Metal/Carbon Hybrid Nanostructures Produced from Plasma-Enhanced Chemical Vapor Deposition over Nafion-Supported Electrochemically Deposited Cobalt Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
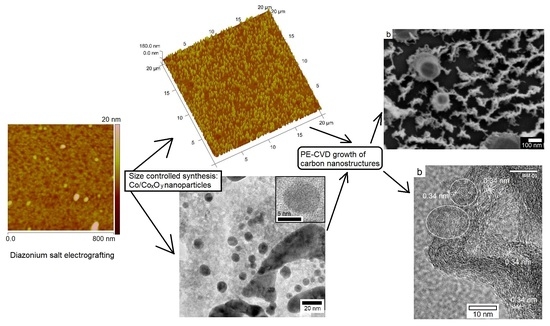
2.1. Aryl Diazonium Grafting and Nafion Membranes Deposition
2.2. NP Synthesis: Effect of Nafion Deposition Process and Electrochemistry Parameters
2.2.1. Dip Coating Versus Spin Coating Process
2.2.2. Indirect Versus Direct Electrochemical Reduction
2.2.3. Effect of Electrochemical Reduction Time
2.3. PECVD Growth of Carbon Nanostructures
3. Experimental
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgement
Conflict of Interest
References
- Mohammed, L.; Gomaa, H.G.; Ragab, D.; Zhu, J. Magnetic nanoparticles for environmental and biomedical applications: A review. Particuology 2017, 30, 1–14. [Google Scholar] [CrossRef]
- Liu, J.; He, K.; Wu, W.; Song, T.B.; Kanatzidis, M.G. In situ synthesis of highly dispersed and ultrafine metal nanoparticles from chalcogels. J. Am. Chem. Soc. 2017, 139, 2900–2903. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Yu, H.; Yan, L.; Zhu, H.; Cheng, X.; Xie, Y.; Long, N.; Shui, M.; Shu, J. High-rate long-life pored nanoribbon VNb9O25 built by interconnected ultrafine nanoparticles as anode for lithium-ion batteries. Appl. Mater. Interfaces 2017, 9, 30608–30616. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Chen, Y.T.; Raja, D.S.; Kang, Y.H.; Tseng, P.C.; Lin, C.H. Metal-organic frameworks to metal/metal oxide embedded carbon matrix: synthesis, characterization and gas sorption properties. Materials 2015, 8, 5336–5347. [Google Scholar] [CrossRef] [PubMed]
- Hitchcock, J.P.; Tasker, A.L.; Stark, K.; Leeson, A.; Baxter, E.A.; Biggs, S.; Cayre, O.J. Adsorption of catalytic nanoparticles onto polymer substrates for controlled deposition of microcapsule metal shells. Langmuir 2018, 34, 1473–1480. [Google Scholar] [CrossRef] [PubMed]
- Stozhko, N.; Bukharinova, M.; Galperin, L.; Khiena Brainina, K. A Nanostructured sensor based on gold nanoparticles and nafion for determination of uric acid. Biosensors 2018, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Allongue, P.; de Villeneuve, C.H.; Cherouvrier, G.; Cortes, R.; Bernard, M.C. Phenyl layers on H–Si(111) by electrochemical reduction of diazonium salts: Monolayer versus multilayer formation. J. Electroanal. Chem. 2003, 550–551, 161–174. [Google Scholar] [CrossRef]
- Hicks, J.M.; Wong, Z.Y.; Scurr, D.J.; Silman, N.; Jackson, S.K.; Mendes, P.M.; Aylott, J.W.; Rawson, F.J. Tailoring the electrochemical properties of carbon nanotube modified indium tin oxide via in situ grafting of aryl diazonium. Langmuir 2017, 33, 4924–4933. [Google Scholar] [CrossRef] [PubMed]
- Rösicke, F.; Sun, G.; Neubert, T.; Janietz, S.; Hinrichs, K.; Rappich, J. Electrochemical functionalization of Au by Aminobenzene and 2-Aminotoluene. J. Phys: Condens. Matter 2016, 28, 094004. [Google Scholar] [CrossRef] [PubMed]
- Gatti, T.; Girardi, G.; Vicentini, N.; Brandiele, R.; Wirix, M.; Durante, C.; Menna, E. Physico-chemical, electrochemical and structural insights into poly(3,4-ethylene dioxythiophene) grafted from molecularly engineered multi-walled carbon nanotube surfaces. J. Nanosci. Nanotechnol. 2018, 18, 1006–1018. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, D.; Jiang, K.; Shen, G. New insights and perspectives into biological materials for flexible electronics. Chem. Soc. Rev. 2010. [Google Scholar] [CrossRef] [PubMed]
- Ulman, A. Formation and structure of self-assembled monolayers. Chem. Rev. 1996, 96, 1533. [Google Scholar] [CrossRef] [PubMed]
- Pinson, J.; Podvorica, F. Attachment of organic layers to conductive or semiconductive surfaces by reduction of diazonium salts. Chem. Soc. Rev. 2005, 34, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Scibona, G.; Fabian, C.; Scuppa, B. Electrochemical behaviour of nafion type membrane. J. Membr. Sci. 1983, 16, 37–50. [Google Scholar] [CrossRef]
- Yoon, M.; Kim, Y.; Kim, Y.M.; Volkov, V.; Song, H.J.; Park, Y.J.; Park, I.W. Superparamagnetic properties of nickel nanoparticles in an ion-exchange polymer Film. Mater. Chem. Phys. 2005, 91, 104–107. [Google Scholar] [CrossRef]
- Shoji, M.; Oyaizu, K.; Nishide, H. Facilitated oxygen transport through a nafion membrane containing Cobaltporphyrin as a fixed oxygen carrier. Polymer 2008, 49, 5659–5664. [Google Scholar] [CrossRef]
- Sode, A.; Ingle, N.J.C.; McCormick, M.; Bizzotto, D.; Gyenge, E.; Ye, S.; Knights, S.; Wilkinson, D.P. Controlling the deposition of Pt nanoparticles within the surface region of Nafion. J. Membr. Sci. 2011, 376, 162–169. [Google Scholar] [CrossRef]
- Yin, C.; Li, J.; Zhou, Y.; Zhang, H.; Fang, P.; He, C. Phase separation and development of proton transport pathways in metal oxide nanoparticle/nafion composite membranes during water uptake. J. Phys. Chem. C 2018. [Google Scholar] [CrossRef]
- Ketpang, K.; Oh, K.; Lim, S.C.; Shanmugam, S. Nafion-porous cerium oxide nanotubes composite membrane for polymer electrolyte fuel cells operated under dry conditions. J. Power Sources 2016, 329, 441–449. [Google Scholar] [CrossRef]
- Tai, C.C.; Chen, C.L.; Liu, C.W.; Huang, Y.R. Investigation the proton transport in highly hydrated nafion membrane doping with SiO2 nanoparticles by molecular dynamics simulation. Thin Solid Films 2018. [Google Scholar] [CrossRef]
- Yin, C.; Wang, L.; Li, J.; Zhou, Y.; Zhang, H.; Fang, P.; He, C. Positron annihilation characteristics, water uptake and proton conductivity of composite Nafion membranes. Phys. Chem. Chem. Phys. 2017, 19, 15953–15961. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Wang, H.; Liu, G.; Wang, Z.; Cheng, J. Simultaneous determination of trace Cd(II) and Pb(II) based on Bi/Nafion/reduced graphene oxide-gold nanoparticle nanocomposite film-modified glassy carbon electrode by one-step electrodeposition. Ionics 2017, 23, 767–777. [Google Scholar] [CrossRef]
- Khulbe, K.C.; Matsuura, T. Removal of heavy metals and pollutants by membrane adsorption techniques. Appl. Water Sci. 2018, 8, 19. [Google Scholar] [CrossRef]
- Matrab, T.; Chancolon, J.; L’hermite, M.M.; Rouzaud, J.N.; Deniau, G.; Boudoue, J.P.; Chehimi, M.M.; Delamar, M. Atom transfer radical polymerization (ATRP) initiated by Aryl Diazonium salts: A new route for surface modification of multiwalled carbon nanotubes by tethered polymer chains. Colloids Surf. A 2006, 287, 217–221. [Google Scholar] [CrossRef]
- Baranton, S.; Belanger, D. In situ generation of diazonium cations in organic electrolyte for electrochemical modification of electrode Surface. Electrochim. Acta 2008, 53, 6961–6967. [Google Scholar] [CrossRef]
- Ren, Z.; Meng, N.; Shehzad, K.; Xu, Y.; Qu, S.; Yu, B.; Luo, J.K. Mechanical properties of nickel-graphene composites synthesized by electrochemical deposition. Nanotechnology 2015, 26, 065706. [Google Scholar] [CrossRef] [PubMed]
- Chamoulaud, G.; Belanger, D. Spontaneous derivatization of a copper electrode with in situ generated diazonium cations in aprotic and aqueous media. J. Phys. Chem. C 2007, 111, 7501–7507. [Google Scholar] [CrossRef]
- Wanunu, M.; Meller, A. Chemically modified solid-state nanopores. Nano Lett. 2007, 7, 1580–1585. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Hu, X.X.; Ding, X.L.; Kong, H.C.; Sha, H.D.; Lin, H.; Wen, W.; Shen, G.; Guo, Z.; Ma, Z.F.; et al. Effects of cobalt precursor on pyrolyzed carbon-supported cobalt-polypyrrole as electrocatalyst toward oxygen reduction reaction. Nanoscale Res. Lett. 2013, 8, 478. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Bae, I.S.; Cho, S.J.; Boo, J.H.; Lee, B.C.; Heo, J.; Chung, I.; Hong, B. Synthesis and characteristics of NH2-functionalized polymer films to align and immobilize DNA molecules. Nanoscale Res. Lett. 2012, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Floch, F.L.; Simonato, J.P.; Bidan, G. Electrochemical signature of the grafting of diazonium salts: A probing parameter for monitoring the electro-addressed functionalization of devices. Electrochim. Acta 2009, 54, 3078–3085. [Google Scholar] [CrossRef]
- Patel, N.; Santini, A.; Bello, V.; Mattei, G.; Miotello, A. Cobalt/cobalt oxide nanoparticles-assembled coatings with various morphology and composition synthesized by pulsed laser deposition. Surf. Coat. Technol. 2013, 235, 784–791. [Google Scholar] [CrossRef]
- Sadasivan, S.; Bellabarba, R.M.; Tooze, R.P. Size dependent reduction–oxidation–reduction behaviour of cobalt oxide nanocrystals. Nanoscale 2013, 5, 11139–11146. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.H.; Moreau, L.M.; Honrao, S.; Hennig, R.G.; Robinson, R.D. The oxidation of cobalt nanoparticles into kirkendall-hollowed CoO and Co3O4: The diffusion mechanisms and atomic structural transformations. J. Phys. Chem. C 2013, 117, 14303. [Google Scholar] [CrossRef]
- Moisala, A.; Nasibulin, A.G.; Kauppinen, E.I. The role of metal nanoparticles in the catalytic production of single-walled carbon nanotubes—A review. J. Phys. Condens. Matter 2003, 15, S3011–S3035. [Google Scholar] [CrossRef]
- Javed, H.; Islam, M.; Mahmood, N.; Achour, A.; Hameed, A.; Khatri, N. Catalytic growth of multi-walled carbon nanotubes using NiFe2O4 nanoparticles and incorporation into epoxy matrix for enhanced mechanical properties. J. Polym. Eng. 2016, 36, 53–64. [Google Scholar] [CrossRef]
- Patole, S.P.; Kim, H.; Choi, J.; Kim, Y.; Baik, S.; Yoo, J.B. Kinetics of catalyst size dependent carbon nanotube growth by growth interruption studies. Appl. Phys. Lett. 2010, 96, 094101. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, J.; Holloway, B.C.; Outlaw, R.A.; Zhao, X.; Hou, K.; Manos, D.M. A mechanism for carbon nanosheet formation. Carbon 2007, 45, 2229–2234. [Google Scholar] [CrossRef]
- Wunderlich, W. Growth model for plasma-CVD growth of carbon nano-tubes on Ni-sheets. Diamond Related Mater 2007, 16, 369–378. [Google Scholar] [CrossRef]
- Kim, C.D.; Lee, H.R.; Min, B.K.; Kim, H.T. Transition of carbon nanotubes growth mode on NH3 plasma-modified Ni films at different plasma powers. Vacuum 2015, 115, 113–116. [Google Scholar] [CrossRef]
- Gohier, A.; Ewels, C.P.; Minea, T.M.; Djouadi, M.A. Carbon nanotube growth mechanism switches from tip- to base-growth with decreasing catalyst particle size. Carbon 2008, 46, 1331–1338. [Google Scholar] [CrossRef]
- Kim, M.J.; Nam, D.H.; Park, H.Y.; Kwon, C.R.; Eom, K.S.; Yoo, S.J.; Jang, J.H.; Kim, H.J.; Cho, E.A.; Kwon, H.S. Cobalt-carbon nanofibers as an efficient support-free catalyst for oxygen reduction reaction with a systematic study of active site formation. J. Mater. Chem. A 2015, 3, 14284. [Google Scholar] [CrossRef]
- Durukan, M.B.; Yuksel, R.; Unalan, H.E. Cobalt oxide nanoflakes on single walled carbon nanotube thin films for supercapacitor electrodes. Electrochim. Acta 2016, 222, 1475–1482. [Google Scholar] [CrossRef]
- Madhu, R.; Veeramani, V.; Chen, S.M.; Manikandan, A.; Lo, A.Y.; Chueh, Y.L. Honeycomb-like porous carbon−cobalt oxide nanocomposite for high-performance enzymeless glucose sensor and supercapacitor applications. Appl. Mater. Interfaces 2015, 7, 15812–15820. [Google Scholar] [CrossRef] [PubMed]
- Ouldhamadouche, N.; Achour, A.; Aissa, K.A.; Islam, M.; Ahmadpourian, M.A.; Arman, A.; Soussou, M.A.M.; Chaker, M.; Brizoual, L.L.; Djouadi, M.A. AlN film thickness effect on photoluminescence properties of AlN/carbon nanotubes shell/core nanostructures for deep ultra-violet optoelectronic devices. Thin Solid Films 2017, 622, 23–28. [Google Scholar] [CrossRef]
- Su, C.Y.; Lan, W.J.; Chu, C.Y.; Liu, X.J.; Kao, W.Y.; Chen, C.H. Photochemical green synthesis of nanostructured cobalt oxides as hydrogen peroxide redox for bifunctional sensing application. Electrochim. Acta 2016, 190, 588–595. [Google Scholar] [CrossRef]
- Li, W.; Jung, H.; Hoa, N.D.; Kim, D.; Hong, S.K.; Kim, H. Nanocomposite of cobalt oxide nanocrystals and single-walled carbon nanotubes for a gas sensor application. Sens. Actuators B Chem. 2010, 150, 160–166. [Google Scholar] [CrossRef]
- Gohier, A.; Minea, T.M.; Djouadi, M.A.; Jimenez, J.; Granier, A. Growth kinetics of low temperature single-wall and few walled carbon nanotubes grown by plasma enhanced chemical vapor deposition. Phys. E 2007, 37, 34–39. [Google Scholar] [CrossRef]







| Sample ID | HF Etch a | Grafting b | Nafion Deposition Dip/Spin Coating | NP Synthesis IR/DR c | t d, nm | NP Size nm |
|---|---|---|---|---|---|---|
| Si | Si/PNBD | Si/PNBD/Naf | Si/PNBD/Naf/NP | |||
| S1 | √ | √ | × | × | ||
| S2 | √ | √ | 0.5 wt %; Dip | IR 0.1 M CoSO4 (3 h); 0.5 M H3BO3 (−1.5 V, 5 s) | 94 ± 5.7 | 5.4 ± 1.6 |
| S3 | √ | √ | 0.5 wt %; Spin 4000 rpm 2 min | IR 0.1M CoSO4 (3h); 0.5 M H3BO3 (−1.5 V, 5 s) | 180 ± 11 | 4.8 ± 2.0 |
| S4 | √ | √ | 1.0 wt %; Spin 4000 rpm 2 min | IR 0.1 M CoSO4 (3 h); 0.5 M H3BO3 (−1.5 V, 20 s) | 87 ± 5.1 | 128 ± 12 5.3 ± 1.1 |
| S5 | √ | √ | 0.5 wt %; Spin 4000 rpm 2 min | DR 0.1 M CoSO4 0.5 M H3BO3; (−1.5 V, 20 s) | 65.2 ± 8.4 | |
| S6 | √ | √ | 1.0 wt %; Spin 4000 rpm 2 min | DR 1mM CoSO4 0.5 M H3BO3; (−1.5 V, 5 s) | 2.8 ± 0.4 | |
| S7 | √ | √ | 1.0 wt %; Spin 4000 rpm 2 min | DR 1 mM CoSO4 0.5 M H3BO3; (−1.5 V, 20 s) | 9.8 ± 2.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.; Achour, A.; Saeed, K.; Boujtita, M.; Javed, S.; Djouadi, M.A. Metal/Carbon Hybrid Nanostructures Produced from Plasma-Enhanced Chemical Vapor Deposition over Nafion-Supported Electrochemically Deposited Cobalt Nanoparticles. Materials 2018, 11, 687. https://doi.org/10.3390/ma11050687
Islam M, Achour A, Saeed K, Boujtita M, Javed S, Djouadi MA. Metal/Carbon Hybrid Nanostructures Produced from Plasma-Enhanced Chemical Vapor Deposition over Nafion-Supported Electrochemically Deposited Cobalt Nanoparticles. Materials. 2018; 11(5):687. https://doi.org/10.3390/ma11050687
Chicago/Turabian StyleIslam, Mohammad, Amine Achour, Khalid Saeed, Mohammed Boujtita, Sofia Javed, and Mohamed Abdou Djouadi. 2018. "Metal/Carbon Hybrid Nanostructures Produced from Plasma-Enhanced Chemical Vapor Deposition over Nafion-Supported Electrochemically Deposited Cobalt Nanoparticles" Materials 11, no. 5: 687. https://doi.org/10.3390/ma11050687
APA StyleIslam, M., Achour, A., Saeed, K., Boujtita, M., Javed, S., & Djouadi, M. A. (2018). Metal/Carbon Hybrid Nanostructures Produced from Plasma-Enhanced Chemical Vapor Deposition over Nafion-Supported Electrochemically Deposited Cobalt Nanoparticles. Materials, 11(5), 687. https://doi.org/10.3390/ma11050687