Advanced Material-Ordered Nanotubular Ceramic Membranes Covalently Capped with Single-Wall Carbon Nanotubes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Modification
2.3. Membrane Characterization
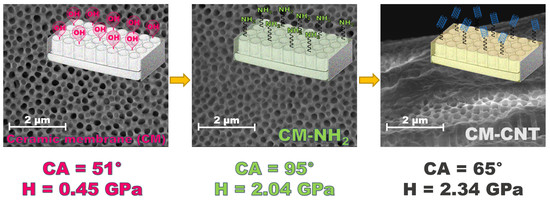
3. Results and Discussion
3.1. Properties of the Pristine Membranes
3.2. Confirmation of Membrane Functionalization
3.3. Properties of the Modified Membranes
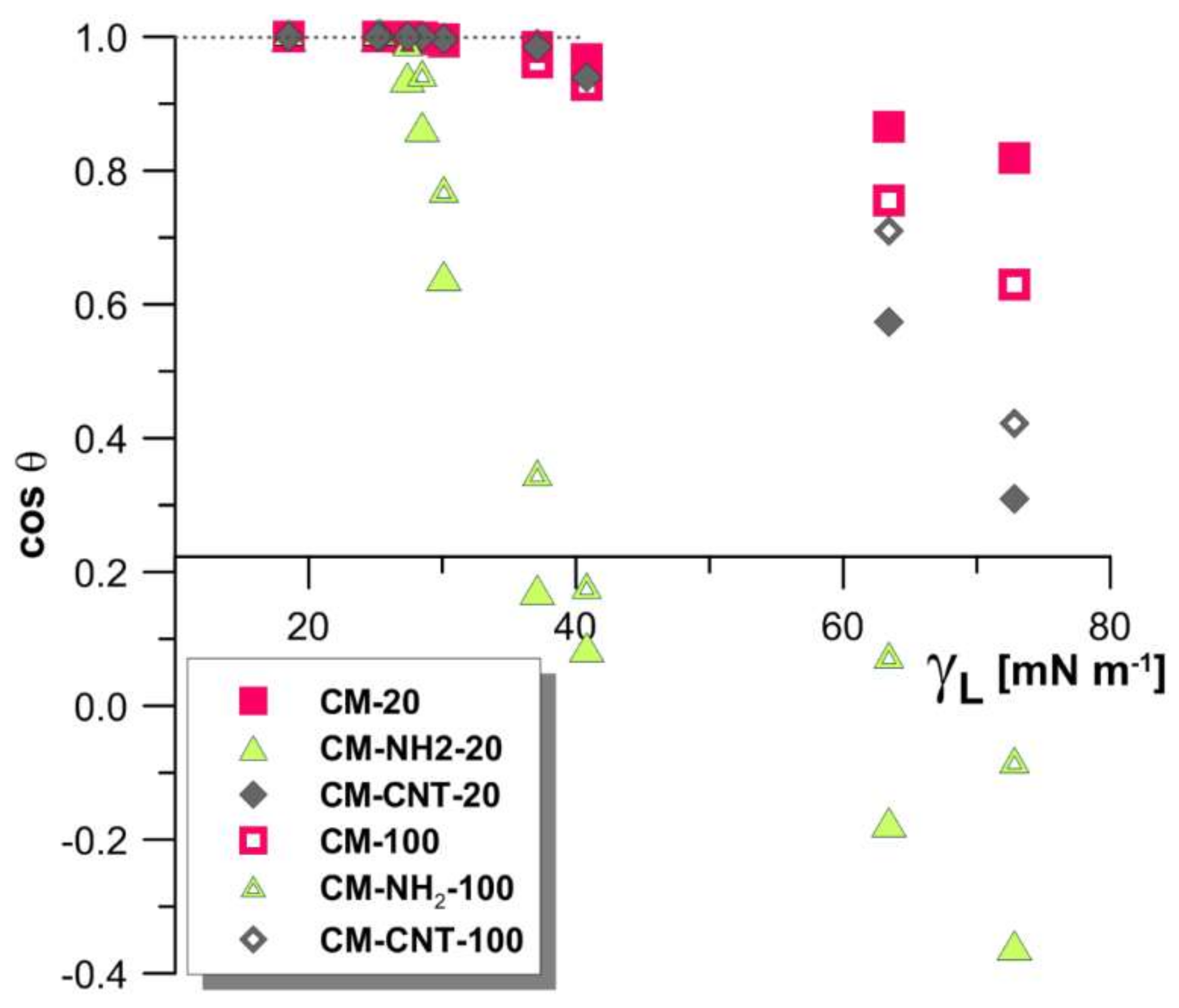
3.3.1. Surface Properties
3.3.2. Physicochemical Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Holt, J.K.; Park, H.G.; Wang, Y.; Stadermann, M.; Artyukhin, A.B.; Grigoropoulos, C.P.; Noy, A.; Bakajin, O. Fast mass transport through sub-2-nanometer carbon nanotubes. Science 2006, 312, 1034–1037. [Google Scholar] [CrossRef] [PubMed]
- Majumder, M.; Chopra, N.; Andrews, R.; Hinds, B.J. Nanoscale hydrodynamics: Enhanced flow in carbon nanotubes. Nature 2005, 438, 44. [Google Scholar] [CrossRef] [PubMed]
- Hinds, B.J.; Chopra, N.; Rantell, T.; Andrews, R.; Gavalas, V.; Bachas, L.G. Aligned multiwalled carbon nanotube membranes. Science 2004, 303, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; McKenzie, D.R. Nanoscale capillary flows in alumina: Testing the limits of classical theory. J. Phys. Chem. Lett. 2016, 7, 2647–2652. [Google Scholar] [CrossRef] [PubMed]
- Esfandiar, A.; Radha, B.; Wang, F.C.; Yang, Q.; Hu, S.; Garaj, S.; Nair, R.R.; Geim, A.K.; Gopinadhan, K. Size effect in ion transport through angstrom-scale slits. Science 2017, 358, 511–513. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Filiz, V.; Bengtson, G.; Shishatskiy, S.; Rahman, M.; Abetz, V. Functionalized carbon nanotubes mixed matrix membranes of polymers of intrinsic microporosity for gas separation. Nanoscale Res. Lett. 2012, 7, 504. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, A.; Salih, H.; Nam, S.; Dastgheib, S.A. Robust carbon nanotube membranes directly grown on hastelloy substrates and their potential application for membrane distillation. Carbon 2016, 106, 243–251. [Google Scholar] [CrossRef]
- Md Jani, A.M.; Losic, D.; Voelcker, N.H. Nanoporous anodic aluminium oxide: Advances in surface engineering and emerging applications. Prog. Mater. Sci. 2013, 58, 636–704. [Google Scholar] [CrossRef]
- Feng, S.; Zhong, Z.; Wang, Y.; Xing, W.; Drioli, E. Progress and perspectives in ptfe membrane: Preparation, modification, and applications. J. Membr. Sci. 2018, 549, 332–349. [Google Scholar] [CrossRef]
- Radha, B.; Esfandiar, A.; Wang, F.C.; Rooney, A.P.; Gopinadhan, K.; Keerthi, A.; Mishchenko, A.; Janardanan, A.; Blake, P.; Fumagalli, L.; et al. Molecular transport through capillaries made with atomic-scale precision. Nature 2016, 538, 222. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-W.; Li, L.; Zhang, J.-W.; Xu, X.; Chen, C.-S. B-sialon ceramic hollow fiber membranes with high strength and low thermal conductivity for membrane distillation. J. Eur. Ceram. Soc. 2016, 36, 59–65. [Google Scholar] [CrossRef]
- Xing, C.; Wang, Y.; Huang, X.; Li, Y.; Li, J. Poly(vinylidene fluoride) nanocomposites with simultaneous organic nanodomains and inorganic nanoparticles. Macromolecules 2016, 49, 1026–1035. [Google Scholar] [CrossRef]
- Wang, H.; Wang, R.; Sun, L.; Liu, Z.; Zhu, Y.; Zhu, Y. Mechanical and tribological characteristics of carbon nanotube-reinforced polyvinylidene fluoride (pvdf)/epoxy composites. RSC Adv. 2016, 6, 45636–45644. [Google Scholar] [CrossRef]
- Begum, S.; Kausar, A.; Ullah, H.; Siddiq, M. Potential of polyvinylidene flouride/carbon nanotube composite in energy, electronics and membrane technology: An overview. Polym.-Plast. Technol. Eng. 2016, 55, 1949–1970. [Google Scholar] [CrossRef]
- Sanchez-Valencia, J.R.; Dienel, T.; Groning, O.; Shorubalko, I.; Mueller, A.; Jansen, M.; Amsharov, K.; Ruffieux, P.; Fasel, R. Controlled synthesis of single-chirality carbon nanotubes. Nature 2014, 512, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi, S.T.; Mehrnia, M.R.; Rashidi, A.M. Preparation of nanofilter from carbon nanotubes for application in virus removal from water. Desalination 2009, 238, 271–280. [Google Scholar] [CrossRef]
- Shin, H.-C.; Liu, M.; Sadanadan, B.; Rao, A.M. Electrochemical insertion of lithium into multi-walled carbon nanotubes prepared by catalytic decomposition. J. Power Sources 2002, 112, 216–221. [Google Scholar] [CrossRef]
- An, K.H.; Kim, W.S.; Park, Y.S.; Choi, Y.C.; Lee, S.M.; Chung, D.C.; Bae, D.J.; Lim, S.C.; Lee, Y.H. Supercapacitors using single-walled carbon nanotube electrodes. Adv. Mater. 2001, 13, 497–500. [Google Scholar] [CrossRef]
- Barisci, J.N.; Wallace, G.G.; Baughman, R.H. Electrochemical studies of single-wall carbon nanotubes in aqueous solutions. J. Electroanal. Chem. 2000, 488, 92–98. [Google Scholar] [CrossRef]
- Ramanathan, T.; Fisher, F.T.; Ruoff, R.S.; Brinson, L.C. Amino-functionalized carbon nanotubes for binding to polymers and biological systems. Chem. Mater. 2005, 17, 1290–1295. [Google Scholar] [CrossRef]
- Sahoo, N.G.; Rana, S.; Cho, J.W.; Li, L.; Chan, S.H. Polymer nanocomposites based on functionalized carbon nanotubes. Prog. Polym. Sci. 2010, 35, 837–867. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Winey, K.I. Polymer nanocomposites containing carbon nanotubes. Macromolecules 2006, 39, 5194–5205. [Google Scholar] [CrossRef]
- Quinn, B.M.; Dekker, C.; Lemay, S.G. Electrodeposition of noble metal nanoparticles on carbon nanotubes. J. Am. Chem. Soc. 2005, 127, 6146–6147. [Google Scholar] [CrossRef] [PubMed]
- Holt, J.K.; Noy, A.; Huser, T.; Eaglesham, D.; Bakajin, O. Fabrication of a carbon nanotube-embedded silicon nitride membrane for studies of nanometer-scale mass transport. Nano Lett. 2004, 4, 2245–2250. [Google Scholar] [CrossRef]
- Tofighy, M.A.; Mohammadi, T. Synthesis and characterization of ceramic/carbon nanotubes composite adsorptive membrane for copper ion removal from water. Korean J. Chem. Eng. 2015, 32, 292–298. [Google Scholar] [CrossRef]
- Lisovsky, A.F. Thermodynamics of isolated pores filling with liquid in sintered composite materials. Metall. Mater. Trans. A 1994, 25, 733–740. [Google Scholar] [CrossRef]
- Bao-Ying, L.; Ching-Wen, L.; Gou-Jen, W. Nanoporous anodic aluminum oxide tube encapsulating a microporous chitosan/collagen composite for long-acting drug release. Biomed. Phys. Eng. Express 2015, 1, 045004. [Google Scholar] [CrossRef]
- Petukhov, D.I.; Eliseev, A.A. Gas permeation through nanoporous membranes in the transitional flow region. Nanotechnology 2016, 27, 085707. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.E.; Shi, Y.T.; Yuan, F.; Liu, J. Mechanochemical behavior of band2ti4o12 powder in ball milling for high κ microwave applications. In Advances and Applications in Electroceramics Ii; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 135–146. [Google Scholar]
- Li, L.; Zhang, M.; Ruan, W. Studies on synergistic effect of cnt and cb nanoparticles on pvdf. Polym. Compos. 2015, 36, 2248–2254. [Google Scholar] [CrossRef]
- Karousis, N.; Tagmatarchis, N.; Tasis, D. Current progress on the chemical modification of carbon nanotubes. Chem. Rev. 2010, 110, 5366–5397. [Google Scholar] [CrossRef] [PubMed]
- Tran, M.Q.; Shaffer, M.S.P.; Bismarck, A. Manufacturing carbon nanotube/pvdf nanocomposite powders. Macromol. Mater. Eng. 2008, 293, 188–193. [Google Scholar] [CrossRef]
- Kadleíková, M.; Breza, J.; Veselý, M. Raman spectra of synthetic sapphire. Microelectron. J. 2001, 32, 955–958. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Saito, R.; Jorio, A. Raman spectroscopy of carbon nanotubes. Phys. Rep. 2005, 409, 47–99. [Google Scholar] [CrossRef]
- Marković, Z.; Kepić, D.; Holclajtner Antunović, I.; Nikolić, M.; Dramićanin, M.; Marinović Cincović, M.; Todorović Marković, B. Raman study of single wall carbon nanotube thin films treated by laser irradiation and dynamic and isothermal oxidation. J. Raman Spectrosc. 2012, 43, 1413–1422. [Google Scholar] [CrossRef]
- Li, Q.; Xue, Q.; Zheng, Q.; Hao, L.; Gao, X. Large dielectric constant of the chemically purified carbon nanotube/polymer composites. Mater. Lett. 2008, 62, 4229–4231. [Google Scholar] [CrossRef]
- Dyshin, A.A.; Eliseeva, O.V.; Bondarenko, G.V.; Kiselev, M.G. Dissolution of single-walled carbon nanotubes in alkanol-cholic acid mixtures. Russ. J. Phys. Chem. A 2015, 89, 1628–1632. [Google Scholar] [CrossRef]
- Ziegler, K.J.; Gu, Z.; Peng, H.; Flor, E.L.; Hauge, R.H.; Smalley, R.E. Controlled oxidative cutting of single-walled carbon nanotubes. J. Am. Chem. Soc. 2005, 127, 1541–1547. [Google Scholar] [CrossRef] [PubMed]
- Fontananova, E.; Grosso, V.; Aljlil, S.A.; Bahattab, M.A.; Vuono, D.; Nicoletta, F.P.; Curcio, E.; Drioli, E.; Di Profio, G. Effect of functional groups on the properties of multiwalled carbon nanotubes/polyvinylidenefluoride composite membranes. J. Membr. Sci. 2017, 541, 198–204. [Google Scholar] [CrossRef]
- Kujawa, J.; Al-Gharabli, S.; Kujawski, W.; Knozowska, K. Molecular grafting of fluorinated and non-fluorinated alkylsiloxanes on various ceramic membrane surfaces for the removal of vocs applying vacuum membrane distillation. ACS Appl. Mater. Interfaces 2017, 9, 6571–6590. [Google Scholar] [CrossRef] [PubMed]
- Kujawa, J.; Kujawski, W. Functionalization of ceramic metal oxide powders and ceramic membranes by perfluoroalkylsilanes and alkylsilanes possessing different reactive groups: Physicochemical and tribological properties. ACS Appl. Mater. Interfaces 2016, 8, 7509–7521. [Google Scholar] [CrossRef] [PubMed]
- Tan, G.; Zhao, L.-D.; Kanatzidis, M.G. Rationally designing high-performance bulk thermoelectric materials. Chem. Rev. 2016, 116, 12123–12149. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.-K.; Mitrovic, S.; Tham, D.; Varghese, J.; Heath, J.R. Reduction of thermal conductivity in phononic nanomesh structures. Nat. Nanotech. 2010, 5, 718. [Google Scholar] [CrossRef] [PubMed]
- Wenwen, L.; Michelle, K.R.; David, R.M. The physics of confined flow and its application to water leaks, water permeation and water nanoflows: A review. Rep. Prog. Phys. 2016, 79, 025901. [Google Scholar]
- Whitby, M.; Cagnon, L.; Thanou, M.; Quirke, N. Enhanced fluid flow through nanoscale carbon pipes. Nano Lett. 2008, 8, 2632–2637. [Google Scholar] [CrossRef] [PubMed]
- Hendren, Z.D.; Brant, J.; Wiesner, M.R. Surface modification of nanostructured ceramic membranes for direct contact membrane distillation. J. Membr. Sci. 2009, 331, 1–10. [Google Scholar] [CrossRef]
- Chibowski, E.; Jurak, M.; Holysz, L.; Szczes, A. Wetting properties of model biological membranes. Curr. Opin. Colloid Interface Sci. 2014, 19, 368–380. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Zhu, H.; Guo, Z.; Liu, W. Adhesion behaviors on superhydrophobic surfaces. Chem. Commun. 2014, 50, 3900–3913. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, Y.; Chen, S.-Y.; Mishra, H.; Schrader, A.M.; Lee, D.W.; Das, S.; Donaldson, S.H.; Israelachvili, J.N. Simple-to-apply wetting model to predict thermodynamically stable and metastable contact angles on textured/rough/patterned surfaces. J. Phys. Chem. C 2017, 121, 5642–5656. [Google Scholar] [CrossRef]
- Wu, C.; Leese, H.S.; Mattia, D.; Dagastine, R.R.; Chan, D.Y.C.; Tabor, R.F. Study of fluid and transport properties of porous anodic aluminum membranes by dynamic atomic force microscopy. Langmuir 2013, 29, 8969–8977. [Google Scholar] [CrossRef] [PubMed]
- Soliveri, G.; Pifferi, V.; Annunziata, R.; Rimoldi, L.; Aina, V.; Cerrato, G.; Falciola, L.; Cappelletti, G.; Meroni, D. Alkylsilane–sio2 hybrids. A concerted picture of temperature effects in vapor phase functionalization. J. Phys. Chem. C 2015, 119, 15390–15400. [Google Scholar] [CrossRef]
- Kujawa, J.; Cerneaux, S.; Kujawski, W.; Bryjak, M.; Kujawski, J. How to functionalize ceramics by perfluoroalkylsilanes for membrane separation process? Properties and application of hydrophobized ceramic membranes. ACS Appl. Mater. Interfaces 2016, 8, 7564–7577. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, X.; Lai, C.; Jiang, X.; Li, X.; Shu, Y. Facile approach to the green synthesis of novel ternary composites with excellent superhydrophobic and thermal stability property: An expanding horizon. Chem. Eng. J. 2017, 309, 240–248. [Google Scholar] [CrossRef]
- Bhushan, B.; Cichomski, M.; Hoque, E.; DeRose, A.; Hoffmann, P.; Mathieu, J. Nanotribological characterization of perfluoroalkylphosphonate self-assembled monolayers deposited on aluminum-coated silicon substrates. Microsyst. Technol. 2006, 12, 588–596. [Google Scholar] [CrossRef]
- Walsh, R. Bond dissociation energy values in silicon-containing compounds and some of their implications. Acc. Chem. Res. 1981, 14, 246–252. [Google Scholar] [CrossRef]
- Kujawa, J.; Cerneaux, S.; Koter, S.; Kujawski, W. Highly efficient hydrophobic titania ceramic membranes for water desalination. ACS Appl. Mater. Interfaces 2014, 6, 14223–14230. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]












| Sample | Adhesion Force (nN) | Nanohardness (GPa) | Young’s Modulus (GPa) |
|---|---|---|---|
| CM-20 | 12.6 ± 0.9 | 1.23 ± 0.09 | 33.08 ± 2.32 |
| CM-NH2-20 | 14.8 ± 1.0 | 5.68 ± 0.40 | 35.09 ± 2.46 |
| CM-CNT-20 | 18.5 ± 1.3 | 6.62 ± 0.46 | 39.14 ± 2.74 |
| CM-100 | 8.3 ± 0.6 | 0.45 ± 0.03 | 42.86 ± 3.00 |
| CM-NH2-100 | 10.8 ± 0.8 | 2.04 ± 0.13 | 45.38 ± 3.18 |
| CM-CNT-100 | 15.7 ± 1.1 | 2.34 ± 0.16 | 48.67 ± 3.41 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Gharabli, S.; Hamad, E.; Saket, M.; Abu El-Rub, Z.; Arafat, H.; Kujawski, W.; Kujawa, J. Advanced Material-Ordered Nanotubular Ceramic Membranes Covalently Capped with Single-Wall Carbon Nanotubes. Materials 2018, 11, 739. https://doi.org/10.3390/ma11050739
Al-Gharabli S, Hamad E, Saket M, Abu El-Rub Z, Arafat H, Kujawski W, Kujawa J. Advanced Material-Ordered Nanotubular Ceramic Membranes Covalently Capped with Single-Wall Carbon Nanotubes. Materials. 2018; 11(5):739. https://doi.org/10.3390/ma11050739
Chicago/Turabian StyleAl-Gharabli, Samer, Eyad Hamad, Munib Saket, Ziad Abu El-Rub, Hassan Arafat, Wojciech Kujawski, and Joanna Kujawa. 2018. "Advanced Material-Ordered Nanotubular Ceramic Membranes Covalently Capped with Single-Wall Carbon Nanotubes" Materials 11, no. 5: 739. https://doi.org/10.3390/ma11050739
APA StyleAl-Gharabli, S., Hamad, E., Saket, M., Abu El-Rub, Z., Arafat, H., Kujawski, W., & Kujawa, J. (2018). Advanced Material-Ordered Nanotubular Ceramic Membranes Covalently Capped with Single-Wall Carbon Nanotubes. Materials, 11(5), 739. https://doi.org/10.3390/ma11050739