The Incorporation of Strontium to Improve Bone-Regeneration Ability of Mesoporous Bioactive Glasses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Sr-Containing MBGs (Sr-MBGs)
2.1.1. Preparation of Sr-Containing MBG Samples by Aerosol-Assisted Spray Drying Method
2.1.2. Preparation of Sr-Containing MBG Samples by Sol-Gel Synthesis (Base-Catalysed)
2.2. Characterization of Sr-MBGs
2.3. Sr2+ Ions Release Tests
2.4. In Vitro Bioactivity of Sr-Containing MBGs
2.5. In Vitro Biological Assessment of Sr-Containing MBGs
2.5.1. Inflammatory Response of Sr-Containing MBGs
2.5.2. Biocompatibility Test of Sr-Containing MBGs
2.5.3. Osteogenic Response to Sr-Containing MBGs
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Sr-Containing MBGs
3.1.1. Morphological and Structural Characterization
3.1.2. Strontium Ion Release from Sr-Containing MBGs
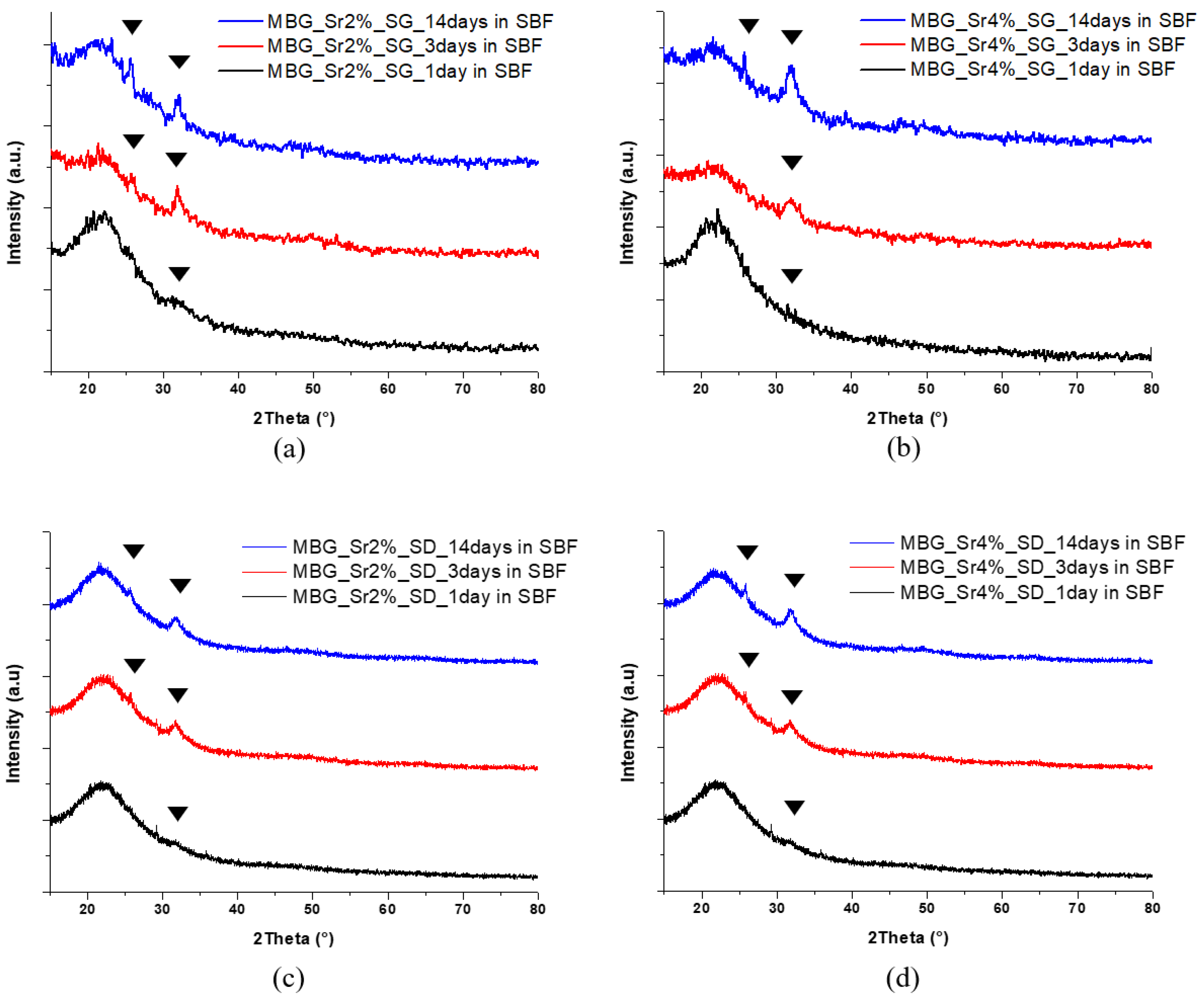
3.1.3. In Vitro Bioactivity of Sr-Containing MBGs
3.2. In Vitro Biological Assessment of Sr-Containing MBGs
3.2.1. Biocompatibility of Sr-Containing MBGs
3.2.2. Inflammatory Response of Sr-Containing MBGs
3.2.3. Pro-Osteogenesis of Sr-Containing MBGs
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Jones, J.R. Reprint of: Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2015, 23, S53–S82. [Google Scholar] [CrossRef] [PubMed]
- Arcos, D.; Vallet-Regí, M. Sol-gel silica-based biomaterials and bone tissue regeneration. Acta Biomater. 2010, 6, 2874–2888. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. Sol-gel materials for bioceramic applications. Curr. Opin. Solid State Mater. Sci. 1997, 2, 604–610. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics. Stress Int. J. Biol. Stress 1998, 28, 1705–1728. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics: From Concept to Clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Brinker, C.J.; Lu, Y.F.; Sellinger, A.; Fan, H.Y. Evaporation Induced Self-Assembly: Nanostructures Made Easy. Adv. Mater. 1999, 11, 579–585. [Google Scholar] [CrossRef]
- Vallet-Regí, M. Nanostructured mesoporous silica matrices in nanomedicine. J. Intern. Med. 2010, 267, 22–43. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Barba, I.; Vallet-Regí, M. Mesoporous bioactive glasses: Relevance of their porous structure compared to that of classical bioglasses. Biomed. Glasses 2015, 1, 140–150. [Google Scholar] [CrossRef]
- Jell, G.; Stevens, M.M. Gene activation by bioactive glasses. J. Mater. Sci. Mater. Med. 2006, 17, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. Genetic design of bioactive glass. J. Eur. Ceram. Soc. 2009, 29, 1257–1265. [Google Scholar] [CrossRef]
- Bari, A.; Bloise, N.; Fiorilli, S.; Novajra, G.; Vallet-Regí, M.; Bruni, G.; Torres-Pardo, A.; González-Calbet, J.M.; Visai, L.; Vitale-Brovarone, C. Copper-containing mesoporous bioactive glass nanoparticles as multifunctional agent for bone regeneration. Acta Biomater. 2017, 55, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Naruphontjirakul, P.; Porter, A.E.; Jones, J.R. In vitro osteogenesis by intracellular uptake of strontium containing bioactive glass nanoparticles. Acta Biomater. 2018, 66, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, N.; Cusano, A.M.; Causa, F.; Caputo, D.; Netti, P.A. Silver-containing mesoporous bioactive glass with improved antibacterial properties. J. Mater. Sci. Mater. Med. 2013, 24, 2129–2135. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef] [PubMed]
- Cacciotti, I. Bivalent cationic ions doped bioactive glasses: The influence of magnesium, zinc, strontium and copper on the physical and biological properties. J. Mater. Sci. 2017, 52, 8812–8831. [Google Scholar] [CrossRef]
- Pontremoli, C.; Boffito, M.; Fiorilli, S.; Laurano, R.; Torchio, A.; Bari, A.; Tonda-Turo, C.; Ciardelli, G.; Vitale-Brovarone, C. Hybrid injectable platforms for the in situ delivery of therapeutic ions from mesoporous glasses. Chem. Eng. J. 2018, 340, 103–113. [Google Scholar] [CrossRef]
- Montalbano, G.; Fiorilli, S.; Caneschi, A.; Vitale-Brovarone, C. Type I collagen and strontium-containing mesoporous glass particles as hybrid system for 3D printing of bone like materials. Materials 2018. submitted. [Google Scholar]
- Wu, C.; Zhou, Y.; Lin, C.; Chang, J.; Xiao, Y. Strontium-containing mesoporous bioactive glass scaffolds with improved osteogenic/cementogenic differentiation of periodontal ligament cells for periodontal tissue engineering. Acta Biomater. 2012, 8, 3805–3815. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, M.; Gentleman, E.; Shahid, S.; Hill, R.G.; Brauer, D.S. Therapeutic Ion-Releasing Bioactive Glass Ionomer Cements with Improved Mechanical Strength and Radiopacity. Front. Mater. 2015, 2. [Google Scholar] [CrossRef]
- O’Donnell, M.D.; Candarlioglu, P.L.; Miller, C.A.; Gentleman, E.; Stevens, M.M. Materials characterisation and cytotoxic assessment of strontium-substituted bioactive glasses for bone regeneration. J. Mater. Chem. 2010, 20, 8934–8941. [Google Scholar] [CrossRef]
- Gentleman, E.; Fredholm, Y.C.; Jell, G.; Lotfibakhshaiesh, N.; O’Donnell, M.D.; Hill, R.G.; Stevens, M.M. The effects of strontium-substituted bioactive glasses on osteoblasts and osteoclasts in vitro. Biomaterials 2010, 31, 3949–3956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.; Chang, J. Multifunctional mesoporous bioactive glasses for effective delivery of therapeutic ions and drug/growth factors. J. Control. Release 2014, 193, 282–295. [Google Scholar] [CrossRef] [PubMed]
- Saidak, Z.; Marie, P.J. Strontium signaling: Molecular mechanisms and therapeutic implications in osteoporosis. Pharmacol. Ther. 2012, 136, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Moghanian, A.; Firoozi, S.; Tahriri, M. Characterization, in vitro bioactivity and biological studies of sol-gel synthesized SrO substituted 58S bioactive glass. Ceram. Int. 2017, 43, 14880–14890. [Google Scholar] [CrossRef]
- Taherkhani, S.; Moztarzadeh, F. Influence of strontium on the structure and biological properties of sol–gel-derived mesoporous bioactive glass (MBG) powder. J. Sol-Gel Sci. Technol. 2016, 78, 539–549. [Google Scholar] [CrossRef]
- Pontiroli, L.; Dadkhah, M.; Novajra, G.; Tcacencu, I.; Fiorilli, S.; Vitale-Brovarone, C. An aerosol-spray-assisted approach to produce mesoporous bioactive glass microspheres under mild acidic aqueous conditions. Mater. Lett. 2017, 190, 111–114. [Google Scholar] [CrossRef]
- Shi, M.; Chen, Z.; Farnaghi, S.; Friis, T.; Mao, X.; Xiao, Y.; Wu, C. Copper-doped mesoporous silica nanospheres, a promising immunomodulatory agent for inducing osteogenesis. Acta Biomater. 2016, 30, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Maçon, A.L.B.; Kim, T.B.; Valliant, E.M.; Goetschius, K.; Brow, R.K.; Day, D.E.; Hoppe, A.; Boccaccini, A.R.; Kim, I.Y.; Ohtsuki, C.; et al. A unified in vitro evaluation for apatite-forming ability of bioactive glasses and their variants. J. Mater. Sci. Mater. Med. 2015, 26, 115. [Google Scholar] [CrossRef] [PubMed]
- Molino, G.; Bari, A.; Baino, F.; Fiorilli, S.; Vitale-Brovarone, C. Electrophoretic deposition of spray-dried Sr-containing mesoporous bioactive glass spheres on glass–ceramic scaffolds for bone tissue regeneration. J. Mater. Sci. 2017, 52, 9103–9114. [Google Scholar] [CrossRef]
- Salinas, A.J.; Shruti, S.; Malavasi, G.; Menabue, L.; Vallet-Regí, M. Substitutions of cerium, gallium and zinc in ordered mesoporous bioactive glasses. Acta Biomater. 2011, 7, 3452–3458. [Google Scholar] [CrossRef] [PubMed]
- Groen, J.C.; Peffer, L.A.A.; Pérez-Ramírez, J. Pore size determination in modified micro- and mesoporous materials. Pitfalls and limitations in gas adsorption data analysis. Microporous Mesoporous Mater. 2003, 60, 1–17. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Fredholm, Y.C.; Karpukhina, N.; Law, R.V.; Hill, R.G. Strontium containing bioactive glasses: Glass structure and physical properties. J. Non-Cryst. Solids 2010, 356, 2546–2551. [Google Scholar] [CrossRef]
- Arcos, D.; López-Noriega, A.; Ruiz-Hernández, E.; Terasaki, O.; Vallet-Regí, M. Ordered mesoporous microspheres for bone grafting and drug delivery. Chem. Mater. 2009, 21, 1000–1009. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, S.; Zhu, Y.; Huang, Y.; Zhu, M.; Tao, C.; Zhang, C. Three-dimensional printing of strontium-containing mesoporous bioactive glass scaffolds for bone regeneration. Acta Biomater. 2014, 10, 2269–2281. [Google Scholar] [CrossRef] [PubMed]
- Lao, J.; Jallot, E.; Nedelec, J.M. Strontium-delivering glasses with enhanced bioactivity: A new biomaterial for antiosteoporotic applications? Chem. Mater. 2008, 20, 4969–4973. [Google Scholar] [CrossRef]
- Jones, J.R.; Hench, L.L. Materials perspective—Biomedical materials for new millennium: Perspective on the future. Mater. Sci. Technol. 2001, 17, 891–900. [Google Scholar] [CrossRef]
- Arepalli, S.K.; Tripathi, H.; Hira, S.K.; Manna, P.P.; Pyare, R.; Singh, S.P. Enhanced bioactivity, biocompatibility and mechanical behavior of strontium substituted bioactive glasses. Mater. Sci. Eng. C 2016, 69, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Jiang, W.; Chen, X.; Li, Y.; Liang, Q. The effects of Sr concentration on physicochemical properties, bioactivity and biocompatibility of sub-micron bioactive glasses spheres. Adv. Powder Technol. 2017, 28, 2713–2722. [Google Scholar] [CrossRef]
- Kaysinger, K.K.; Ramp, W.K. Extracellular pH modulates the activity of cultured human osteoblasts. J. Cell Biochem. 1998, 68, 83–89. [Google Scholar] [CrossRef]
- Kaewamatawong, T.; Kawamura, N.; Okajima, M.; Sawada, M.; Morita, T.; Shimada, A. Acute pulmonary toxicity caused by exposure to colloidal silica: Particle size dependent pathological changes in mice. Toxicol. Pathol. 2005, 33, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Kusaka, T.; Nakayama, M.; Nakamura, K.; Ishimiya, M.; Furusawa, E.; Ogasawara, K. Effect of silica particle size on macrophage inflammatory responses. PLoS ONE 2014, 9, e92634. [Google Scholar] [CrossRef] [PubMed]
- Costantini, L.M.; Gilberti, R.M.; Knecht, D.A. The phagocytosis and toxicity of amorphous silica. PLoS ONE 2011, 6, e14647. [Google Scholar] [CrossRef] [PubMed]
- Morishige, T.; Yoshioka, Y.; Inakura, H.; Tanabe, A.; Narimatsu, S.; Yao, X.; Monobe, Y.; Imazawa, T.; Tsunoda, S.I.; Tsutsumi, Y.; et al. Suppression of nanosilica particle-induced inflammation by surface modification of the particles. Arch. Toxicol. 2012, 86, 1297–1307. [Google Scholar] [CrossRef] [PubMed]
- Waters, K.M.; Masiello, L.M.; Zangar, R.C.; Tarasevich, B.J.; Karin, N.J.; Quesenberry, R.D.; Bandyopadhyay, S.; Teeguarden, J.G.; Pounds, J.G.; Thrall, B.D. Macrophage responses to silica nanoparticles are highly conserved across particle sizes. Toxicol. Sci. 2009, 107, 553–569. [Google Scholar] [CrossRef] [PubMed]
- Renaudin, G.; Laquerrière, P.; Filinchuk, Y.; Jallot, E.; Nedelec, J.M. Structural characterization of sol–gel derived Sr-substituted calcium phosphates with anti-osteoporotic and anti-inflammatory properties. J. Mater. Chem. 2008, 18, 3593–3600. [Google Scholar] [CrossRef] [Green Version]
- Lerner, U.H. Inflammation-induced bone remodeling in periodontal disease and the influence of post-menopausal osteoporosis. J. Dent. Res. 2006, 85, 596–607. [Google Scholar] [CrossRef] [PubMed]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Lacey Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Cochran, D.L. Inflammation and Bone Loss in Periodontal Disease. J. Periodontol. 2008, 79, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Nakashima, T.; Hiroshi, N.; Penninger, J.M. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol. Med. 2006, 12, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, K.; Jin, H.M.; Song, I.; Youn, B.U.; Lee, J.; Kim, N. Silibinin inhibits osteoclast differentiation mediated by TNF family members. Mol. Cells 2009, 28, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Sage, H.; Vernon, R.B.; Funk, S.E.; Everitt, E.A.; Angello, J. SPARC, a secreted protein associated with cellular proliferation, inhibits cell spreading in vitro and exhibits Ca+2-dependent binding to the extracellular matrix. J. Cell Biol. 1989, 109, 341–356. [Google Scholar] [CrossRef] [PubMed]
- Keim, R.J.; Swords, N.A.; Orfeo, T.; Mann, K.G. Osteonectin in matrix remodeling: A plasminogen-osteonectin-collagen complex. J. Biol. Chem. 1994, 269, 30147–30153. [Google Scholar]
- Sila-Asna, M.; Bunyaratvej, A.; Maeda, S.; Kitaguchi, H.; Bunyaratavej, N. Osteoblast Differentiation and Bone Formation Gene Expression in Strontium-inducing Bone Marrow Mesenchymal Stem Cell. Kobe J. Med. Sci. 2007, 53, 25–35. [Google Scholar] [PubMed]
- Doi, Y.; Okuda, R.; Takezawa, Y.; Shibata, S.; Moriwaki, Y.; Wakamatsu, N.; Shimizu, N.; Moriyama, K.; Shimokawa, H. Osteonectin inhibiting de novo formation of apatite in the presence of collagen. Calcif. Tissue Int. 1989, 44, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Liang, Q.; Li, Y.; Fan, J.; Wang, G.; Pan, H.; Ruan, C. Strontium incorporation improves the bone-forming ability of scaffolds derived from porcine bone. Colloids Surf. B Biointerfaces 2018, 162, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Baghaban Eslaminejad, M.; Kamali, A.; Hosseini, S.; Sayahpour, F.A.; Baharvand, H. Synergistic effect of strontium, bioactive glass and nano-hydroxyapatite promotes bone regeneration of critical-sized radial bone defects. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Querido, W.; Rossi, A.L.; Farina, M. The effects of strontium on bone mineral: A review on current knowledge and microanalytical approaches. Micron 2016, 80, 122–134. [Google Scholar] [CrossRef] [PubMed]








| Reagent | MBG_Sr2%_SD (g) | MBG_Sr4%_SD (g) |
|---|---|---|
| SrCl2·6H2O | 0.32 | 0.64 |
| Ca(NO3)2·4H2O | 1.86 | 1.57 |
| Reagent | MBG_Sr2%_SG (g) | MBG_Sr4%_SG (g) |
|---|---|---|
| SrCl2·6H2O | 0.84 | 1.68 |
| Ca(NO3)2·4H2O | 4.88 | 4.13 |
| Parameters | MBG_Sr2%_SG | MBG_Sr4%_SG | MBG_Sr2%_SD | MBG_Sr4%_SD |
|---|---|---|---|---|
| BET surface area | 803 m2·g−1 | 551 m2·g−1 | 167 m2·g−1 | 154 m2·g−1 |
| Average Pore size | 4.8 nm | 4.1 nm | 8.3 nm | 7.8 nm |
| Pore volume | 0.82 cm³·g−1 | 0.45 cm³·g−1 | 0.18 cm³·g−1 | 0.17 cm³·g−1 |
| Sample | Sr2+ Incorporated mol % | Sr2+ Incorporated ppm | Sr2+ Released at 3 h (ppm) | Sr2+ Released at 72 h (ppm) |
|---|---|---|---|---|
| MBG_Sr2%_SG | 1.0% | 2.40 | 2.19 | 2.36 |
| MBG_Sr4%_SG | 2.0% | 7.60 | 6.68 | 7.60 |
| MBG_Sr2%_SD | 2.0% | 7.20 | 6.30 | 6.90 |
| MBG_Sr4%_SD | 3.7% | 13.20 | 12.1 | 13.2 |
| Sample | RANKL/OPG |
|---|---|
| Polystyrene 72 h | 1.000 |
| MBG_Sr2%_SG 72 h | 0.181 |
| MBG_Sr2%_SD 72 h | 0.230 |
| Polystyrene 7 days | 2.291 |
| MBG_Sr2%_SG 7 days | 0.563 |
| MBG_Sr2%_SD 7 days | 0.275 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiorilli, S.; Molino, G.; Pontremoli, C.; Iviglia, G.; Torre, E.; Cassinelli, C.; Morra, M.; Vitale-Brovarone, C. The Incorporation of Strontium to Improve Bone-Regeneration Ability of Mesoporous Bioactive Glasses. Materials 2018, 11, 678. https://doi.org/10.3390/ma11050678
Fiorilli S, Molino G, Pontremoli C, Iviglia G, Torre E, Cassinelli C, Morra M, Vitale-Brovarone C. The Incorporation of Strontium to Improve Bone-Regeneration Ability of Mesoporous Bioactive Glasses. Materials. 2018; 11(5):678. https://doi.org/10.3390/ma11050678
Chicago/Turabian StyleFiorilli, Sonia, Giulia Molino, Carlotta Pontremoli, Giorgio Iviglia, Elisa Torre, Clara Cassinelli, Marco Morra, and Chiara Vitale-Brovarone. 2018. "The Incorporation of Strontium to Improve Bone-Regeneration Ability of Mesoporous Bioactive Glasses" Materials 11, no. 5: 678. https://doi.org/10.3390/ma11050678
APA StyleFiorilli, S., Molino, G., Pontremoli, C., Iviglia, G., Torre, E., Cassinelli, C., Morra, M., & Vitale-Brovarone, C. (2018). The Incorporation of Strontium to Improve Bone-Regeneration Ability of Mesoporous Bioactive Glasses. Materials, 11(5), 678. https://doi.org/10.3390/ma11050678