Evanescent Properties of Optical Diffraction from 2-Dimensional Hexagonal Photonic Crystals and Their Sensor Applications
Abstract
:1. Introduction
2. Materials and Methods
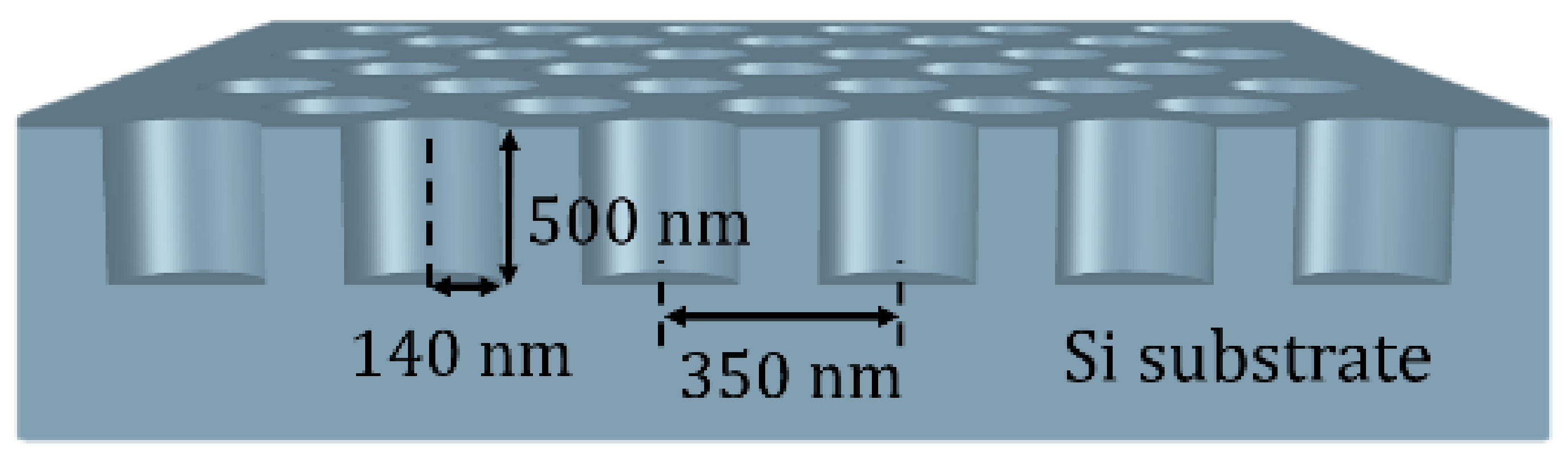
2.1. Design and Fabrication of the Hexagonal 2D Photonic Crystal
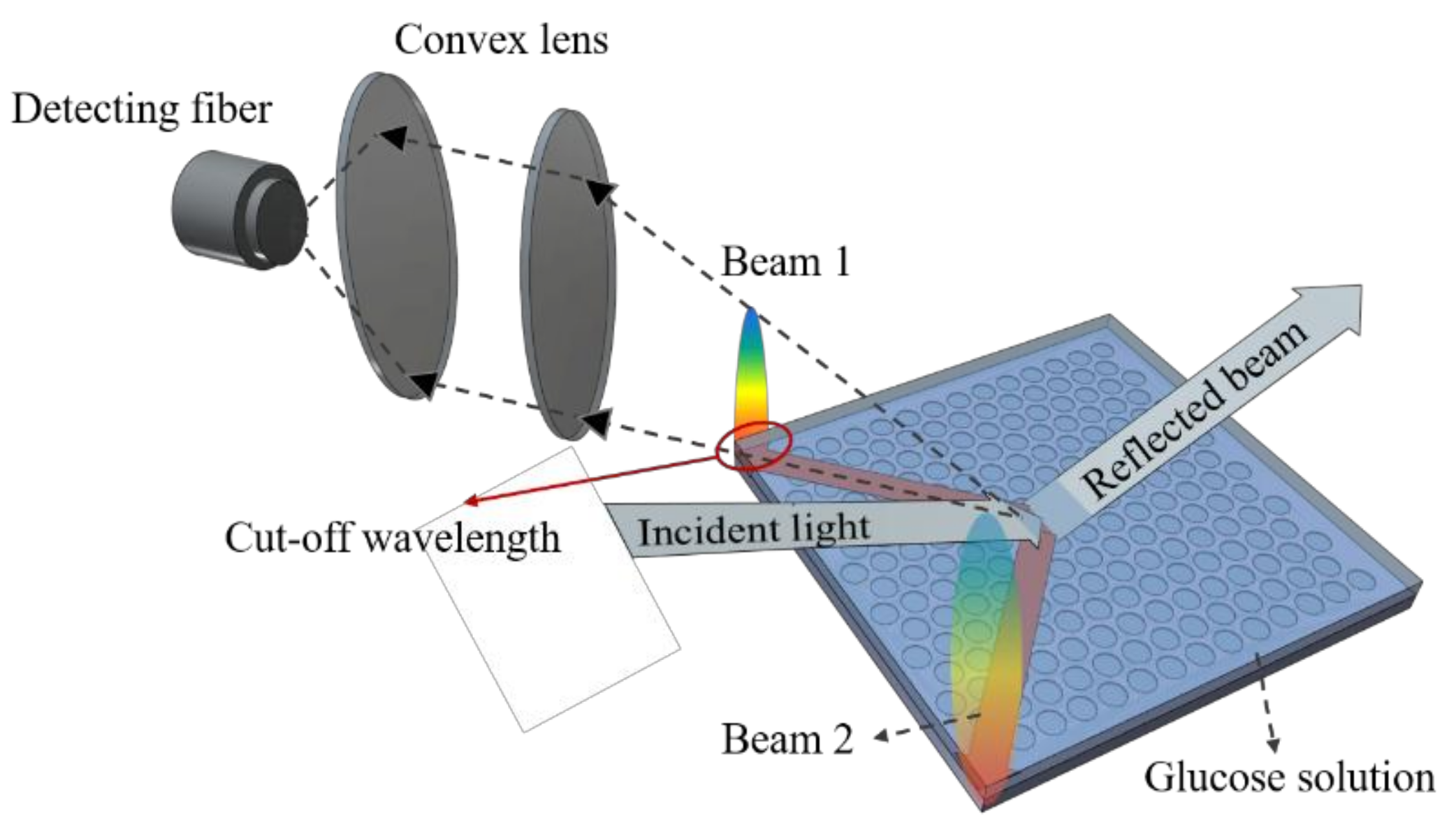
2.2. Measurement Setup
3. Results and Discussion
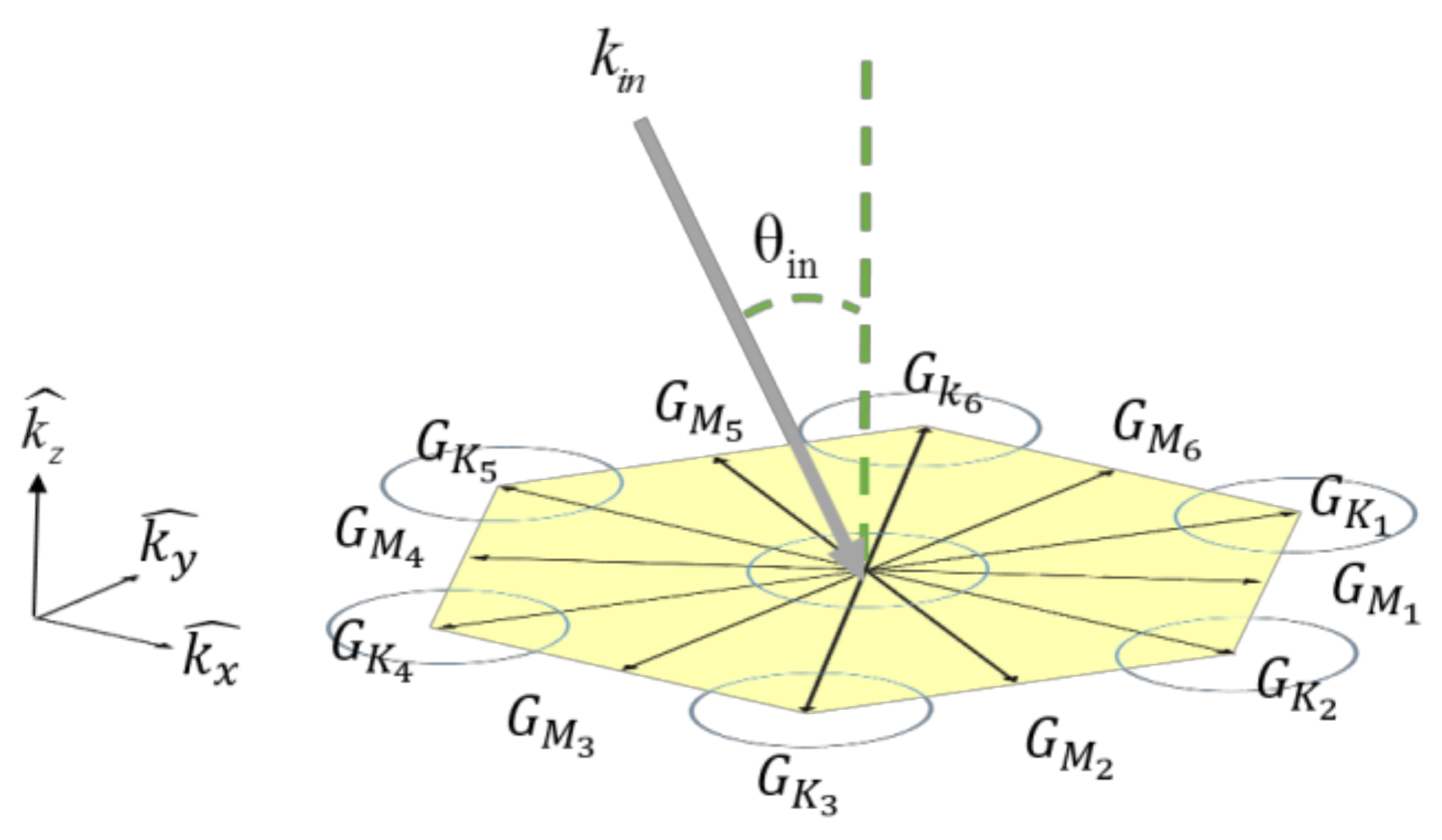
3.1. Optical Bhavior of the Hexagonal PHC
3.2. Principle of PhC Sensing
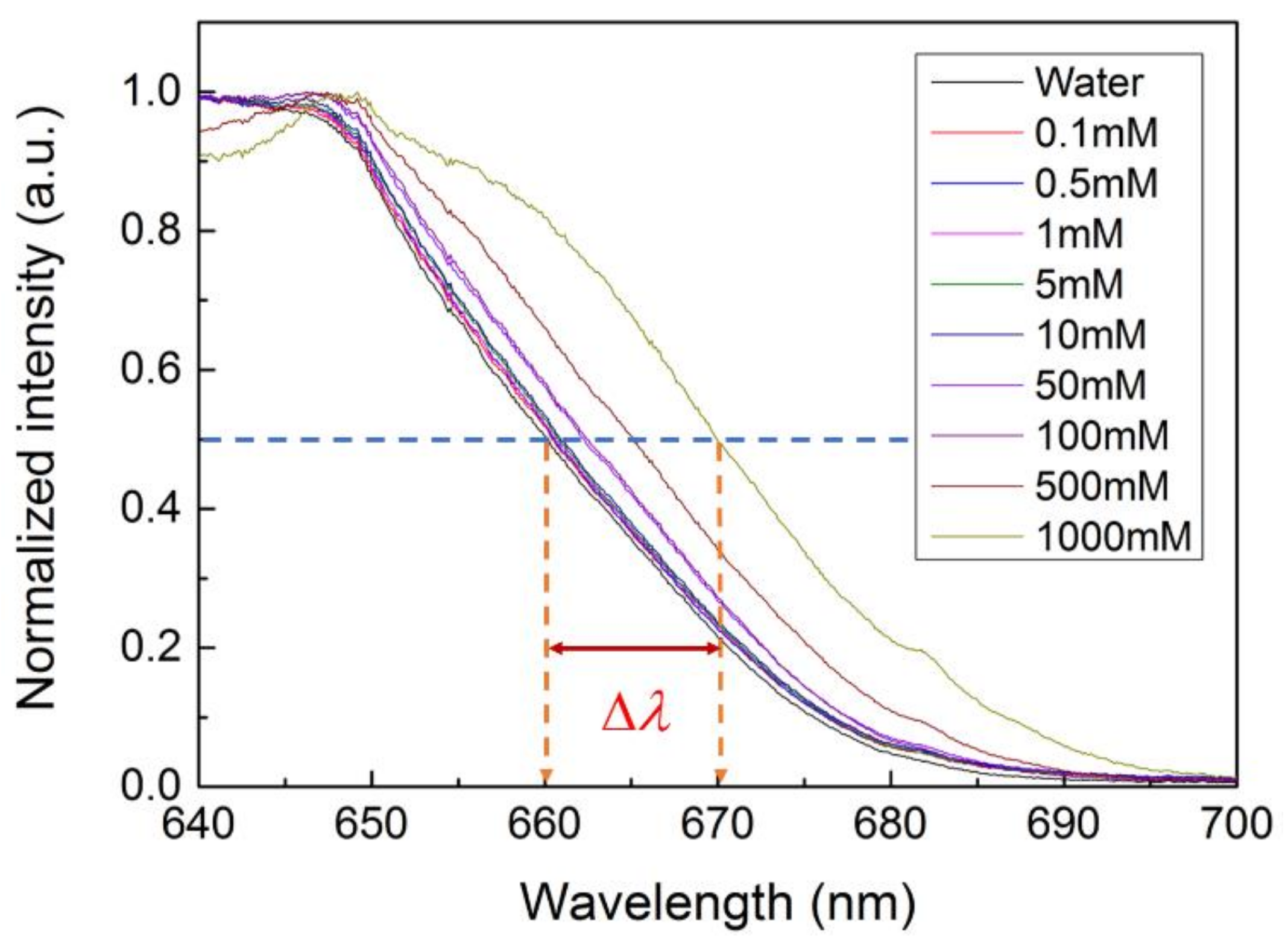
3.3. Cut-Off Wavelength Measurement and Data Acquisition
3.4. Detection Limit and Sensitivity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hsiao, F.-L.; Lee, C.K. Computational study of photonic crystals nano-ring resonator for biochemical sensing. IEEE Sens. J. 2010, 10, 1185–1191. [Google Scholar] [CrossRef]
- Cullen, D.C.; Lowe, C.R. A direct surface plasmon—Polariton immunosensor: Preliminary investigation of the non-specific adsorption of serum components to the sensor interface. Sens. Actuators B Chem. 1990, 1, 576–579. [Google Scholar] [CrossRef]
- Kouno, T.; Sakai, M.; Kishino, K.; Hara, K. Sensing operations based on hexagonal GaN microdisks acting as whispering-gallery mode optical microcavities. Opt. Lett. 2015, 40, 2866–2869. [Google Scholar] [CrossRef] [PubMed]
- Miri, N.; Mohammadzaheri, M. Optical sensing using microspheres with different size and material. IEEE Sens. J. 2014, 14, 3593–3598. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, K.-Q. Photonic crystal structures with tunable structure color as colorimetric sensors. Sensors 2013, 13, 4192–4213. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, X.; Gu, Z. Photonic crystals in bioassays. Adv. Funct. Mater. 2010, 20, 2970–2988. [Google Scholar] [CrossRef]
- Choi, E.; Choi, Y.; Nejad, Y.H.P.; Shin, K.; Park, J. Label-free specific detection of immunoglobulin G antibody using nanoporous hydrogel photonic crystals. Sens. Actuators B Chem. 2013, 180, 107–113. [Google Scholar] [CrossRef]
- Cullen, D.C.; Brown, R.G.; Lowe, C.R. Detection of immuno-complex formation via surface plasmon resonance on gold-coated diffraction gratings. Biosensors 1987, 3, 211–225. [Google Scholar] [CrossRef]
- Jory, M.J.; Vukusic, P.S.; Sambles, J.R. Development of a prototype gas sensor using surface plasmon resonance on gratings. Sens. Actuators B Chem. 1994, 17, 203–209. [Google Scholar] [CrossRef]
- Troia, B.; Paolicelli, A.; Leonardis, F.D.; Passaro, V.M.N. Photonic crystals for optical sensing: A review. In Advances in Photonic Crystals; Passaro, V.M.N., Ed.; InTech: London, UK, 2013; ISBN 978-953-51-0954-9. [Google Scholar]
- Scullion, M.G.; Di Falco, A.; Krauss, T.F. Slotted photonic crystal cavities with integrated microfluidics for biosensing applications. Biosens. Bioelectron. 2011, 27, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, Q. Biosensors and bioelectronics on smartphone for portable biochemical detection. Biosens. Bioelectron. 2016, 75, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Gallegos, D.; Long, K.D.; Yu, H.; Clark, P.P.; Lin, Y.; George, S.; Nath, P.; Cunningham, B.T. Label-free biodetection using a smartphone. Lab Chip 2013, 13, 2124–2132. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K.; Painter, O. Momentum space design of high-Q photonic crystal optical cavities. Opt. Express 2002, 10, 670–684. [Google Scholar] [CrossRef] [PubMed]
- OK Biotech Co., Ltd. OKMETER Direct Blood Glucose Monitoring System. Available online: http://www.okbiotech.com/en/product-372268/OKmeter-Blood-Glucose-Monitoring-System-Blood-Glucose-Meter.html (accessed on 3 April 2018).






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, Y.-Y.; Chen, Y.-T.; Chen, C.-C.; Huang, J.-J. Evanescent Properties of Optical Diffraction from 2-Dimensional Hexagonal Photonic Crystals and Their Sensor Applications. Materials 2018, 11, 549. https://doi.org/10.3390/ma11040549
Liao Y-Y, Chen Y-T, Chen C-C, Huang J-J. Evanescent Properties of Optical Diffraction from 2-Dimensional Hexagonal Photonic Crystals and Their Sensor Applications. Materials. 2018; 11(4):549. https://doi.org/10.3390/ma11040549
Chicago/Turabian StyleLiao, Yu-Yang, Yung-Tsan Chen, Chien-Chun Chen, and Jian-Jang Huang. 2018. "Evanescent Properties of Optical Diffraction from 2-Dimensional Hexagonal Photonic Crystals and Their Sensor Applications" Materials 11, no. 4: 549. https://doi.org/10.3390/ma11040549
APA StyleLiao, Y.-Y., Chen, Y.-T., Chen, C.-C., & Huang, J.-J. (2018). Evanescent Properties of Optical Diffraction from 2-Dimensional Hexagonal Photonic Crystals and Their Sensor Applications. Materials, 11(4), 549. https://doi.org/10.3390/ma11040549




