Stability of GO Modified by Different Dispersants in Cement Paste and Its Related Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
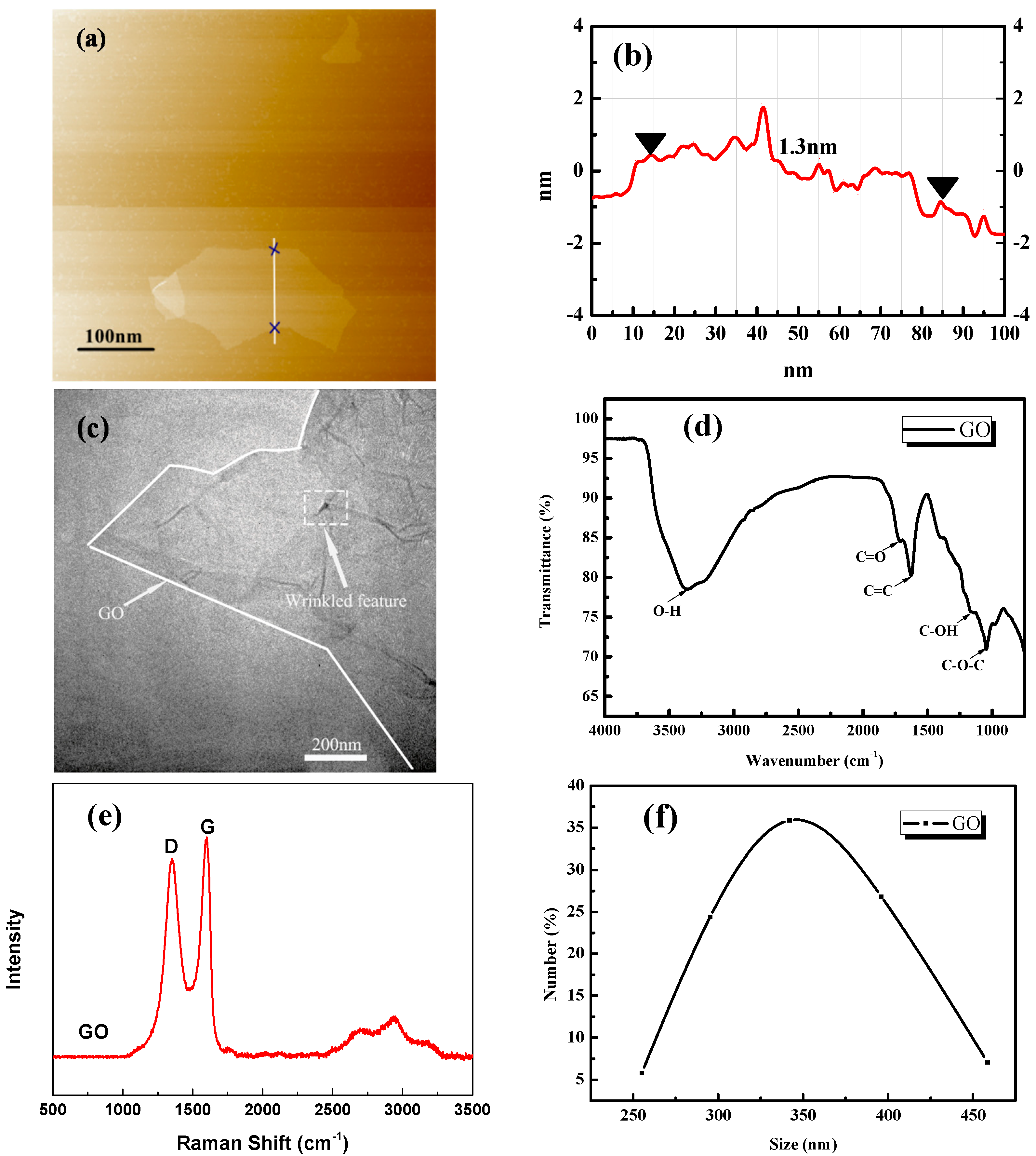
2.1.1. Graphene Oxide (GO)
2.1.2. Dispersing Agents (DAs)
2.1.3. Cement
2.2. Preparation of GO Suspensions
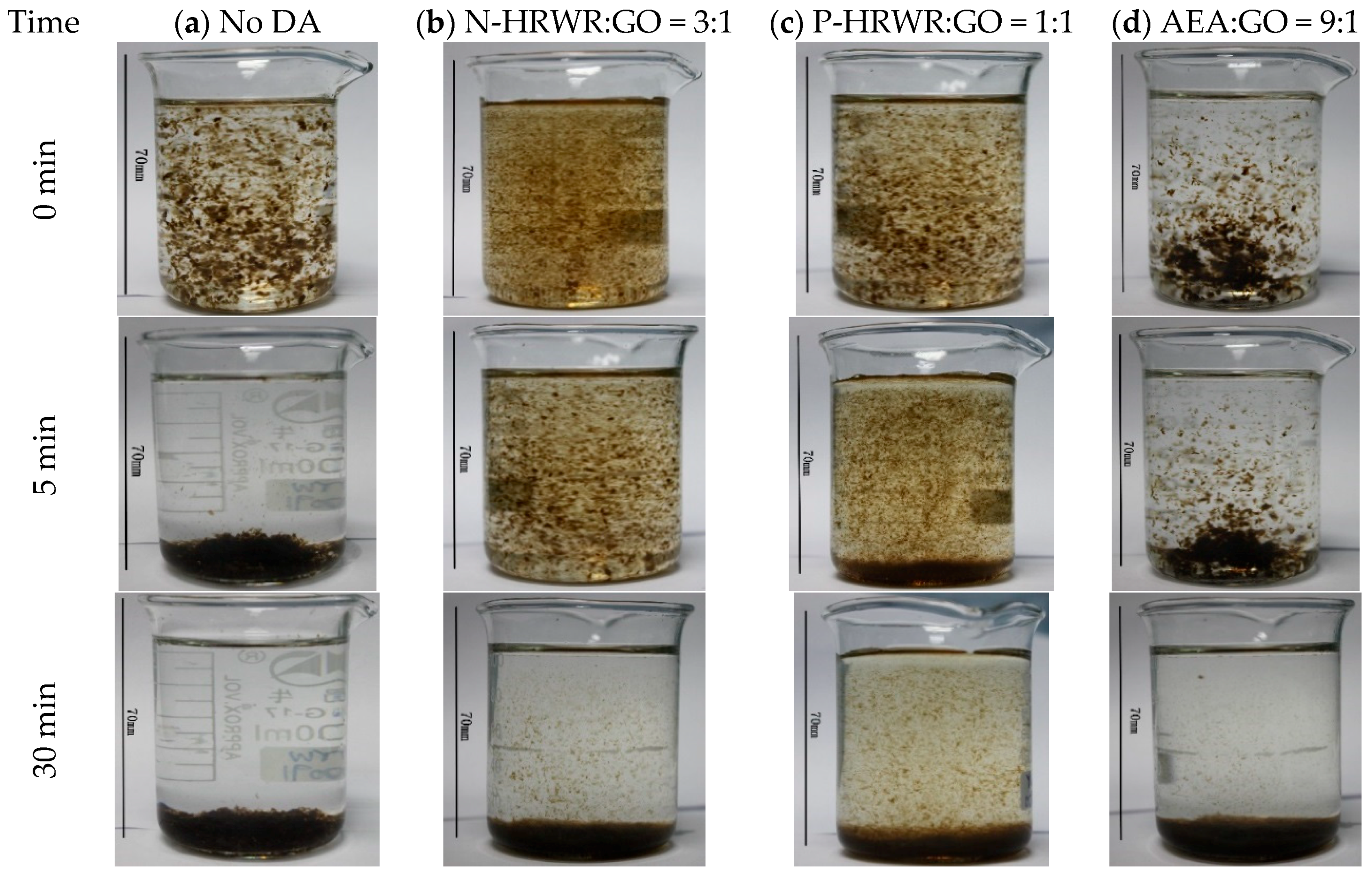
2.2.1. DA Type and DA to GO Mass Ratio
2.2.2. Effect of Cement Addition and Solution pH
2.3. Testing Methods
2.3.1. UV–Vis Absorption Spectrum
2.3.2. Characterization of the Specific Surface Area of GO
3. Results and Discussion
3.1. Dispersion State of GO in Aqueous Solutions
3.1.1. Selection of Wavenumber
3.1.2. Effect of DA Types
3.1.3. Effect of DA Concentrations
3.2. Dispersion State of GO in Sumulated Concrete Pore Solution (SCPS)
3.3. Dispersion State of GO in Cement Paste
3.3.1. Effect of Cement Addition on GO Dispersion
3.3.2. Mechanisms of GO Agglomeration in Cement Paste
4. Conclusions
- (1)
- The dispersion of GO in aqueous solutions was positively influenced by P-HRWA, N-HRWA, and AEA, respectively; among the DAs, the AEA provided the most improvement of dispersion in aqueous solutions.
- (2)
- Compared with the dispersion of GO in SCPS and cement pastes, homogeneous dispersion of GO in aqueous solution did not guarantee uniform dispersion in cement pastes. Although AEA allowed GO to reach the optimum state of dispersion in aqueous solutions, lowering the surface tension of the aqueous solution was AEA’s main advantage, which did not exist in the highly alkaline and high ionic strength solution.
- (3)
- The dual effects of electronic repulsion and steric hindrance of P-HRWR resulted in better GO dispersion in the high alkalinity and high ionic strength of cement paste.
- (4)
- With the increase of ions and pH, the P-HRWR separated from the GO. Thus, GO were absorbed on the surface of the cement particles, and GO sedimentation generated.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sanchez, F.; Sobolev, K. Nanotechnology in concrete—A review. Constr. Build. Mater. 2010, 24, 2060–2071. [Google Scholar] [CrossRef]
- Raki, L.; Beaudoin, J.; Alizadeh, R.; Makar, J.; Sato, T. Cement and concrete nanoscience and nanotechnology. Materials 2010, 3, 918–942. [Google Scholar] [CrossRef]
- Long, W.J.; Wei, J.J.; Ma, H.Y.; Xing, F. Dynamic mechanical properties and microstructure of graphene oxide nanosheets reinforced cement composites. Nanomaterials 2017, 7, 407. [Google Scholar] [CrossRef] [PubMed]
- Senff, L.; Hotza, D.; Llucas, S.; Ferreira, V.M.; Labrincha, J.A. Effect of nano-SiO2, and nano-TiO2, addition on the rheological behavior and the hardened properties of cement mortars. Mater. Sci. Eng. 2012, 532, 354–361. [Google Scholar] [CrossRef]
- Kong, D.; Du, X.; Wei, S.; Zhang, H.; Yang, Y.; Shah, S.P. Influence of nano-silica agglomeration on microstructure and properties of the hardened cement-based materials. Constr. Build. Mater. 2012, 37, 707–715. [Google Scholar] [CrossRef]
- Al, J.K.; Shoukry, H. Use of nano-structured waste materials for improving mechanical, physical and structural properties of cement mortar. Constr. Build. Mater. 2014, 73, 636–644. [Google Scholar] [CrossRef]
- Pisello, A.L.; D’Alessandro, A.; Sambuco, S.; Rallini, M.; Ubertini, F.; Asdrubali, F.; Materazzi, A.L.; Cotana, F. Multipurpose experimental characterization of smart nanocomposite cement-based materials for thermal-energy efficiency and strain-sensing capability. Sol. Energy Mater. Sol. Cells 2017, 161, 77–88. [Google Scholar] [CrossRef]
- Lee, S.J.; You, I.; Zi, G.; Yoo, D.Y. Experimental investigation of the piezoresistive properties of cement composites with hybrid carbon fibers and nanotubes. Sensors 2017, 17, 2516. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Ding, S.; Yu, X. Intrinsic self-sensing concrete and structures: A review. Measurement 2015, 59, 110–128. [Google Scholar] [CrossRef]
- Shang, Y.; Zhang, D.; Yang, C.; Liu, Y.; Liu, Y. Effect of graphene oxide on the rheological properties of cement pastes. Constr. Build. Mater. 2015, 96, 20–28. [Google Scholar] [CrossRef]
- Li, X.; Korayem, A.; Li, C. Incorporation of graphene oxide and silica fume into cement paste: A study of dispersion and compressive strength. Constr. Build. Mater. 2016, 123, 327–335. [Google Scholar] [CrossRef]
- Ghafari, E.; Ghahari, S.; Feng, Y.; Severgnini, F.; Lu, N. Effect of Zinc oxide and Al-Zinc oxide nanoparticles on the rheological properties of cement paste. Compos. Part B 2016, 105, 160–166. [Google Scholar] [CrossRef]
- Chithra, S.; Kumar, S.; Chinnaraju, K. The effect of colloidal nano-silica on workability, mechanical and durability properties of high performance concrete with copper slag as partial fine aggregate. Constr. Build. Mater. 2016, 113, 794–804. [Google Scholar] [CrossRef]
- Konsta, G.M.; Batis, G.; Danoglidis, P. Effect of CNT and CNF loading and count on the corrosion resistance, conductivity and mechanical properties of nanomodified OPC mortars. Constr. Build. Mater. 2017, 147, 48–57. [Google Scholar] [CrossRef]
- Sun, X.; Wu, Q.; Zhang, J.; Yan, Q.; Wu, Y.; Lee, S. Rheology, curing temperature and mechanical performance of oil well cement: Combined effect of cellulose nanofibers and graphene nano-platelets. Mater. Des. 2016, 114, 92–101. [Google Scholar] [CrossRef]
- Wang, Q.; Cui, X.; Wang, J.; Li, S.; Lv, C.; Dong, Y. Effect of fly ash on rheological properties of graphene oxide cement paste. Constr. Build. Mater. 2017, 138, 35–44. [Google Scholar] [CrossRef]
- Chuah, S.; Pan, Z.; Sanjayan, J.G.; Wang, C.M.; Duan, W.H. Nano reinforced cement and concrete composites and new perspective from graphene oxide. Constr. Build. Mater. 2014, 73, 113–124. [Google Scholar] [CrossRef]
- Long, W.J.; Li, H.D.; Fang, C.L.; Xing, F. Uniformly dispersed and re-agglomerated graphene oxide-based cement pastes: A comparison of rheological properties, mechanical properties and microstructure. Nanomaterials 2018, 8, 31. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, J.; Zhu, L.; Jia, C. Preparation of cement composites with ordered microstructures via doping with graphene oxide nanosheets and an investigation of their strength and durability. Materials 2016, 9, 924. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.; Sanjayan, J.G.; Duan, W.H.; Nazari, A. Incorporating graphene oxide in cement composites: A study of transport properties. Constr. Build. Mater. 2015, 84, 341–347. [Google Scholar] [CrossRef]
- Li, W.; Li, X.; Chen, S.; Chen, S.J.; Liu, Y.M.; Duan, W.H.; Shah, S.P. Effects of graphene oxide on early-age hydration and electrical resistivity of Portland cement paste. Constr. Build. Mater. 2017, 136, 506–514. [Google Scholar] [CrossRef]
- Gao, Y.; Ren, X.; Tan, X.; Hayat, T.; Alsaedi, A.; Chen, C. Insights into key factors controlling GO stability in natural surface waters. J. Hazard. Mater. 2017, 335, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.C.; Siddiqui, N.A.; Marom, G.; Kim, J.K. Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: A review. Compos. Part A 2010, 41, 1345–1367. [Google Scholar] [CrossRef]
- Stephens, C.; Brown, L.; Sanchez, F. Quantification of the re-agglomeration of carbon nanofiber aqueous dispersion in cement pastes and effect on the early age flexural response. Carbon 2016, 107, 482–500. [Google Scholar] [CrossRef]
- Parveen, S.; Rana, S.; Fangueiro, R.; Paiva, M.C. Microstructure and mechanical properties of carbon nanotube reinforced cementitious composites developed using a novel dispersion technique. Cem. Concr. Res. 2015, 73, 215–227. [Google Scholar] [CrossRef]
- Zhao, L.; Guo, X.L.; Ge, C.; Li, Q.; Guo, L.P.; Shu, X.; Liu, J.P. Investigation of the effectiveness of PC@GO on the reinforcement for cement composites. Constr. Build. Mater. 2016, 113, 470–478. [Google Scholar] [CrossRef]
- Lu, L.; Dong, O. Properties of Cement Mortar and Ultra-High Strength Concrete Incorporating Graphene Oxide Nanosheets. Nanomaterials 2017, 7, 187. [Google Scholar] [CrossRef] [PubMed]
- Babak, F.; Abolfazl, H.; Alimorad, R.; Parviz, G. Preparation and mechanical properties of graphene oxide: Cement nanocomposites. Sci. World J. 2013, 2014, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Fangueiro, R. A Review on nanomaterial dispersion, microstructure, and mechanical properties of carbon nanotube and nanofiber reinforced cementitious composites. J. Nanomater. 2013, 2013, 1–19. [Google Scholar] [CrossRef]
- Bastos, G.; Patiño-Barbeito, F.; Patiño-Cambeiro, F.; Armesto, J. Nano-Inclusions Applied in Cement-Matrix Composites: A Review. Materials 2016, 9, 1015. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Zhang, Y. Influences of dispersant types and concentrations on stability of graphene water-based slurry. Mater. Rev. 2016, 30, 167–171. [Google Scholar] [CrossRef]
- Pourbaix, M.; Franklin, J. Atlas of Electrochemical Equilibria in Aqueous Solutions; Pergamon Press: Oxford, UK; New York, NY, USA, 1966; Volume 1, p. 471. [Google Scholar]
- Sahoo, G.; Balasubramaniam, R. On the corrosion behaviour of phosphoric irons in simulated concrete pore solution. Corros. Sci. 2008, 50, 131–143. [Google Scholar] [CrossRef]
- Freire, L.; Carmezim, M.J.; Ferreira, M.G.S.; Montemor, M.F. The electrochemical behaviour of stainless steel AISI 304 in alkaline solutions with different pH in the presence of chlorides. Electrochim. Acta 2011, 56, 5280–5289. [Google Scholar] [CrossRef]
- Li, H.; Zhang, M.; Ou, J. Abrasion resistance of concrete containing nano-particles for pavement. Wear 2006, 260, 1262–1266. [Google Scholar] [CrossRef]
- Deng, K.; Li, C.; Qiu, X.; Zhou, J.; Hou, Z. Electrochemical preparation, characterization and application of electrodes modified with nickel–cobalt hexacyanoferrate/graphene oxide–carbon nanotubes. J. Electroanal. Chem. 2015, 755, 197–202. [Google Scholar] [CrossRef]
- Wang, M.; Wang, R.; Yao, H.; Farhan, S.; Zheng, S.; Du, C. Study on the three dimensional mechanism of graphene oxide nanosheets modified cement. Constr. Build. Mater. 2016, 126, 730–739. [Google Scholar] [CrossRef]
- Sharma, S.; Kothiyal, N. Comparative effects of pristine and ball-milled graphene oxide on physico-chemical characteristics of cement mortar nanocomposites. Constr. Build. Mater. 2016, 115, 256–268. [Google Scholar] [CrossRef]
- Kudin, K.; Ozbas, B.; Schniepp, H. Raman spectra of graphite oxide and functionalized graphene sheets. Nano Lett. 2007, 8, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, Q.; Yang, M.G.; Huang, C.H.; Yang, Y.G.; Wang, M.Z. Structural evolution during annealing of thermally reduced graphene nanosheets for application in supercapacitors. Carbon 2012, 50, 3572–3584. [Google Scholar] [CrossRef]
- Ferrari, A.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2008, 61, 14095–14107. [Google Scholar] [CrossRef]
- GB50119-2013 Code for Concrete Admixture Application; Ministry of Housing and Urban-Rural Development of the People’s Republic of China: Beijing, China, 2013.
- Łaźniewska, P.B. The methodology for assessing the impact of new generation superplasticizers on air content in self-compacting concrete. Constr. Build. Mater. 2014, 53, 488–502. [Google Scholar] [CrossRef]
- Yoshioka, K.; Sakai, E.; Daimon, M.; Kitahara, A. Role of steric hindrance in the performance of superplasticizers for concrete. J. Am. Ceram. Soc. 1997, 80, 2667–2671. [Google Scholar] [CrossRef]
- Mindess, S.; Young, J.; Darwin, D. Concrete, 2nd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2003; ISBN 0130646326. [Google Scholar]
- Yan, B.L.; Jiang, L.Z.; Xiao, Z.M.; Liu, C.; Zhang, Q.Y.; Chen, P.; Huo, C.M.; Xi, J.S.; Song, L.C.; Wang, X.; et al. GB/T 175-2007 Common Portland Cement; Standards Press of China: Beijing, China, 2007. [Google Scholar]
- Konsta, G.M.; Metaxa, Z.; Shah, S. Highly dispersed carbon nanotube reinforced cement-based materials. Cem. Concr. Res. 2016, 40, 1052–1059. [Google Scholar] [CrossRef]
- Metaxa, Z.; Konsta, G.M.; Shah, S. Carbon nanofiber cementitious composites: Effect of debulking procedure on dispersion and reinforcing efficiency. Cem. Concr. Compos. 2013, 36, 25–32. [Google Scholar] [CrossRef]
- Konsta, G.M.; Metaxa, Z.; Shah, S. Nanoimaging of highly dispersed carbon nanotube reinforced cement based materials. In Proceedings of the 7th RILEM International Symposium on Fibre Reinforced Concrete, Chennai, India, 17–19 September 2008; pp. 125–131. [Google Scholar]
- Zou, B.; Chen, S.J.; Korayem, A.H.; Collins, F.; Wang, C.M.; Duan, W.H. Effect of ultrasonication energy on engineering properties of carbon nanotube reinforced cement pastes. Carbon 2015, 85, 212–220. [Google Scholar] [CrossRef]
- Lothenbach, B.; Winnefeld, F. Thermodynamic modelling of the hydration of Portland cement. Cem. Concr. Res. 2006, 36, 209–226. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Q.; Zhu, H.; Liu, H.; Chen, Y.; Yang, M. Dispersing multi-walled carbon nanotubes with water-soluble block copolymers and their use as supports for metal nanoparticles. Carbon 2007, 45, 285–292. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, L.; Chen, Y. Fabrication of ceramic oxide-coated SWNT composites by sol-gel process with a polymer glue. J. Nanopart. Res. 2011, 13, 3731–3740. [Google Scholar] [CrossRef]
- Chuah, S.; Li, W.; Chen, S.J.; Sanjayan, J.G.; Duan, W.H. Investigation on dispersion of graphene oxide in cement composite using different surfactant treatments. Constr. Build. Mater. 2018, 161, 519–527. [Google Scholar] [CrossRef]
- Ren, X.; Li, J.; Tan, X. Impact of Al2O3 on the aggregation and deposition of graphene oxide. Environ. Sci. Technol. 2014, 48, 5493–5500. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, O.; Sierra, G.; Tobón, J. Influence of super plasticizer and Ca(OH)2 on the stability of functionalized multi-walled carbon nanotubes dispersions for cement composites applications. Constr. Build. Mater. 2013, 47, 771–778. [Google Scholar] [CrossRef]
- Grunlan, J.; Liu, L.; Regev, O. Weak polyelectrolyte control of carbon nanotube dispersion in water. J. Colloid Interface Sci. 2008, 317, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Yuan, B.; Wang, X.; Hu, Y. Enhanced mechanical properties, water stability and repeatable shape recovery behavior of Ca2+, crosslinking graphene oxide-based nacre-mimicking hybrid film. Mater. Des. 2017, 115, 46–51. [Google Scholar] [CrossRef]









| Appearance | Solid Content (mass %) | pH | Viscosity | Absorbance Ratio A230/A600 | Carbon (%) | Molar Ratio (O/C) |
|---|---|---|---|---|---|---|
| Brown paste | 43 ± 1 | ≥1.2 | ≥2000 | ≥45 | 47 ± 5 | 0.6 ± 1 |
| Type | Aspect | Solid Content, % | pH | Recommended Content |
|---|---|---|---|---|
| RMC-3 | Dark-brown liquid | 49.98 | 5–6 | 0.5%–1.5% |
| CP-WRM50 | Slight-yellow liquid | 50.79 | 4–5 | |
| KS-20 | Yellow brown solid | 95 | 6–7 a | 0.75%–1.5% |
| K12 | White solid | 96 | 7–8 a | 0.03%–0.1% |
| Composition | CaO | SiO2 | Al2O3 | Fe2O3 | MgO | SO3 | f-CaO | Na2O | LOI |
|---|---|---|---|---|---|---|---|---|---|
| Content (%) | 64.65 | 21.88 | 4.49 | 3.45 | 2.36 | 2.44 | 0.28 | 0.51 | 1.31 |
| Fineness (0.08/%) | Specific Surface Area (m2/kg) | Density (g/cm3) | Setting Times (min) | Stability | |||||
| 0.8 | 344 | 3.15 | Final | Initial | Qualified | ||||
| 130 | 205 | ||||||||
| Sample | GO (g) | DAs (g) | Water (g) |
|---|---|---|---|
| pGO | 0.03 | 0 | 999.97 |
| GO-N-1 | 0.03 | N-HRWR, 0.03 | 999.97 |
| GO-N-3 | 0.03 | N-HRWR, 0.09 | 999.97 |
| GO-N-9 | 0.03 | N-HRWR, 0.27 | 999.97 |
| GO-A-1 | 0.03 | AEA, 0.03 | 999.97 |
| GO-A-3 | 0.03 | AEA, 0.09 | 999.97 |
| GO-A-9 | 0.03 | AEA, 0.27 | 999.97 |
| GO-P-1 | 0.03 | P-HRWR, 0.03 | 999.97 |
| GO-P-3 | 0.03 | P-HRWR, 0.09 | 999.97 |
| GO-P-9 | 0.03 | P-HRWR, 0.27 | 999.97 |
| Sample | Sample Style (Diameter) | Solvent | Particle Weight Concentration | Density of Particle (g/mL) | Specific Surface Area (m2/g) |
|---|---|---|---|---|---|
| pGO | GO (350 nm) | Water | 0.00003 | 2.2 | 2112.45 |
| pGO-JZ30min | GO (350 nm) | Water | 0.00003 | 2.2 | 1824.47 |
| GO-N-1-JZ30min | GO (350 nm) | Water | 0.00003 | 2.2 | 1954.47 |
| GO-N-3-JZ30min | GO (350 nm) | Water | 0.00003 | 2.2 | 1978.14 |
| GO-N-9-JZ30min | GO (350 nm) | Water | 0.00003 | 2.2 | 2001.17 |
| GO-A-1-JZ30min | GO (350 nm) | Water | 0.00003 | 2.2 | 2025.31 |
| GO-A-3-JZ30min | GO (350 nm) | Water | 0.00003 | 2.2 | 2077.97 |
| GO-A-9-JZ30min | GO (350 nm) | Water | 0.00003 | 2.2 | 2021.15 |
| GO-P-1-JZ30min | GO (350 nm) | Water | 0.00003 | 2.2 | 2000.47 |
| GO-P-3-JZ30min | GO (350 nm) | Water | 0.00003 | 2.2 | 2003.14 |
| GO-P-9-JZ30min | GO (350 nm) | Water | 0.00003 | 2.2 | 2217.63 |
| Sample | Sample Style (Diameter) | Solvent | Particle Weight Concentration | Density of Particle (g/mL) | Specific Surface Area (m2/g) |
|---|---|---|---|---|---|
| pGO-JZ30min | GO (350 nm) | Water | 0.00003 | 2.2 | 54.87 |
| GO-N-3-JZ30min | GO (350 nm) | Water-N | 0.00003 | 2.2 | 215.47 |
| GO-A-9-JZ30min | GO (350 nm) | Water-A | 0.00003 | 2.2 | 60.47 |
| GO-P-1-JZ30min | GO (350 nm) | Water-P | 0.00003 | 2.2 | 1205.35 |
| Sample | Sample Style (Diameter) | Solvent | Particle Weight Concentration | Density of Particle (g/mL) | Specific Surface Area (m2/g) |
|---|---|---|---|---|---|
| GO-N-3-C-JZ30min | GO (350 nm) | Water-N-C | 0.00003 | 2.2 | 515.47 |
| GO-A-9-C-JZ30min | GO (350 nm) | Water-A-C | 0.00003 | 2.2 | 87.95 |
| GO-P-1-C-JZ30min | GO (350 nm) | Water-P-C | 0.00003 | 2.2 | 1745.12 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, W.-J.; Fang, C.; Wei, J.; Li, H. Stability of GO Modified by Different Dispersants in Cement Paste and Its Related Mechanism. Materials 2018, 11, 834. https://doi.org/10.3390/ma11050834
Long W-J, Fang C, Wei J, Li H. Stability of GO Modified by Different Dispersants in Cement Paste and Its Related Mechanism. Materials. 2018; 11(5):834. https://doi.org/10.3390/ma11050834
Chicago/Turabian StyleLong, Wu-Jian, Changle Fang, Jingjie Wei, and Haodao Li. 2018. "Stability of GO Modified by Different Dispersants in Cement Paste and Its Related Mechanism" Materials 11, no. 5: 834. https://doi.org/10.3390/ma11050834
APA StyleLong, W.-J., Fang, C., Wei, J., & Li, H. (2018). Stability of GO Modified by Different Dispersants in Cement Paste and Its Related Mechanism. Materials, 11(5), 834. https://doi.org/10.3390/ma11050834





