A One-Component, Fast-Cure, and Economical Epoxy Resin System Suitable for Liquid Molding of Automotive Composite Parts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterizations
2.3. Sample Preparation
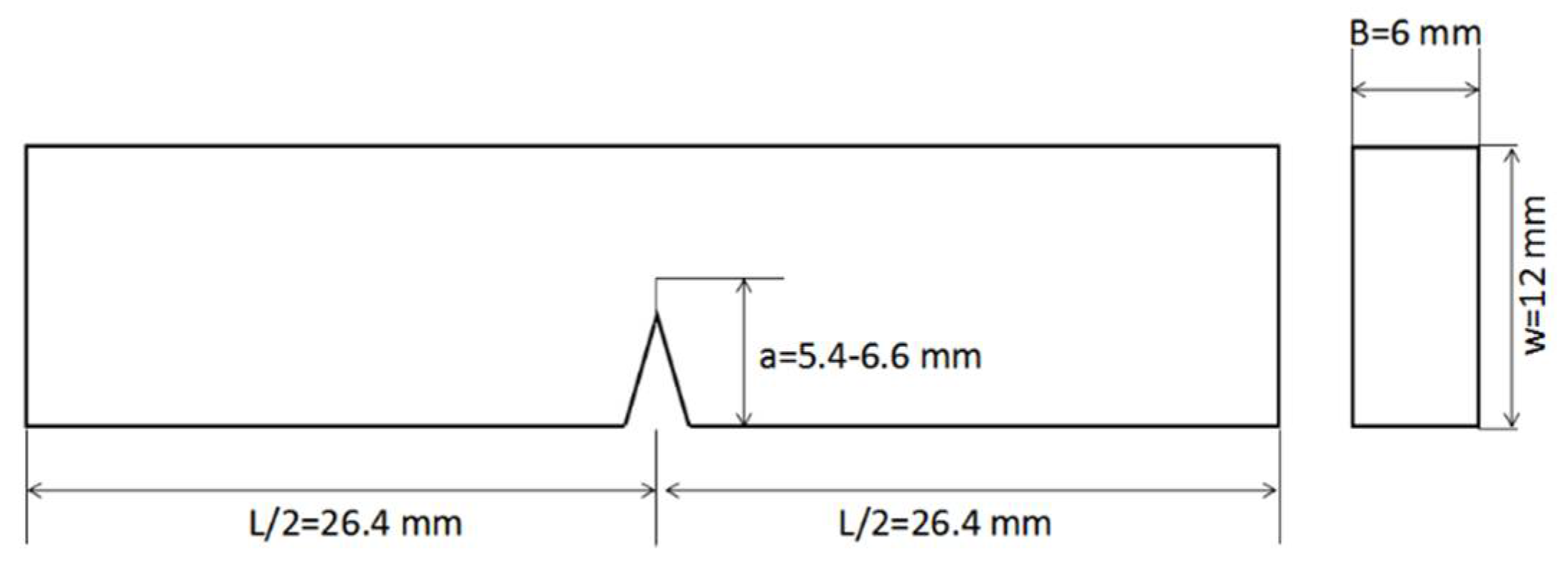
2.3.1. Preparation of Neat Resin Test Coupons
2.3.2. Preparation of the Carbon Fiber Composite Laminates
3. Results and Discussion
3.1. Comparison of the Two Imidazoles
3.2. Evaluation of Different Levels of 1C2E4MIM
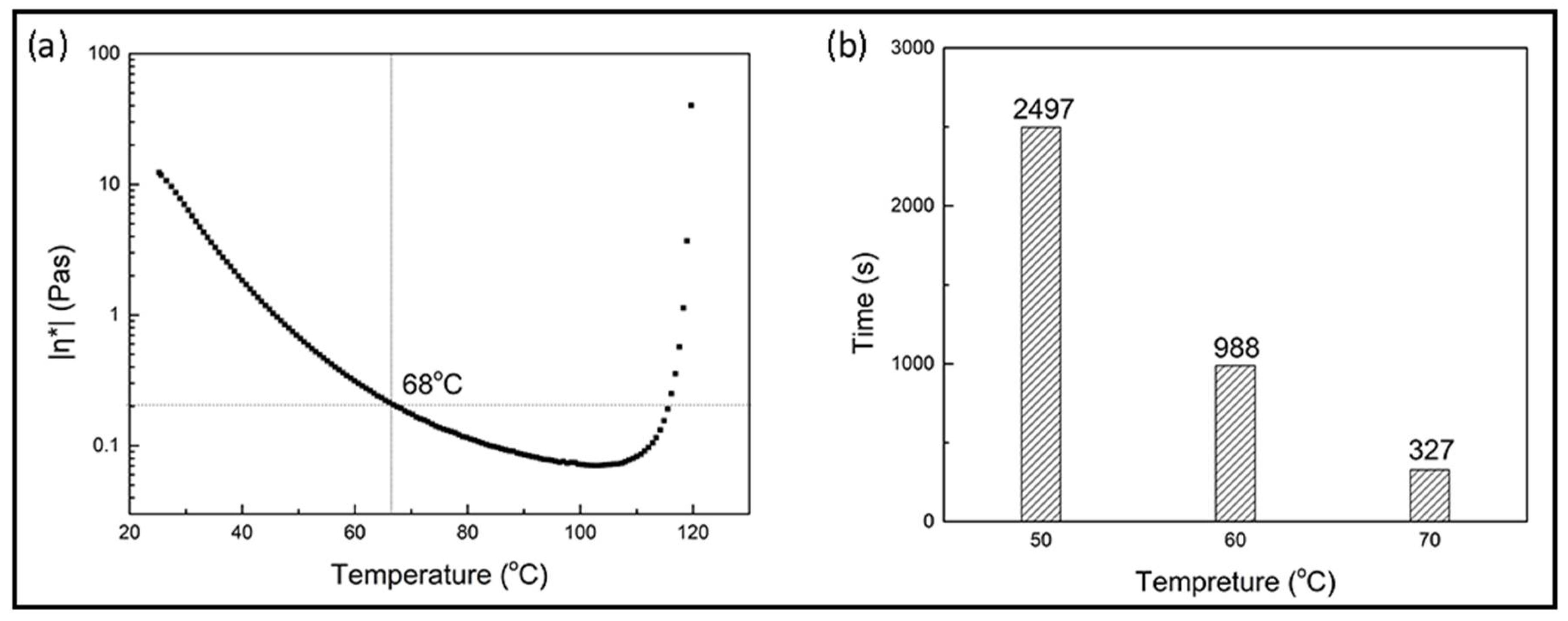
3.2.1. Curing Behaviors
3.2.2. Thermo-Mechanical Properties
3.2.3. Neat Resin Mechanical Properties
3.3. Evaluation of Resin and Composite with 4 wt % 1C2E4MIM
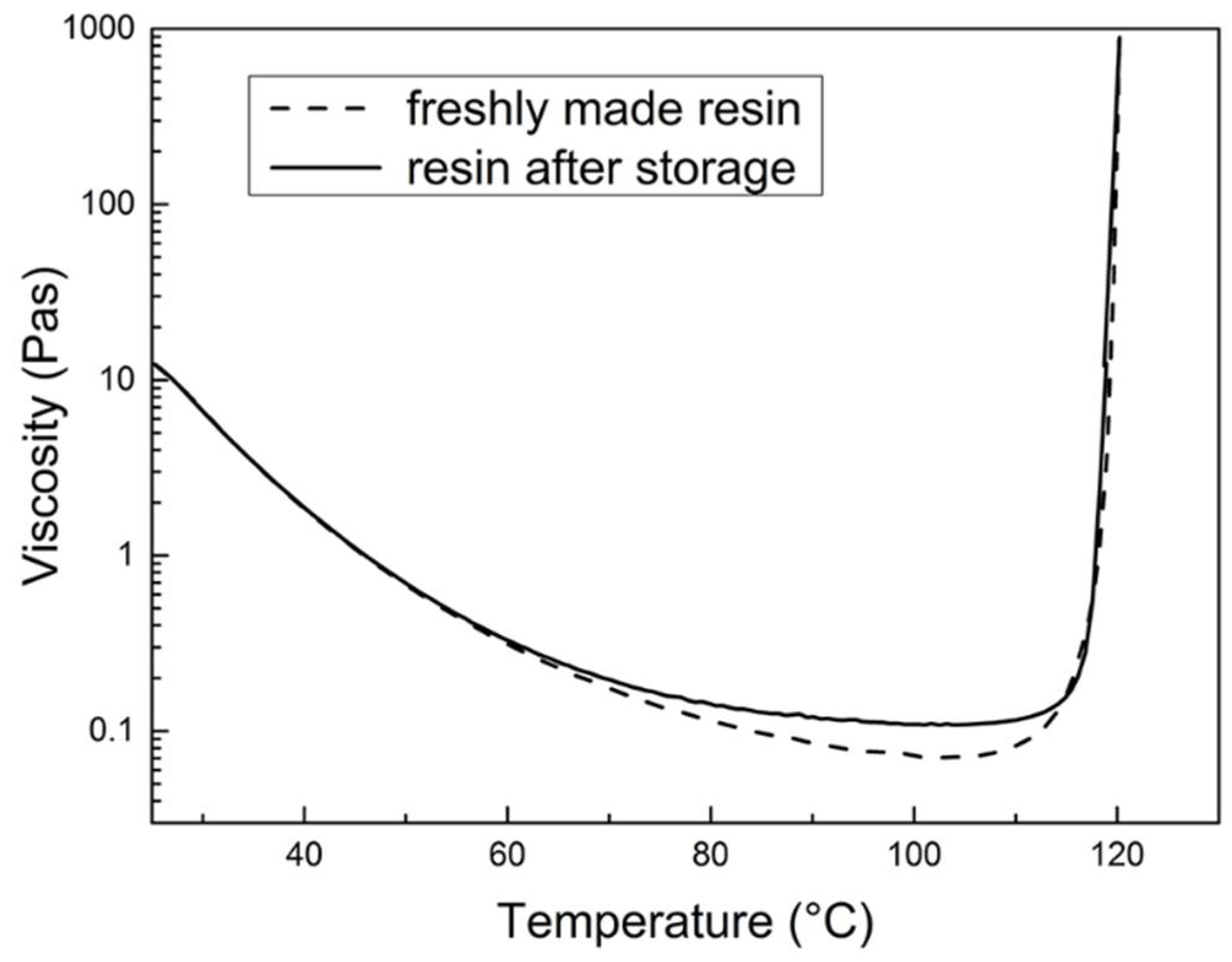
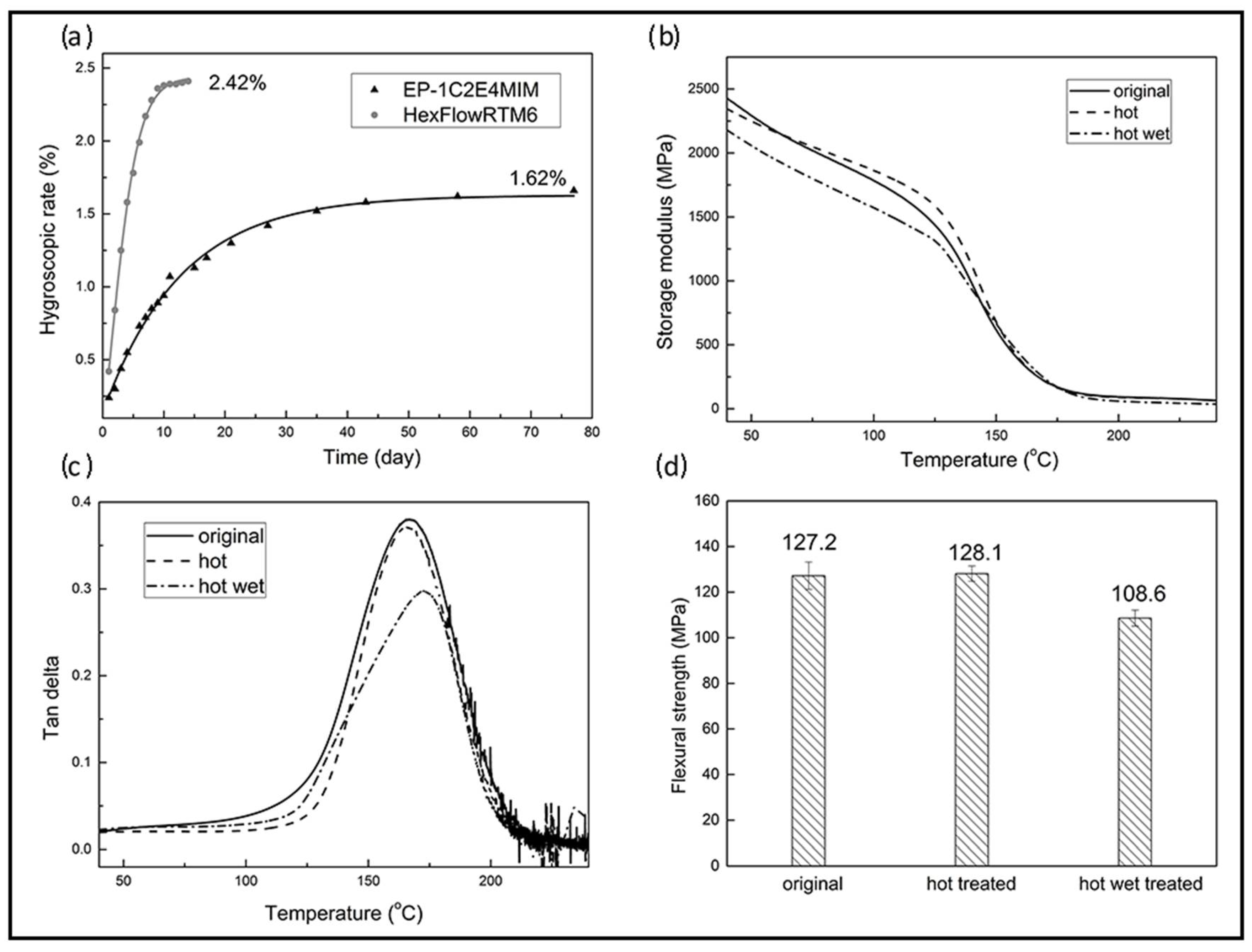
3.3.1. Shelf Life
3.3.2. Water Absorption and Hot-Wet Properties
3.3.3. Mechanical Properties of Carbon Fabric-Reinforced Composites
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Guzmán, D.; Ramis, X.; Fernández-Francos, X.; Serra, A. New catalysts for diglycidyl ether of bisphenol A curing based on thiol–epoxy click reaction. Eur. Polym. J. 2014, 59, 377–386. [Google Scholar] [CrossRef]
- Wu, Z.; Li, S.; Liu, M.; Wang, H.; Wang, Z.; Liu, X. Study on liquid oxygen compatibility of bromine-containing epoxy resins for the application in liquid oxygen tank. Polym. Adv. Technol. 2016, 27, 98–108. [Google Scholar] [CrossRef]
- Garschke, C.; Parlevliet, P.P.; Weimer, C.; Fox, B.L. Cure kinetics and viscosity modelling of a high-performance epoxy resin film. Polym. Test. 2013, 32, 150–157. [Google Scholar] [CrossRef]
- Bassampour, Z.S.; Budy, S.M.; Son, D.Y. Degradable epoxy resins based on bisphenol A diglycidyl ether and silyl ether amine curing agents. J. Appl. Polym. Sci. 2017, 134, 44620. [Google Scholar] [CrossRef]
- Ye, W.; Wei, W.; Fei, X.; Lu, R.; Liu, N.; Luo, J.; Zhu, Y.; Liu, X. Six-arm star-shaped polymer with cyclophosphazene core and poly(ε-caprolactone) arms as modifier of epoxy thermosets. J. Appl. Polym. Sci. 2017, 134, 44384. [Google Scholar] [CrossRef]
- Drakonakis, V.M.; Velisaris, C.N.; Seferis, J.C.; Doumanidis, C.C. Feather-inspired carbon fiber reinforced polymers with nanofibrous fractal interlayer. Polym. Compos. 2016, 37, 168–181. [Google Scholar] [CrossRef]
- Feraboli, P.; Masini, A. Development of carbon/epoxy structural components for a high performance vehicle. Compos. Part B 2004, 35, 323–330. [Google Scholar] [CrossRef]
- Scattina, A.; Peroni, L.; Peroni, M.; Avalle, M. Numerical Analysis of Hybrid Joining in Car Body Applications. J. Adhes. Sci. Technol. 2012, 25, 2409–2433. [Google Scholar] [CrossRef]
- Drakonakis, V.M.; Velisaris, C.N.; Seferis, J.C.; Doumanidis, C.C.; Wardle, B.L.; Papanicolaou, G.C. Matrix hybridization in the interlayer for carbon fiber reinforced composites. Polym. Compos. 2010, 31, 1965–1976. [Google Scholar] [CrossRef] [Green Version]
- Jain, R.; Choudhary, V.; Narula, A.K. Curing and thermal behavior of DGEBA in presence of dianhydrides and aromatic diamine. J. Appl. Polym. Sci. 2007, 105, 3804–3808. [Google Scholar] [CrossRef]
- Suzuki, K.; Sugita, Y.; Sanda, F.; Endo, T. One-component epoxy resin with imine as water-initiated latent hardener: Improvement of the mechanical and adhesive properties by the addition of methacrylate copolymer. J. Appl. Polym. Sci. 2005, 96, 1943–1949. [Google Scholar] [CrossRef]
- Visakh, P.M.; Nazarenko, O.B.; Amelkovich, Y.A.; Melnikova, T.V. Effect of zeolite and boric acid on epoxy-based composites. Polym. Adv. Technol. 2016, 27, 1098–1101. [Google Scholar] [CrossRef]
- Sipaut, C.S.; Padavettan, V.; Rahman, I.A.; Mansa, R.F.; Dayou, J.; Jafarzadeh, M. An optimized preparation of bismaleimide-diamine co-polymer matrices. Polym. Adv. Technol. 2014, 25, 673–683. [Google Scholar] [CrossRef]
- McCoy, J.D.; Ancipink, W.B.; Clarkson, C.M.; Kropka, J.M.; Celina, M.C.; Giron, N.H.; Hailesilassie, L.; Fredj, N. Cure mechanisms of diglycidyl ether of bisphenol A (DGEBA) epoxy with diethanolamine. Polymer 2016, 105, 243–254. [Google Scholar] [CrossRef]
- Zhang, K.; Gu, Y.; Li, M.; Zhang, Z. Effect of rapid curing process on the properties of carbon fiber/epoxy composite fabricated using vacuum assisted resin infusion molding. Mater. Des. 2014, 54, 624–631. [Google Scholar] [CrossRef]
- De, B.; Karak, N. Novel high performance tough hyperbranched epoxy by an A2+ B3polycondensation reaction. J. Mater. Chem. A 2013, 1, 348–353. [Google Scholar] [CrossRef]
- Agius, S.L.; Joosten, M.; Trippit, B.; Wang, C.H.; Hilditch, T. Rapidly cured epoxy/anhydride composites: Effect of residual stress on laminate shear strength. Compos. Part A 2016, 90, 125–136. [Google Scholar] [CrossRef]
- Amirova, L.R.; Burilov, A.R.; Amirova, L.M.; Bauer, I.; Habicher, W.D. Kinetics and mechanistic investigation of epoxy-anhydride compositions cured with quaternary phosphonium salts as accelerators. J. Polym. Sci. Part A 2016, 54, 1088–1097. [Google Scholar] [CrossRef]
- Naffakh, M.; Dumon, M.; Gérard, J.-F. Modeling the chemorheological behavior of epoxy/liquid aromatic diamine for resin transfer molding applications. J. Appl. Polym. Sci. 2006, 102, 4228–4237. [Google Scholar] [CrossRef]
- Fache, M.; Montérémal, C.; Boutevin, B.; Caillol, S. Amine hardeners and epoxy cross-linker from aromatic renewable resources. Eur. Polym. J. 2015, 73, 344–362. [Google Scholar] [CrossRef]
- Li, G.; Li, B.; Liu, Y.; Liu, D.; Yang, X. Improved surface quality and hygrothermal performance of epoxy based prepreg by liquefied dicyandiamide with enhanced solubility and dispersibility. Mater. Des. 2016, 102, 115–121. [Google Scholar] [CrossRef]
- Okuhira, H.; Kii, T.; Ochi, M.; Takeyama, H. Characterization of epoxy resin hardening with ketimine latent hardeners. J. Adhes. Sci. Technol. 2004, 18, 205–211. [Google Scholar] [CrossRef]
- Naumann, S.; Speiser, M.; Schowner, R.; Giebel, E.; Buchmeiser, M.R. Air Stable and Latent Single-Component Curing of Epoxy/Anhydride Resins Catalyzed by Thermally Liberated N-Heterocyclic Carbenes. Macromolecules 2014, 47, 4548–4556. [Google Scholar] [CrossRef]
- Lin, K.-F.; Wang, W.-H. Imidazole and Chromium Acetylacetonate as Additives for the Cure and Fracture Toughness of Epoxy Resins and Their Composites. Polym. Compos. 1995, 16, 269–275. [Google Scholar] [CrossRef]
- Mahendran, A.R.; Wuzella, G.; Kandelbauer, A.; Aust, N. Thermal cure kinetics of epoxidized linseed oil with anhydride hardener. J. Therm. Anal. Calorim. 2011, 107, 989–998. [Google Scholar] [CrossRef]
- Ooi, S.K.; Cook, W.D.; Simon, G.P.; Such, C.H. DSC studies of the curing mechanisms and kinetics of DGEBA using imidazole curing agents. Polymer 2000, 41, 3639–3649. [Google Scholar] [CrossRef]
- Arimitsu, K.; Fuse, S.; Kudo, K.; Furutani, M. Imidazole derivatives as latent curing agents for epoxy thermosetting resins. Mater. Lett. 2015, 161, 408–410. [Google Scholar] [CrossRef]
- Lei, D.; Ma, W.; Wang, L.; Zhang, D. Preparation of 2-ethyl-4-methylimidazole derivatives as latent curing agents and their application in curing epoxy resin. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Wong, F.F.; Chen, K.-L.; Lin, C.M.; Yeh, M.-Y. New investigation of 1-substituted imidazole derivatives as thermal latent catalysts for epoxy-phenolic resins. J. Appl. Polym. Sci. 2007, 104, 3292–3300. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Z. Silicon-Containing Cycloaliphatic Epoxy Resins with Systematically Varied Functionalities: Synthesis and Structure/Property Relationships. Macromol. Chem. Phys. 2011, 212, 926–936. [Google Scholar] [CrossRef]
- Prolongo, S.G.; del Rosario, G.; Ureña, A. Comparative study on the adhesive properties of different epoxy resins. Int. J. Adhes. Adhes. 2006, 26, 125–132. [Google Scholar] [CrossRef]
- ASTM. Standard Test Method for Tensile Properties of Plastics; Designation: D638-10; ASTM: West Conshohocken, PA, USA, 2010. [Google Scholar]
- ASTM. Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials; Designation: D790-10; ASTM: West Conshohocken, PA, USA, 2010. [Google Scholar]
- ASTM. Standard Test Methods for Plane-Strain Fracture Toughness and Strain Energy Release Rate of Plastic Materials; Designation: D5045-99; ASTM: West Conshohocken, PA, USA, 1999. [Google Scholar]
- ASTM. Standard Test Method for Tensile Properties of Polymer Matrix Composite Materials; Designation: D3039/D3039M-08; ASTM: West Conshohocken, PA, USA, 2008. [Google Scholar]
- ASTM. Compressive Properties of Polymer Matrix Composite Materials with Unsupported Gage Section by Shear Loading; Designation: D3410/D3410M-03; ASTM: West Conshohocken, PA, USA, 2003. [Google Scholar]
- ASTM. Standard Test Method for Short-Beam Strength of Polymer Matrix Composite Materials and Their Laminates1; Designation: D2344/D2344M-00; ASTM: West Conshohocken, PA, USA, 2006. [Google Scholar]
- Li, X.; Yan, Y.; Guo, L.; Xu, C. Effect of strain rate on the mechanical properties of carbon/epoxy composites under quasi-static and dynamic loadings. Polym. Test. 2016, 52, 254–264. [Google Scholar] [CrossRef]





| 1C2E4MIM (wt %) | Ti(1) (oC) | Tmax(2) (oC) | ΔH (3) (J/g) | Time to Reach Complete Cure at 120 °C (min) | Tg(4) (°C) |
|---|---|---|---|---|---|
| 2% | 135.1 | 155.1 | 128.8 | 27 | 71.6 |
| 4% | 135.0 | 150.4 | 353.3 | 15 | 166.4 |
| 6% | 134.3 | 147.5 | 441.1 | 8 | 137.1, 165.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Liu, W.; Qiu, Y.; Wei, Y. A One-Component, Fast-Cure, and Economical Epoxy Resin System Suitable for Liquid Molding of Automotive Composite Parts. Materials 2018, 11, 685. https://doi.org/10.3390/ma11050685
Wang Y, Liu W, Qiu Y, Wei Y. A One-Component, Fast-Cure, and Economical Epoxy Resin System Suitable for Liquid Molding of Automotive Composite Parts. Materials. 2018; 11(5):685. https://doi.org/10.3390/ma11050685
Chicago/Turabian StyleWang, Yiru, Wanshuang Liu, Yiping Qiu, and Yi Wei. 2018. "A One-Component, Fast-Cure, and Economical Epoxy Resin System Suitable for Liquid Molding of Automotive Composite Parts" Materials 11, no. 5: 685. https://doi.org/10.3390/ma11050685
APA StyleWang, Y., Liu, W., Qiu, Y., & Wei, Y. (2018). A One-Component, Fast-Cure, and Economical Epoxy Resin System Suitable for Liquid Molding of Automotive Composite Parts. Materials, 11(5), 685. https://doi.org/10.3390/ma11050685








