Fabrication and Sintering Behavior of Er:SrF2 Transparent Ceramics using Chemically Derived Powder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Nanoparticles, Powder and Ceramics
2.2. Characterization
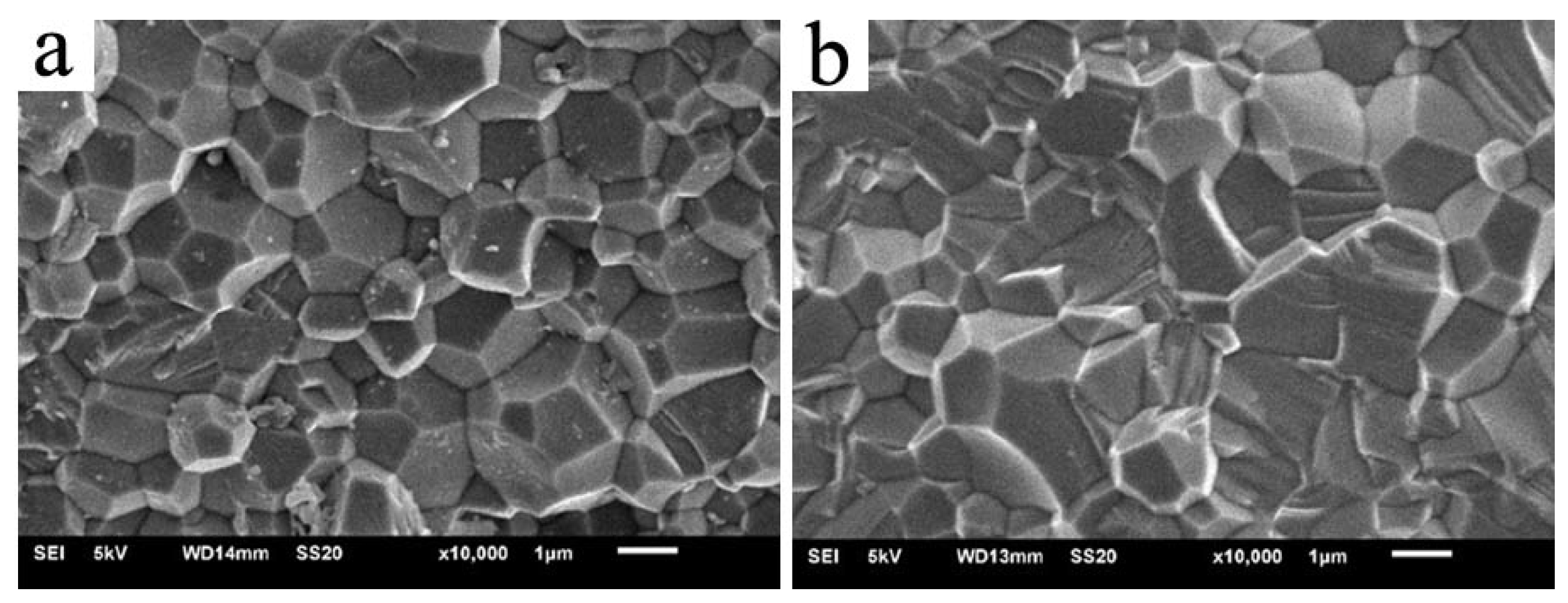
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Basiev, T.T. Continuously tunable cw lasing near 2.75 μm in diode-pumped Er3+:SrF2 and Er3+:CaF2 crystals. Quantum Electron. 2006, 36, 591–594. [Google Scholar] [CrossRef]
- Li, T.; Beil, K.; Kränkel, C.; Huber, G. Efficient high-power continuous wave Er:Lu2O3 laser at 2.85 μm. Opt. Lett. 2012, 37, 2568–2570. [Google Scholar] [CrossRef] [PubMed]
- Sulc, J.; Němec, M.; Svejkar, R.; Jelínková, H.; Doroshenko, M.E.; Fedorov, P.P.; Osiko, V.V. Diode-pumped Er:CaF2 ceramic 2.7 μm tunable laser. Opt. Lett. 2013, 38, 3406–3409. [Google Scholar] [CrossRef] [PubMed]
- Godard, A. Infrared (2–12 μm) solid-state laser sources: A review. C. R. Phys. 2007, 8, 1100–1128. [Google Scholar] [CrossRef]
- Chen, Y.F.; Huang, T.M.; Kao, C.F.; Wang, C.L. Optimization in scaling fiber-coupled laser-diode end-pumped lasers to higher power: Influence of thermal effect. IEEE J. Quantum Electron. 1997, 33, 1424–1429. [Google Scholar] [CrossRef]
- Jun, M.H.; Meng, J.Q.; Li, L.X.; Zhu, X.L.; Tian, L.Y. Study on compensation of thermal lens in high power high repetition solid-state laser. High Power Laser Part. Beams 2005, 17, 175–179. [Google Scholar]
- Wyss, E.; Roth, M.; Graf, T.; Weber, H.P. Thermooptical compensation methods for high-power lasers. IEEE J. Quantum Electron. 2002, 38, 1620–1628. [Google Scholar] [CrossRef]
- Ikesue, A.; Kinoshita, T.; Kamata, K.; Yoshida, K. Fabrication and Optical Properties of High-Performance Polycrystalline Nd:YAG Ceramics for Solid-State Lasers. J. Am. Ceram. Soc. 1995, 78, 1033–1040. [Google Scholar] [CrossRef]
- Chen, S.; Wu, Y.Q. New opportunities for transparent ceramics. Am. Ceram. Soc. Bull. 2013, 92, 32–37. [Google Scholar]
- Ikesue, A.; Aung, Y.L. Synthesis of Yb:YAG Ceramics Without Sintering Additives and their Performance. J. Am. Ceram. Soc. 2017, 100, 26–30. [Google Scholar] [CrossRef]
- Ikesue, A.; Kamata, K. Microstructure and optical properties of hot isostatically pressed Nd:YAG ceramics. J. Am. Ceram. Soc. 1996, 79, 1927–1933. [Google Scholar] [CrossRef]
- Luo, D.W.; Zhang, J.; Xu, C.W.; Qin, X.P.; Tang, D.Y.; Ma, J. Fabrication and laser properties of transparent Yb:YAG ceramics. Opt. Mater. 2012, 34, 936–939. [Google Scholar] [CrossRef]
- Hu, Z.W.; Xu, X.D.; Wang, J.; Liu, P.; Li, D.Z.; Wan, X.D.; An, L.Q.; Zhang, J.; Xu, J.; Tang, D.Y. Spark plasma sintering of Sm3+ doped Y2O3 transparent ceramics for visible light lasers. Ceram. Int. 2017, 43, 12057–12060. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Luo, D.W.; Yang, H.; Tang, D.Y.; Kong, L.B. Densification and microstructural evolution of yttria transparent ceramics: The effect of ball milling conditions. J. Eur. Ceram. Soc. 2015, 35, 1011–1019. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Ning, K.J.; Ma, J.; Luo, D.W.; Yin, D.L.; Yang, H.; Tang, D.Y.; Kong, L.B. Densification of zirconia doped yttria transparent ceramics using co-precipitated powders. Ceram. Int. 2016, 42, 10770–10778. [Google Scholar] [CrossRef]
- Yan, D.Y.; Xu, X.D.; Lu, H.; Wang, Y.W.; Liu, P.; Zhang, J. Fabrication and properties of Y2O3 transparent ceramic by sintering aid combinations. Ceram. Int. 2016, 42, 16640–16643. [Google Scholar] [CrossRef]
- An, L.Q.; Ito, A.; Zhang, J.; Tang, D.Y.; Goto, T. Highly transparent Nd3+:Lu2O3 produced by spark plasma sintering and its laser oscillation. Opt. Mater. Express 2014, 4, 1420–1426. [Google Scholar] [CrossRef]
- Zhu, C.H.; Song, J.H.; Mei, B.C.; Li, W.W.; Liu, Z.D. Fabrication and optical characterizations of CaF2-SrF2-NdF3 transparent ceramic. Mater. Lett. 2016, 167, 115–117. [Google Scholar] [CrossRef]
- Li, W.W.; Mei, B.C.; Song, J.H.; Wang, Z. Fabrication and optical property of highly transparent SrF2 ceramic. Mater. Lett. 2015, 159, 210–212. [Google Scholar] [CrossRef]
- Basiev, T.T.; Doroshenko, M.E.; Konyushkin, V.A.; Osiko, V.V.; Fedorov, P.P.; Demidenko, V.A.; Dukel’skii, K.V.; Mironov, I.A.; Smirnov, A.N. Fluoride optical nanoceramics. Russ. Chem. Bull. 2008, 57, 877–886. [Google Scholar] [CrossRef]
- Basiev, T.T.; Doroshenko, M.E.; Konyushkin, V.A.; Osiko, V.V. SrF2:Nd3+ laser fluoride ceramics. Opt. Lett. 2010, 35, 4009–4011. [Google Scholar] [CrossRef] [PubMed]
- Sarthou, J.; Duquesne, J.Y.; Becerra, L.; Gredin, P.; Mortier, M. Thermal conductivity measurements of Yb:CaF2 transparent ceramics using the 3 omega method. J. Appl. Phys. 2017, 121, 245108. [Google Scholar] [CrossRef]
- D’Acapito, F.; Pelli-Cresi, S.; Blanc, W.; Benabdesselam, M.; Mady, F.; Gredin, P.; Mortier, M. The incorporation site of Er in nanosized CaF2. J. Phys. Condens. Matter 2016, 28, 485301. [Google Scholar] [CrossRef] [PubMed]
- Lupei, V.; Lupei, A.; Ikesue, A. Transparent polycrystalline ceramic laser materials. Opt. Mater. 2008, 30, 1781–1786. [Google Scholar] [CrossRef]
- Ikesue, A.; Aung, Y.L. Ceramic laser materials. Nat. Photonics 2008, 2, 721–727. [Google Scholar] [CrossRef]
- Kong, J.; Tang, D.Y.; Lu, J.; Ueda, K.; Yagi, H.; Yanagitani, T. Diode-end-pumped 4.2-W continuous-wave Yb:Y2O3 ceramic laser. Opt. Lett. 2004, 29, 1212–1214. [Google Scholar] [CrossRef] [PubMed]
- Basiev, T.T.; Doroshenko, M.E.; Fedorov, P.P.; Konyushkin, V.A.; Kuznetsov, S.V.; Osiko, V.V.; Akchurin, M.S. Efficient laser based on CaF2-SrF2-YbF3 nanoceramics. Opt. Lett. 2008, 33, 521–523. [Google Scholar] [CrossRef] [PubMed]
- Lyberis, A.; Patriarche, G.; Gredin, P.; Vivien, D.; Mortier, M. Origin of light scattering in ytterbium doped calcium fluoride transparent ceramic for high power lasers. J. Eur. Ceram. Soc. 2011, 31, 1619–1630. [Google Scholar] [CrossRef]
- Cardinali, V.; Marmois, E.; Le Garrec, B.; Bourdet, G. Determination of the thermo-optic coefficient dn/dT of ytterbium doped ceramics (Sc2O3, Y2O3, Lu2O3, YAG), crystals (YAG, CaF2) and neodymium doped phosphate glass at cryogenic temperature. Opt. Mater. 2012, 34, 990–994. [Google Scholar] [CrossRef]
- Tropf, W.J. Temperature dependent refractive index model for BaF2, CaF2, MgF2, SrF2, LiF, NaF, KCl, ZnS and ZnSe. Opt. Eng. 1995, 34, 1369–1373. [Google Scholar] [CrossRef]
- Hatch, S.E.; Parsons, W.F.; Weagley, R.J. HOT-PRESSED POLYCRYSTALLINE CaF2:Dy2+ LASER. Appl. Phys. Lett. 1964, 5, 153–154. [Google Scholar] [CrossRef]
- Akchurin, M.S.; Basiev, T.T.; Demidenko, A.A.; Doroshenko, M.E.; Fedorov, P.P.; Garibin, E.A.; Gusev, P.E.; Kuznetsov, S.V.; Krutov, M.A.; Mironov, I.A.; et al. CaF2:Yb laser ceramics. Opt. Mater. 2013, 35, 444–450. [Google Scholar] [CrossRef]
- Dukel’skii, K.V.; Mironov, I.A.; Demidenko, V.A.; Smirnov, A.N.; Fedorov, P.P.; Osiko, V.V.; Basiev, T.T.; Orlovskii, Y.V. Optical fluoride nanoceramic. J. Opt. Technol. 2008, 75, 728–736. [Google Scholar] [CrossRef]
- Mortier, M.; Bensalah, A.; Dantelle, G.; Patriarche, G.; Vivien, D. Rare-earth doped oxyfluoride glass-ceramics and fluoride ceramics: Synthesis and optical properties. Opt. Mater. 2007, 29, 1263–1270. [Google Scholar] [CrossRef]
- Aubry, P.; Bensalah, A.; Gredin, P.; Patriarche, G.; Vivien, D.; Mortier, M. Synthesis and optical characterizations of Yb-doped CaF2 ceramics. Opt. Mater. 2009, 31, 750–753. [Google Scholar] [CrossRef]
- Ju, Q.W.; Mei, B.C.; Yu, H.; Qian, X.B.; Wang, J.Y.; Ma, F.K.; Su, L.B. Spectral Properties of Pr, R3+:SrF2 (R = Y, Gd) Laser Crystals. J. Inorg. Mater. 2017, 32, 943–948. [Google Scholar]
- Zhang, F.; Liu, J.; Li, W.W.; Mei, B.C.; Jiang, D.P.; Qian, X.B.; Su, L.B. Dual-wavelength continuous-wave and passively Q-switched Nd, Y:SrF2 ceramic laser. Opt. Eng. 2016, 55, 106114. [Google Scholar] [CrossRef]
- Chen, S.; Gaume, R. Transparent bulk-size nanocomposites with high inorganic loading. Appl. Phys. Lett. 2015, 107, 241906. [Google Scholar] [CrossRef]
- Krell, A.; Waetzig, K.; Klimke, J. Influence of the structure of MgO·nAl2O3 spinel lattices on transparent ceramics processing and properties. J. Eur. Ceram. Soc. 2012, 32, 2887–2898. [Google Scholar] [CrossRef]
- Kochawattana, S.; Stevenson, A.; Lee, S.H.; Ramirez, M.; Gopalan, V.; Dumm, J.; Castillo, V.K.; Quarles, G.J.; Messing, G.L. Sintering and grain growth in SiO2 doped Nd:YAG. J. Eur. Ceram. Soc. 2008, 28, 1527–1534. [Google Scholar] [CrossRef]
- Chen, S.; Wu, Y.Q. Influence of temperature on the spark plasma sintering of calcium fluoride ceramics. J. Mater. Res. 2014, 29, 2297–2302. [Google Scholar] [CrossRef]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Liu, P.; Wang, J.; Xu, X.; Li, D.; Zhang, J.; Nie, X. Fabrication and Sintering Behavior of Er:SrF2 Transparent Ceramics using Chemically Derived Powder. Materials 2018, 11, 475. https://doi.org/10.3390/ma11040475
Liu J, Liu P, Wang J, Xu X, Li D, Zhang J, Nie X. Fabrication and Sintering Behavior of Er:SrF2 Transparent Ceramics using Chemically Derived Powder. Materials. 2018; 11(4):475. https://doi.org/10.3390/ma11040475
Chicago/Turabian StyleLiu, Jun, Peng Liu, Jun Wang, Xiaodong Xu, Dongzhen Li, Jian Zhang, and Xinming Nie. 2018. "Fabrication and Sintering Behavior of Er:SrF2 Transparent Ceramics using Chemically Derived Powder" Materials 11, no. 4: 475. https://doi.org/10.3390/ma11040475
APA StyleLiu, J., Liu, P., Wang, J., Xu, X., Li, D., Zhang, J., & Nie, X. (2018). Fabrication and Sintering Behavior of Er:SrF2 Transparent Ceramics using Chemically Derived Powder. Materials, 11(4), 475. https://doi.org/10.3390/ma11040475




