A.; Lazzeri, L. Application of response surface methodology to evaluate the effect of dry-spinning parameters on poly (ε-caprolactone) fiber properties. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Padmanabhan, T.; Kamaraj, V.; Magwood, L., Jr.; Starly, B. Experimental investigation on the operating variables of a near-field electrospinning process via response surface methodology. J. Manuf. Process. 2011, 13, 104–112. [Google Scholar] [CrossRef]
- Gu, S.Y.; Ren, J.; Vancso, G.J. Process optimization and empirical modeling for electrospun polyacrylonitrile (pan) nanofiber precursor of carbon nanofibers. Eur. Polym. J. 2005, 41, 2559–2568. [Google Scholar] [CrossRef]
- Agarwal, S.; Wendorff, J.H.; Greiner, A. Use of electrospinning technique for biomedical applications. Polymer 2008, 49, 5603–5621. [Google Scholar] [CrossRef]
- Smith, C.D.; Masouros, S.; Hill, A.M.; Amis, A.A.; Bull, A.M.J. A biomechanical basis for tears of the human acetabular labrum. Br. J. Sports Med. 2009, 43, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Petersen, W.; Petersen, F.; Tillmann, B. Structure and vascularization of the acetabular labrum with regard to the pathogenesis and healing of labral lesions. Arch. Orthop. Trauma Surg. 2003, 123, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zhang, P.; Jiang, Y.; Xu, F.; Yin, J.; Zuo, Y. Mechanical properties of nylon-6/sio2 nanofibers prepared by electrospinning. Mater. Lett. 2009, 63, 34–36. [Google Scholar] [CrossRef]
- Arinstein, A.; Zussman, E. Electrospun polymer nanofibers: Mechanical and thermodynamic perspectives. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 691–707. [Google Scholar] [CrossRef]
- Burman, M.; Arinstein, A.; Zussman, E. Do surface effects explain the unique elasticity of polymer nanofibers? EPL 2011, 96, 16006. [Google Scholar] [CrossRef]
- Katti, D.S.; Robinson, K.W.; Ko, F.K.; Laurencin, C.T. Bioresorbable nanofiber-based systems for wound healing and drug delivery: Optimization of fabrication parameters. J. Biomed. Mater. Res. Part B Appl. Biomater. 2004, 70B, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Boland, E.D.; Wnek, G.E.; Simpson, D.G.; Pawlowski, K.J.; Bowlin, G.L. Tailoring tissue engineering scaffolds using electrostatic processing techniques: A study of poly(glycolic acid) electrospinning. J. Macromol. Sci. Part A 2001, 38, 1231–1243. [Google Scholar] [CrossRef]
- Silva, C.S.R.; Luz, G.M.; Gamboa-martÍnez, T.C.; Mano, J.F.; GÓmez ribelles, J.L.; GÓmez-tejedor, J.A. Poly(ɛ-caprolactone) electrospun scaffolds filled with nanoparticles. Production and optimization according to taguchi's methodology. J. Macromol. Sci. Part B 2013, 53, 781–799. [Google Scholar] [CrossRef]
- Nottelet, B.; Pektok, E.; Mandracchia, D.; Tille, J.C.; Walpoth, B.; Gurny, R.; Möller, M. Factorial design optimization and in vivo feasibility of poly(ε-caprolactone)-micro- and nanofiber-based small diameter vascular grafts. J. Biomed. Mater. Res. Part A 2009, 89A, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Bölgen, N.; Menceloğlu, Y.Z.; Acatay, K.; Vargel, İ.; Pişkin, E. In vitro and in vivo degradation of non-woven materials made of poly(ε-caprolactone) nanofibers prepared by electrospinning under different conditions. J. Biomater. Sci. Polym. Ed. 2005, 16, 1537–1555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeun, J.P.; Lim, Y.M.; Nho, Y.C. Study on morphology of electrospun poly(caprolactone) nanofiber. J. Ind. Eng. Chem. 2005, 11, 573–578. [Google Scholar]
- Kong, L.; Ziegler, G.R. Quantitative relationship between electrospinning parameters and starch fiber diameter. Carbohydr. Polym. 2013, 92, 1416–1422. [Google Scholar] [CrossRef] [PubMed]
- Williamson, M.R.; Coombes, A.G.A. Gravity spinning of polycaprolactone fibres for applications in tissue engineering. Biomaterials 2004, 25, 459–465. [Google Scholar] [CrossRef]
- Dusunceli, N.; Colak, O.U. Modelling effects of degree of crystallinity on mechanical behavior of semicrystalline polymers. Int. J. Plast. 2008, 24, 1224–1242. [Google Scholar] [CrossRef]
- Ouchi, T.; Ichimura, S.; Ohya, Y. Synthesis of branched poly(lactide) using polyglycidol and thermal, mechanical properties of its solution-cast film. Polymer 2006, 47, 429–434. [Google Scholar] [CrossRef]
- Thomas, V.; Jose, M.V.; Chowdhury, S.; Sullivan, J.F.; Dean, D.R.; Vohra, Y.K. Mechano-morphological studies of aligned nanofibrous scaffolds of polycaprolactone fabricated by electrospinning. J. Biomater. Sci. Polym. Ed. 2006, 17, 969–984. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.X.; Yang, X.P.; Zhang, F.; Wu, S.Z.; Waclawik, E. Stretching-induced orientation to improve mechanical properties of electrospun pan nanocomposites. Int. J. Mod. Phys. B 2008, 22, 5913–5918. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- De Vrieze, S.; Van Camp, T.; Nelvig, A.; Hagström, B.; Westbroek, P.; De Clerck, K. The effect of temperature and humidity on electrospinning. J. Mater. Sci. 2009, 44, 1357–1362. [Google Scholar] [CrossRef]
- Wang, C.; Chien, H.-S.; Hsu, C.-H.; Wang, Y.-C.; Wang, C.-T.; Lu, H.-A. Electrospinning of polyacrylonitrile solutions at elevated temperatures. Macromolecules 2007, 40, 7973–7983. [Google Scholar] [CrossRef]
- Pakravan, M.; Heuzey, M.-C.; Ajji, A. A fundamental study of chitosan/peo electrospinning. Polymer 2011, 52, 4813–4824. [Google Scholar] [CrossRef]
- Ramazani, S.; Karimi, M. Investigating the influence of temperature on electrospinning of polycaprolactone solutions. E-Polymers 2014, 14, 323–333. [Google Scholar] [CrossRef]
- Mit-uppatham, C.; Nithitanakul, M.; Supaphol, P. Ultrafine electrospun polyamide-6 fibers: Effect of solution conditions on morphology and average fiber diameter. Macromol. Chem. Phys. 2004, 205, 2327–2338. [Google Scholar] [CrossRef]
- Lee, K.H.; Kim, H.Y.; Khil, M.S.; Ra, Y.M.; Lee, D.R. Characterization of nano-structured poly(ε-caprolactone) nonwoven mats via electrospinning. Polymer 2003, 44, 1287–1294. [Google Scholar] [CrossRef]
- Li, W.-J.; Mauck, R.L.; Cooper, J.A.; Yuan, X.; Tuan, R.S. Engineering controllable anisotropy in electrospun biodegradable nanofibrous scaffolds for musculoskeletal tissue engineering. J. Biomech. 2007, 40, 1686–1693. [Google Scholar] [CrossRef] [PubMed]
- Anindyajati, A.; Boughton, P.; Ruys, A. The effect of rotating collector design on tensile properties and morphology of electrospun polycaprolactone fibres. MATEC Web Conf. 2015, 27, 2002. [Google Scholar] [CrossRef]







| Code | Parameter | Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| A | Concentration (% w/v) | 10 | 12.5 | 15 |
| B | Flow rate (mL/h) | 3 | 4.5 | 6 |
| C | Distance (cm) | 10 | 12.5 | 15 |
| D | Temperature (°C) | 23 | 34 | 45 |
| E | Mandrel rotation speed (RPM) | 1000 | 1500 | 2000 |
| Run | Conc. (% w/v) | Flow Rate (mL/h) | Distance (cm) | Temp. (°C) | Rotation (RPM) | Average Fibre Ø (µm) | Young’s Modulus (MPa) |
|---|---|---|---|---|---|---|---|
| 1 | 10 | 3 | 10 | 45 | 1000 | 0.903 | 17.38 |
| 2 | 12.5 | 4.5 | 12.5 | 45 | 1500 | 1.742 | 26.55 |
| 3 | 12.5 | 4.5 | 12.5 | 34 | 1500 | 1.436 | 45.83 |
| 4 | 15 | 3 | 15 | 45 | 1000 | 1.741 | 19.79 |
| 5 | 10 | 3 | 15 | 45 | 2000 | 1.810 | 17.87 |
| 6 | 10 | 3 | 10 | 23 | 2000 | 1.239 | 21.68 |
| 7 | 10 | 4.5 | 12.5 | 34 | 1500 | 1.051 | 24.49 |
| 8 | 12.5 | 4.5 | 12.5 | 34 | 1500 | 1.330 | 27.66 |
| 9 | 12.5 | 4.5 | 12.5 | 34 | 1500 | 2.775 | 57.92 |
| 10 | 15 | 6 | 10 | 45 | 1000 | 2.401 | 31.69 |
| 11 | 12.5 | 4.5 | 12.5 | 23 | 1500 | 3.661 | 63.34 |
| 12 | 10 | 6 | 15 | 23 | 2000 | 2.249 | 55.49 |
| 13 | 12.5 | 4.5 | 15 | 34 | 1500 | 2.127 | 23.55 |
| 14 | 10 | 6 | 10 | 23 | 1000 | 2.128 | 40.70 |
| 15 | 15 | 3 | 15 | 23 | 2000 | 2.628 | 93.08 |
| 16 | 12.5 | 4.5 | 12.5 | 34 | 2000 | 2.955 | 51.81 |
| 17 | 15 | 3 | 10 | 23 | 1000 | 2.732 | 70.22 |
| 18 | 15 | 4.5 | 12.5 | 34 | 1500 | 2.726 | 53.99 |
| 19 | 12.5 | 4.5 | 12.5 | 34 | 1500 | 2.571 | 32.56 |
| 20 | 12.5 | 4.5 | 10 | 34 | 1500 | 2.400 | 44.98 |
| 21 | 12.5 | 6 | 12.5 | 34 | 1500 | 2.023 | 58.49 |
| 22 | 15 | 6 | 10 | 23 | 2000 | 2.475 | 120.02 |
| 23 | 10 | 6 | 15 | 45 | 1000 | 2.403 | 30.26 |
| 24 | 12.5 | 4.5 | 12.5 | 34 | 1500 | 2.509 | 21.02 |
| 25 | 10 | 6 | 10 | 45 | 2000 | 3.118 | 53.15 |
| 26 | 12.5 | 3 | 12.5 | 34 | 1500 | 2.134 | 30.59 |
| 27 | 15 | 6 | 15 | 23 | 1000 | 2.562 | 127.75 |
| 28 | 12.5 | 4.5 | 12.5 | 34 | 1000 | 2.171 | 15.68 |
| 29 | 10 | 3 | 15 | 23 | 1000 | 2.100 | 36.42 |
| 30 | 12.5 | 4.5 | 12.5 | 34 | 1500 | 2.181 | 20.93 |
| 31 | 15 | 3 | 10 | 45 | 2000 | 2.665 | 70.41 |
| 32 | 15 | 6 | 15 | 45 | 2000 | 2.478 | 51.63 |
| Terms | Iteration Steps | Terms | Iteration Steps | ||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 0 | 1 | 2 | ||
| Model | 0.416 | 0.002 | 0.000 | A*C | 0.464 | - | - |
| Linear | 0.130 | 0.002 | 0.000 | A*D | 0.560 | - | - |
| Square | 0.869 | - | - | A*E | 0.810 | - | - |
| 2-way interactions | 0.473 | - | - | B*C | 0.503 | - | - |
| Constant | 0.000 * | 0.000 | 0.000 | B*D | 0.425 | - | - |
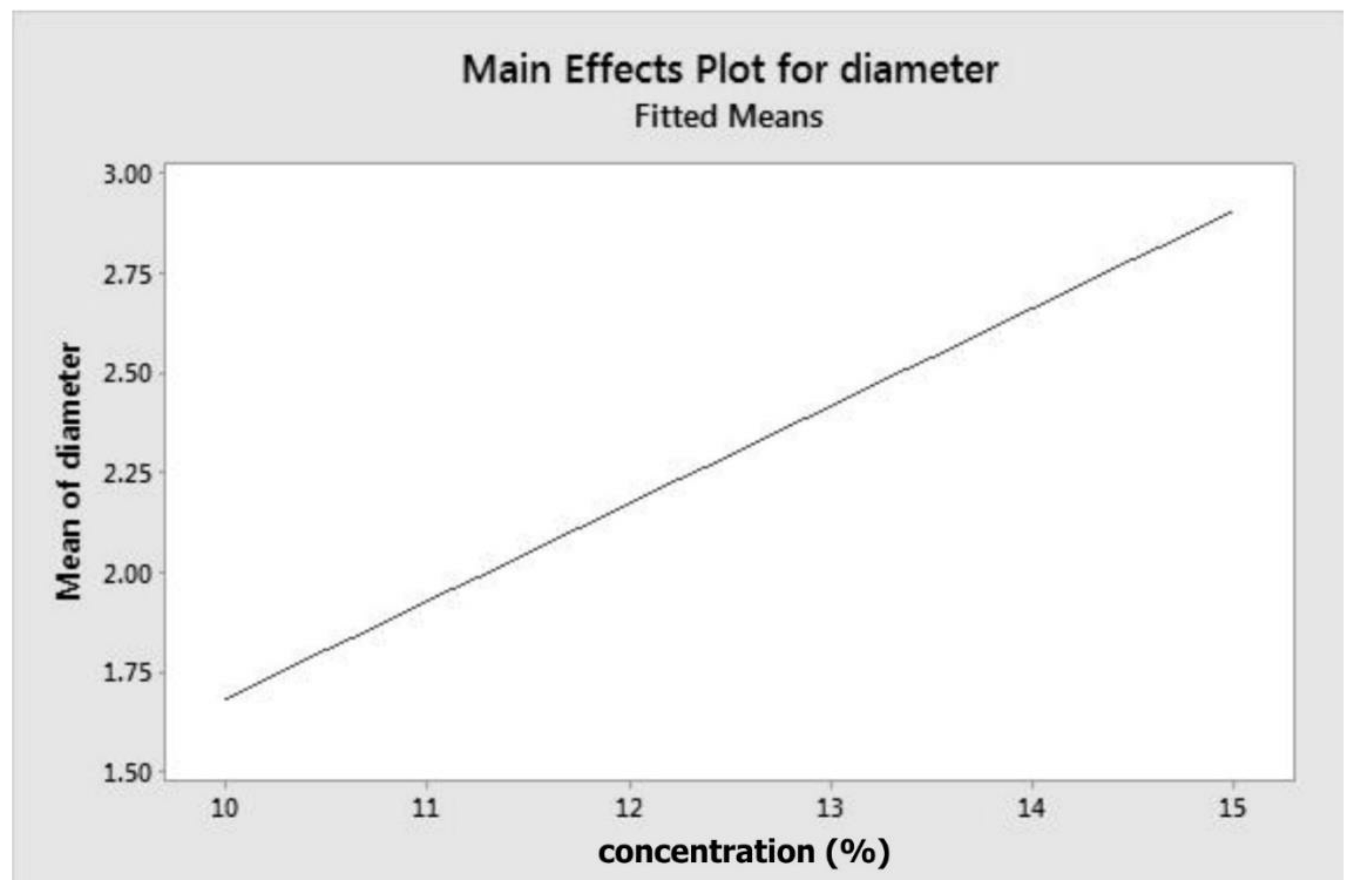
| Concentration (A) | 0.012 * | 0.002 | 0.000 | B*E | 0.983 | - | - |
| Flow-rate (B) | 0.427 | - | - | C*D | 0.313 | - | - |
| Distance (C) | 0.809 | - | - | C*E | 0.476 | - | - |
| Temperature (D) | 0.548 | - | - | D*E | 0.112 | - | - |
| Rotation (E) | 0.414 | - | - | Model evaluation | |||
| A*A | 0.578 | - | - | Lack of Fit | 0.628 | 0.771 | 0.893 |
| B*B | 0.782 | - | - | R2 | 67.73% | 26.68% | 39.12% |
| C*C | 0.916 | - | - | R2 adjusted | 9.06% | 24.24% | 37.02% |
| D*D | 0.735 | - | - | R2 predicted | 0.00% | 16.82% | 31.77% |
| E*E | 0.237 | - | - | Unusual observation | - | 1 | - |
| A*B | 0.086 | - | - | ||||
| Terms | Iteration Steps | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| p-Value | |||||
| Model | 0.001 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * |
| Linear | 0.000 * | 0.000 * | 0.000 * | 0.000* | 0.000 * |
| Square | 0.047 * | - | - | - | - |
| 2-way interactions | 0.026 * | 0.003 * | 0.004 * | 0.001 * | 0.000 * |
| Constant | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * |
| Concentration (A) | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * |
| Flow-rate (B) | 0.003 * | 0.006 * | 0.009 * | 0.001 * | 0.000 * |
| Distance (C) | 0.785 | - | - | - | - |
| Temperature (D) | 0.000 * | 0.000 * | 0.000 * | 0.000 * | 0.000 * |
| Rotation (E) | 0.016 * | 0.034 * | 0.041 * | 0.031 * | 0.006 * |
| A*A | 0.649 | - | - | - | - |
| B*B | 0.273 | - | - | - | - |
| C*C | 0.863 | - | - | - | - |
| D*D | 0.253 | - | - | - | - |
| E*E | 0.812 | - | - | - | - |
| A*B | 0.861 | - | - | - | - |
| A*C | 0.884 | - | - | - | - |
| A*D | 0.002 * | 0.003 * | 0.004 * | 0.001 * | 0.000 * |
| A*E | 0.225 | - | - | - | - |
| B*C | 0.521 | - | - | - | - |
| B*D | 0.121 | - | - | - | - |
| B*E | 0.851 | - | - | - | - |
| C*D | 0.039 * | 0.075 | - | - | - |
| C*E | 0.060 | - | - | - | - |
| D*E | 0.132 | - | - | - | - |
| Model evaluation | |||||
| Lack of Fit | 0.855 | 0.518 | 0.460 | 0.800 | 0.893 |
| R2 | 93.32% | 76.03% | 72.72% | 77.79% | 82.09% |
| R2 adjusted | 81.18% | 70.28% | 67.47% | 73.16% | 78.20% |
| R2 predicted | 0.00% | 56.21% | 54.43% | 66.06% | 73.24% |
| Unusual obsv. | - | 1 | 2 | 1 | - |
| Run Order | Distance (cm) | Day | Fibre Diameter (µm) | Elastic Modulus (MPa) |
|---|---|---|---|---|
| 1 | 10 | 1 | 1.202 | 34.46 |
| 2 | 12.5 | 1 | 1.127 | 16.19 |
| 3 | 15 | 1 | 1.116 | 10.06 |
| 4 | 15 | 2 | 1.408 | 23.71 |
| 5 | 12.5 | 2 | 1.396 | 27.92 |
| 6 | 10 | 2 | 1.562 | 30.37 |
| 7 | 12.5 | 3 | 1.635 | 34.70 |
| 8 | 15 | 3 | 1.601 | 33.50 |
| 9 | 10 | 3 | 1.457 | 22.66 |
| Mean ± SD | 1.391 ± 0.199 | 25.95 ± 8.61 | ||
| Prediction | 1.680 | 25.08 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anindyajati, A.; Boughton, P.; Ruys, A.J. Modelling and Optimization of Polycaprolactone Ultrafine-Fibres Electrospinning Process Using Response Surface Methodology. Materials 2018, 11, 441. https://doi.org/10.3390/ma11030441
Anindyajati A, Boughton P, Ruys AJ. Modelling and Optimization of Polycaprolactone Ultrafine-Fibres Electrospinning Process Using Response Surface Methodology. Materials. 2018; 11(3):441. https://doi.org/10.3390/ma11030441
Chicago/Turabian StyleAnindyajati, Adhi, Philip Boughton, and Andrew J. Ruys. 2018. "Modelling and Optimization of Polycaprolactone Ultrafine-Fibres Electrospinning Process Using Response Surface Methodology" Materials 11, no. 3: 441. https://doi.org/10.3390/ma11030441
APA StyleAnindyajati, A., Boughton, P., & Ruys, A. J. (2018). Modelling and Optimization of Polycaprolactone Ultrafine-Fibres Electrospinning Process Using Response Surface Methodology. Materials, 11(3), 441. https://doi.org/10.3390/ma11030441






