Nucleation and Growth of Porous MnO2 Coatings Prepared on Nickel Foam and Evaluation of Their Electrochemical Performance
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
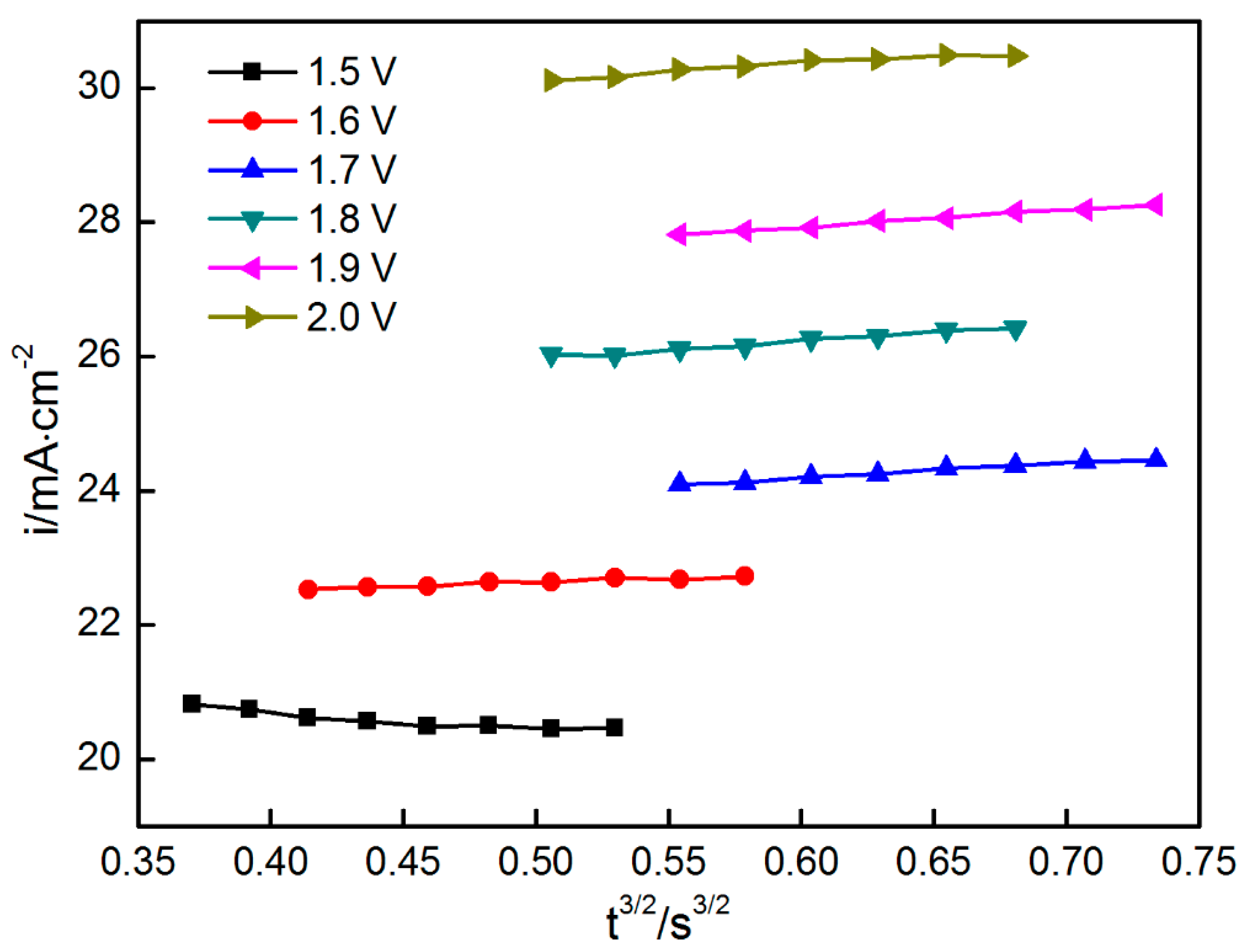
3.1. Formation Process of MnO2
3.2. Nucleation and Growth Mechanism
3.3. Chemical Compositions and Phase Constituents of the Deposit
3.4. Morphological Evolution of MnO2
3.5. Evaluation of Electrochemical Performance
4. Conclusions
- As shown by analyzing of chronoamperometry and SEM, the process of deposition of MnO2 on nickel foam contained four stages. After an extremely short incubation period (the first stage), the exposed nickel foam was instantly covered by a large number of MnO2, then nuclei were connected after a very short time (less than s) at the second stage. With the continuously competitive growth of boundaries and grains, the morphology of MnO2 electrode surface changed from fine sheet network structure to honeycomb structure (the third stage). At the fourth stage, the electrodeposition of MnO2 was diffusion-controlled, resulting in the formation of porous MnO2;
- MnO2 electrode with had a very high specific surface area (about 6 times that before the electrode deposition), which resulted in a high specific capacitance. MnO2 coatings deposited for different time (30, 60, 120, 300 s) exhibited a similar specific capacitance (CV: about 224 F/g; galvanostatic charge-discharge: about 264 F/g). Comparatively speaking, the value of MnO2 deposited for 600 s was highest (CV: 270 F/g; galvanostatic charge-discharge: 400 F/g).
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- El-Kady, M.F.; Strong, V.; Dubin, S.; Kaner, R.B. Laser scribing of high-performance and flexible graphene-based electrochemical capacitors. Science 2012, 335, 1326–1330. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Ganesh, K.J.; Cai, W.; Ferreira, P.J.; Pirkle, A.; Wallace, R.M.; Cychosz, K.A.; Thommes, M.; et al. Carbon-based supercapacitors produced by activation of graphene. Science 2011, 332, 1537–1541. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Liu, J.; Zeng, Z.; Ng, C.F.; Ma, L.; Zhang, H.; Lin, J.; Shen, Z.; Fan, H.J. Three-dimensional graphene foam supported Fe3O4 lithium battery anodes with long cycle life and high rate capability. Nano Lett. 2013, 13, 6136–6143. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Zhu, C.; Luo, J.; Zeng, Z.; Guan, C.; Ng, C.F.; Zhang, H.; Fan, H.J. Synthesis of free-standing metal sulfide nanoarrays via anion exchange reaction and their electrochemical energy storage application. Small 2014, 10, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Service, R.F. New ‘supercapacitor’ promises to pack more electrical punch. Science 2006, 313, 902–905. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 2, 2520–2531. [Google Scholar] [CrossRef] [PubMed]
- Pandolfo, A.G.; Hollenkamp, A.F. Carbon properties and their role in supercapacitors. J. Power Sources 2006, 157, 11–27. [Google Scholar] [CrossRef]
- Hulicova, D.; Yamashita, J.; Soneda, Y.; Hatori, A.H.; Kodama, M. Supercapacitors prepared from melamine-based Carbon. Chem. Mater. 2005, 17, 1241–1247. [Google Scholar] [CrossRef]
- Yoon, S.; Lee, J.; Hyeon, T.; Oh, S.M. Electric double-layer capacitor performance of a new mesoporous carbon. J. Electrochem. Soc. 2000, 147, 2507–2512. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, X.; Pan, L.; Li, H.; Sun, Z.; Sun, C.; Tay, B.K. Carbon nanotube-zinc oxide electrode and gel polymer electrolyte for electrochemical supercapacitors. J. Alloys Compd. 2009, 480, L17–L19. [Google Scholar] [CrossRef]
- Zhou, H.H.; Chen, H.; Luo, S.L.; Lu, G.W.; Wei, W.Z.; Kuang, Y.F. The effect of the polyaniline morphology on the other performance of polyanilinesurpercapacitors. J. Solid State Electr. 2005, 9, 574–578. [Google Scholar] [CrossRef]
- Gedela, V.; Puttapati, S.K.; Nagavolu, C. A unique solar radiation exfoliated reduced graphene oxide/polyanilinenanofibers composite electrode material for supercapacitors. Mater. Lett. 2015, 152, 177–180. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.A. Extremely facile synthesis of manganese dioxide-polyanilinenano-reticulation with enhanced electrochemical properties. J. Alloys Compd. 2016, 677, 281–287. [Google Scholar] [CrossRef]
- Xu, C.J.; Kang, F.; Li, B.; Du, H. Recent progress on manganese dioxide based supercapacitors. J. Mater. Res. 2010, 25, 1421–1432. [Google Scholar] [CrossRef]
- Wang, Y.G.; Xia, Y.Y. Electrochemical capacitance characterization of NiO with ordered mesoporous structure synthesized by template SBA-15. Electrochim. Acta 2006, 51, 3223–3227. [Google Scholar] [CrossRef]
- Lin, C.; Ritter, J.A.; Popov, B.N. Characterization of sol-gel-derived cobalt oxide xerogels as electrochemical capacitors. J. Electrochem. Soc. 1998, 145, 4097–4103. [Google Scholar] [CrossRef]
- Jow, J.J.; Lai, H.H.; Chen, H.R.; Wang, C.C.; Wu, M.S.; Ling, T.R. Effect of hydrothermal treatment on the performance of RuO2-Ta2O5/Ti electrodes for use in supercapacitors. Electrochim. Acta 2010, 55, 2793–2798. [Google Scholar] [CrossRef]
- Hassan, S.; Suzuki, M.; El-Moneim, A.A. Synthesis of MnO2-chitosan nanocomposite by one-step electrodeposition for electrochemical energy storage application. J. Power Sources 2014, 246, 68–73. [Google Scholar] [CrossRef]
- Lv, P.; Zhang, P.; Feng, Y.Y.; Li, Y.; Feng, W. High-performance electrochemical capacitors using electrodeposited MnO2 on carbon nanotube array grown on carbon fabric. Electrochim. Acta 2012, 78, 515–523. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Zhang, W.H.; Li, D.S.; Gao, L.; Hou, C.L.; Zhang, Y.H.; Liu, Y.Q. Facile synthesis of MnO2/rGO/Ni composite foam with excellent pseudocapacitive behavior for supercapacitors. J. Alloys Compd. 2015, 649, 579–584. [Google Scholar] [CrossRef]
- Wang, C.L.; Li, F.T.; Wang, Y.N.; Qu, H.L.; Yi, X.L.; Lu, Y.; Qiu, Y.; Zou, Z.J.; Yu, B.H.; Luo, Y.S. Facile synthesis of nanographene sheet hybrid α-MnO2 nanotube and nanoparticle as high performance electrode materials for supercapacitor. J. Alloys Compd. 2015, 634, 12–18. [Google Scholar] [CrossRef]
- Wei, C.; Pang, H.; Lu, Q.; Liang, S.; Gao, F. Two-dimensional β-MnO2 nanowire network with enhanced electrochemical capacitance. Sci. Rep. 2013, 3, 2193. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Wang, Y.; Lin, J.; Lu, L. Hydrothermal synthesis of MnO2/CNT nanocomposite with a CNT core/porous MnO2 sheath hierarchy architecture for supercapacitors. Nanoscale Res. Lett. 2012, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.W.; Kong, L.B.; Zhou, W.J.; Li, H.L. Hydrothermal synthesis and pseudocapacitance properties of α-MnO2 hollow spheres and hollow urchins. J. Phys. Chem. C 2007, 111, 19141–19147. [Google Scholar] [CrossRef]
- Bello, A.; Fashedemi, O.O.; Lekitima, J.N.; Fabiane, M.; Arhin, D.D.; Ozoemena, K.I.; Gogotsi, Y.; Johnson, A.T.C.; Manyala, N. High-performance symmetric electrochemical capacitor based on graphene foam and nanostructured manganese oxide. AIP Adv. 2013, 3, 1094–1103. [Google Scholar] [CrossRef]
- Reddy, R.N.; Reddy, R.G. Sol-gel MnO2 as an electrode material for electrochemical capacitors. J. Power Sources 2003, 124, 330–337. [Google Scholar] [CrossRef]
- Tang, X.H.; Li, H.J.; Liu, Z.H.; Yang, Z.P.; Wang, Z.L. Preparation and capacitive property of manganese oxide nanobelt bundles with birnessite-type structure. J. Power Sources 2011, 196, 855–859. [Google Scholar] [CrossRef]
- Toupin, M.; Brousse, T.; Belanger, D. Influence of microstucture on the chargestorage properties of chemically synthesized manganese dioxide. Chem. Mater. 2002, 14, 3946–3952. [Google Scholar] [CrossRef]
- Fan, X.Y.; Wang, X.L.; Li, G.; Yu, A.P.; Chen, Z.W. High-performance flexible electrode based on electrodeposition of polypyrrole/MnO2 on carbon cloth for supercapacitors. J. Power Sources 2016, 326, 357–364. [Google Scholar] [CrossRef]
- Mishra, R.K.; Prajapati, C.S.; Shahi, R.R.; Kushwaha, A.K.; Sahay, P.P. Influence of electrodeposition modes on the electrochemical performance of MnO2 films prepared using anionic MnO4− (Mn7+) precursor. Ceram. Int. 2017, 44, 5710–5718. [Google Scholar] [CrossRef]
- Xiao, K.; Li, J.W.; Chen, G.F.; Liu, Z.Q.; Li, N.; Su, Y.Z. Amorphous MnO2 supported on 3D-Ni nanodendrites for large areal capacitance supercapacitors. Electrochim. Acta 2014, 149, 341–348. [Google Scholar] [CrossRef]
- Dupont, M.; Hollenkamp, A.F.; Donne, S.W. Electrochemically active surface area effects on the performance of manganese dioxide for electrochemical capacitor applications. Electrochim. Acta 2013, 104, 140–147. [Google Scholar] [CrossRef]
- Adelkhani, H.; Ghaemi, M. Characterization of manganese dioxide electrodeposited by pulse and direct current for electrochemical capacitor. J. Alloys Compd. 2010, 493, 175–178. [Google Scholar] [CrossRef]
- Wang, G.L.; Wang, W.; Zhao, Y.F.; Shao, G.J.; Liu, T.T.; Ma, Z.P. Pulsed electrodeposition of mesoporous cobalt-doped manganese dioxide as supercapacitor electrode material. Ionics 2014, 20, 243–249. [Google Scholar] [CrossRef]
- Li, Q.; Lu, X.F.; Xu, H.; Tong, Y.X.; Li, G.R. Carbon/MnO2 double-walled nanotube arrays with fast ion and electron transmission for high-performance supercapacitors. ACS Appl. Mater. Interface 2014, 6, 2726–2733. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.H.; Hu, L.B.; Lu, N.; Wang, H.L.; Vosgueritchian, M.; Yang, Y.; Cui, Y.; Bao, Z.N. Enhancing the supercapacitor performance of graphene/MnO2 nanostructured electrodes by conductive wrapping. Nano Lett. 2011, 11, 4438–4442. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.W.; Lu, S.T.; Zhang, H.B.; Varanasi, C.V.; Liu, J. Synergistic effects from graphene and carbon nanotubes enable flexible and robust electrodes for highperformance supercapacitors. Nano Lett. 2012, 12, 4206–4211. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.L.; Wei, X.; Jiang, Y.M.; Wu, X.Y.; Han, L.N.; Wang, K.X.; Chen, J.S. Cobaltdoped MnO2 hierarchical yolk-shell spheres with improved supercapacitive performance. J. Phys. Chem. C 2015, 119, 8465–8471. [Google Scholar] [CrossRef]
- Wessels, K.; Minnermann, M.; Rathousky, J.; Wark, M.; Oekermann, T. Influence of Calcination Temperature on the Photoelectrochemical and Photocatalytic Properties of Porous TiO2 Films Electrodeposited from Ti(IV)-Alkoxide Solution. J. Phys. Chem. C 2008, 112, 15122–15128. [Google Scholar] [CrossRef]
- Nirmal Peiris, T.A.; Senthilarasu, S.; UpulWijayantha, K.G. Enhanced performance of flexible dyesensitized solar cells: Electrodeposition of Mg(OH)2 on a nanocrystalline TiO2 electrode. J. Phys. Chem. C 2012, 116, 1211–1218. [Google Scholar] [CrossRef]
- Yang, J.; Lia, L.F.; Ruan, H.C.; Xie, F.Y.; Wei, M.D. Nanostructured porous MnO2 on Ni foam substrate with a high massloading via a CV electrodeposition route for supercapacitor application. Electrochim. Acta 2014, 136, 189–194. [Google Scholar] [CrossRef]
- Tizfahm, J.; Aghazadeh, M.; Mohammad, G.M.; Mohammad, R.Z.; Parviz, N.; Farnoush, F. Electrochemical preparation and evaluation of the supercapacitive performance of MnO2 nanoworms. Mater. Lett. 2016, 167, 153–156. [Google Scholar] [CrossRef]
- Clark, M.; Ivey, D.G. Nucleation and growth of electrodeposited Mn oxide rods for supercapacitor electrodes. Nanotechnology 2015, 26, 384001. [Google Scholar] [CrossRef] [PubMed]
- Dupont, M.F.; Donne, S.W. Nucleation and growth of electrodeposited manganese dioxide for electrochemical capacitors. Electrochim. Acta 2014, 120, 219–225. [Google Scholar] [CrossRef]
- Nijjer, S.; Thonstad, J.; Haarberg, G.M. Oxidation of manganese (II) and reduction of manganese dioxide in sulphuric acid. Electrochim. Acta 2000, 46, 395–399. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons Inc.: New York, NY, USA, 2001; pp. 114–116. [Google Scholar]
- Tuyen, N.; Michel, B.; Carmezim, M.J.; Montemor, M.F. NixCo1−x(OH)2 nanosheets on carbon nanofoam paper as high areal capacity electrodes for hybrid supercapacitors. Energy 2017, 126, 208–216. [Google Scholar] [CrossRef]
- Tuyen, N.; Carmezim, M.J.; Montemor, M.F. Current transient and in situ AFM studies of initial growth stages of electrochemically deposited nickel cobalt hydroxide nanosheet films. Phys. Chem. Chem. Phys. 2016, 18, 12368–12374. [Google Scholar] [CrossRef]
- Rao, S.R.; Cui, H.S.; Lin, S. Floatation of base metal oxide by long hydrocarbon chain xanthate. Met. Ore Dress. Abroad 2003, 6, 22–24. [Google Scholar]
- Xia, X. The relation between chemical, physical properties and electrochemical activity for manganese dioxides (VIII). Battery Bimon. 2007, 37, 271–274. [Google Scholar]
- Aghazadeh, M.; Maragheh, M.G.; Ganjali, M.R.; Norouzi, P.; Faridbod, F. Electrochemical preparation of MnO2 nanobelts through pulse base-electrogeneration and evaluation of their electrochemical performance. Appl. Surf. Sci. 2016, 364, 141–147. [Google Scholar] [CrossRef]














© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.; Li, J.; Xu, Y. Nucleation and Growth of Porous MnO2 Coatings Prepared on Nickel Foam and Evaluation of Their Electrochemical Performance. Materials 2018, 11, 716. https://doi.org/10.3390/ma11050716
Huang W, Li J, Xu Y. Nucleation and Growth of Porous MnO2 Coatings Prepared on Nickel Foam and Evaluation of Their Electrochemical Performance. Materials. 2018; 11(5):716. https://doi.org/10.3390/ma11050716
Chicago/Turabian StyleHuang, Wenxin, Jun Li, and Yunhe Xu. 2018. "Nucleation and Growth of Porous MnO2 Coatings Prepared on Nickel Foam and Evaluation of Their Electrochemical Performance" Materials 11, no. 5: 716. https://doi.org/10.3390/ma11050716




