Energy Storage Analysis of a Mixed R161/MOF-5 Nanoparticle Nanofluid Based on Molecular Simulations
Abstract
:1. Introduction
2. Method and Computational Details
2.1. Simulation Models
2.2. MD Simulation Details
2.3. GCMC Simulation Details
3. Results and Discussion
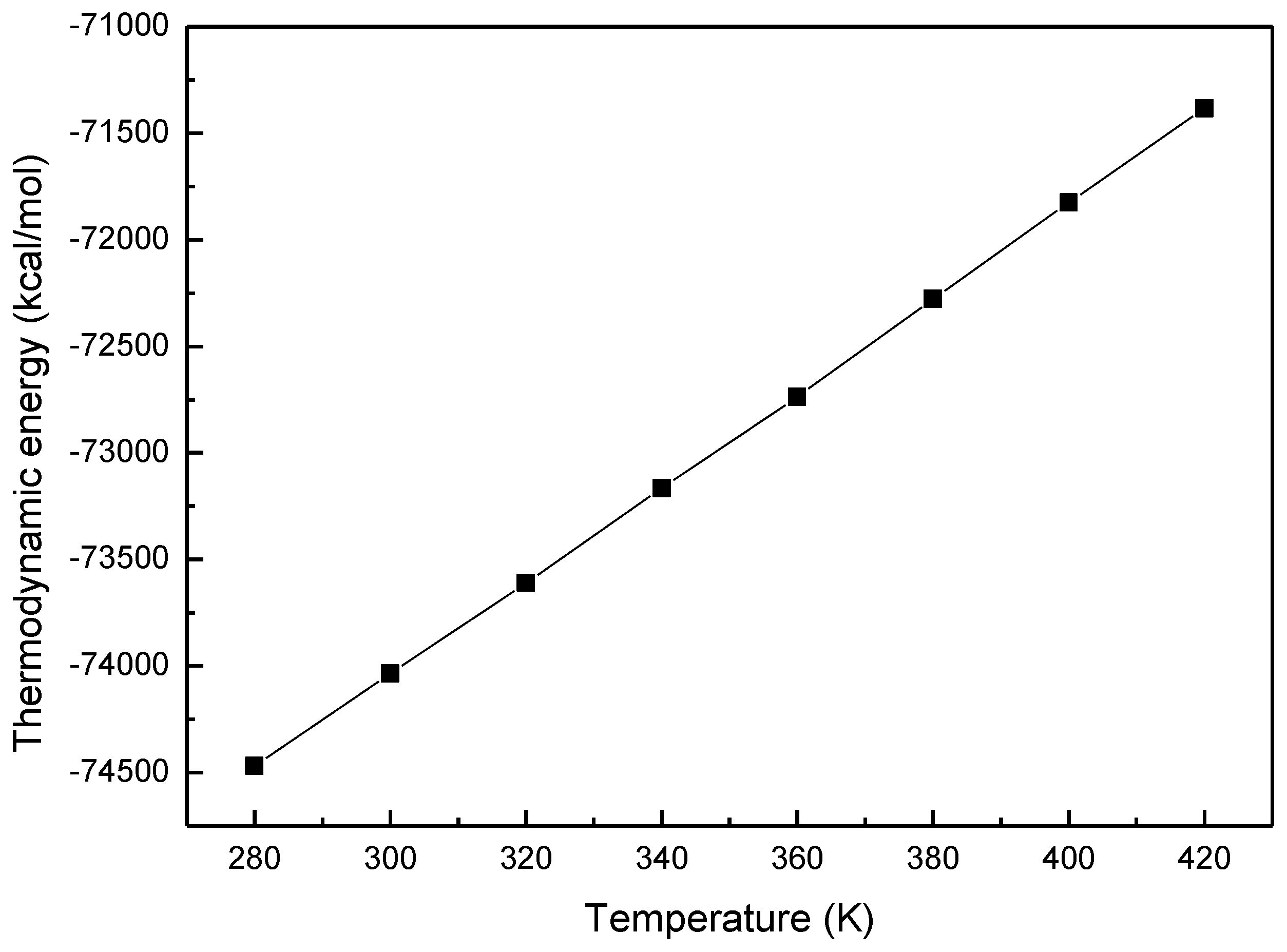
3.1. Thermodynamic Energy of MOF-5
3.2. Adsorption Isotherms and Enthalpy of Desorption
3.3. Thermal Energy Storage
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| ΔhMOHCs | The enthalpy of MOHCs (kJ/kg) |
| ΔhFluid | The enthalpy of pure organic fluid (kJ/kg) |
| Δhdesorption | The enthalpy of desorption (kJ/mol) |
| T | Temperature (K) |
| Cp | The heat capacity of MOFs (kcal/(mol·K)) |
| qst | The adsorption heat (kcal/(mol·K)) |
| x | The mass fraction of MOF in MOHCs |
| ρ | Density (g/cm3) |
Appendix A. Adsorption Isotherms Fitted by the Langmuir Equation
| Temperature (K) | Constant a | Constant b |
|---|---|---|
| 280 | 0.00690 | 21.027 |
| 300 | 0.00584 | 20.798 |
| 320 | 0.00564 | 20.567 |
| 340 | 0.00435 | 20.187 |
| 360 | 0.00364 | 19.670 |
| 380 | 0.00325 | 19.008 |
| 400 | 0.00299 | 18.340 |
| 420 | 0.00245 | 17.641 |
References
- Zhang, C.; Liu, C.; Wang, S.; Xu, X.; Li, Q. Thermo-economic comparison of subcritical organic Rankine cycle based on different heat exchanger configurations. Energy 2017, 123, 728–741. [Google Scholar] [CrossRef]
- Chan, C.W.; Ling-Chin, J.; Roskilly, A.P. A review of chemical heat pumps, thermodynamic cycles and thermal energy storage technologies for low grade heat utilisation. Appl. Therm. Eng. 2013, 50, 1257–1273. [Google Scholar] [CrossRef]
- Xuan, Y.; Li, Q. Heat Transfer Enhancement of Nanofluids. Int. J. Heat Fluid Flow 2000, 21, 58–64. [Google Scholar] [CrossRef]
- Choi, S.U.S.; Zhang, Z.G.; Yu, W.; Lockwood, F.E.; Grulke, E.A. Anomalous thermal conductivity enhancement in nanotube suspensions. Appl. Phys. Lett. 2001, 79, 2252–2254. [Google Scholar] [CrossRef]
- Li, Q.; Yu, Y.; Liu, Y.; Liu, C.; Lin, L. Thermal properties of the mixed n-octadecane/Cu nanoparticle nanofluids during phase transition: A molecular dynamics study. Materials 2017, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Celen, A.; Cebi, A.; Aktas, M.; Mahian, O.; Dalkilic, A.S.; Wongwises, S. A review of nanorefrigerants: Flow characteristics and applications. Int. J. Refrig. 2014, 44, 125–140. [Google Scholar] [CrossRef]
- Milanese, M.; Iacobazzi, F.; Colangelo, G.; Risi, A.D. An investigation of layering phenomenon at the liquid-solid interface in Cu and CuO based nanofluids. Int. J. Heat Mass Transf. 2016, 103, 564–571. [Google Scholar] [CrossRef]
- Iacobazzi, F.; Milanese, M.; Colangelo, G.; Lomascolo, M.; Risi, A.D. An explanation of the Al2O3 nanofluid thermal conductivity based on the phonon theory of liquid. Energy 2016, 116, 786–794. [Google Scholar] [CrossRef]
- Li, Q.; Liu, C. Molecular dynamics simulation of heat transfer with effects of fluid–lattice interactions. Int. J. Heat Mass Transf. 2012, 55, 8088–8092. [Google Scholar] [CrossRef]
- Li, Q.; Liu, C.; Chen, X. Molecular dynamics simulation of sulphur nucleation in S–H2S system. Mol. Phys. 2014, 112, 947–955. [Google Scholar] [CrossRef]
- Li, Q.; Xiao, Y.; Shi, X.; Song, S. Rapid evaporation of water on graphene/graphene-oxide: A molecular dynamics study. Nanomaterials 2017, 7, 265. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, B.; Liu, L. Nanoscale fluid mechanics and energy conversion. Appl. Mech. Rev. 2014, 66, 050803. [Google Scholar] [CrossRef]
- McGrail, B.P.; Thallapally, P.K.; Blanchard, J.; Nune, S.K.; Jenks, J.J.; Dang, L.X. Metal-organic heat carrier nanofluids. Nano Energy 2013, 2, 845–855. [Google Scholar] [CrossRef]
- Annapureddy, H.V.R.; Motkuri, R.K.; Nguyen, P.T.M.; Truong, T.B.; Thallapally, P.K.; McGrail, B.P.; Dang, L.X. Computational studies of adsorption in metal organic frameworks and interaction of nanoparticles in condensed phases. Mol. Simul. 2014, 40, 571–584. [Google Scholar] [CrossRef]
- James, S.L. Metal-organic frameworks. Chem. Soc. Rev. 2003, 32, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Kaye, S.S.; Daily, A.; Yaghi, O.M.; Long, J.R. Impact of preparation and handling on the hydrogen storage properties of Zn4O (1, 4-benzenedicarboxylate) 3 (MOF-5). J. Am. Chem. Soc. 2007, 129, 14176–14177. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.M.; Liu, J.; Xiao, K.; Harris, A.T. Microwave enhanced synthesis of MOF-5 and its CO2 capture ability at moderate temperatures across multiple capture and release cycles. Chem. Eng. J. 2010, 156, 465–470. [Google Scholar] [CrossRef]
- Getman, R.B.; Bae, Y.S.; Wilmer, C.E.; Snurr, R.Q. Review and analysis of molecular simulations of methane, hydrogen, and acetylene storage in metal-organic frameworks. Chem. Rev. 2012, 112, 703–723. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, D.; Smit, B. Understanding Molecular Simulation: From Algorithms to Applications, 2nd ed.; Academic Press: New York, NY, USA, 2002. [Google Scholar]
- Henninger, S.K.; Habib, H.A.; Janiak, C. MOFs as adsorbents for low temperature heating and cooling applications. J. Am. Chem. Soc. 2009, 131, 2776–2777. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Standards and Technology (NIST). Available online: http://webbook.nist.gov/chemistry/fluid/ (accessed on 15 February 2018).
- Li, Q.; Wang, M.; Liang, Y.; Lin, L.; Fu, T.; Wei, P.; Peng, T. Molecular dynamics simulations of aggregation of copper nanoparticles with different heating rates. Phys. E Low-Dimens. Syst. Nanostruct. 2017, 90, 137–142. [Google Scholar] [CrossRef]
- Lei, G.; Liu, C.; Li, Q.; Xu, X. Graphyne nanostructure as a potential adsorbent for separation of H2S/CH4 mixture: Combining grand canonical Monte Carlo simulations with ideal adsorbed solution theory. Fuel 2016, 182, 210–219. [Google Scholar] [CrossRef]
- Accelrys, I. Materials Studio; Accelrys Software Inc.: San Diego, CA, USA, 2010. [Google Scholar]
- Sun, H. COMPASS: An ab Initio force-field optimized for condensed-phase applicationsoverview with details on alkane and benzene compounds. J. Phys. Chem. B 1998, 102, 7338–7364. [Google Scholar] [CrossRef]
- Ewald, P.P. Die berechnung optischer und elektrostatischer gitterpotentiale. Ann. Phys. 1921, 369, 253–287. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Postma, J.P.M.; Gunsteren, W.F.V.; Dinola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Mu, B.; Walton, K.S. Thermal analysis and heat capacity study of metal–organic frameworks. J. Phys. Chem. C 2011, 115, 22748–22754. [Google Scholar] [CrossRef]

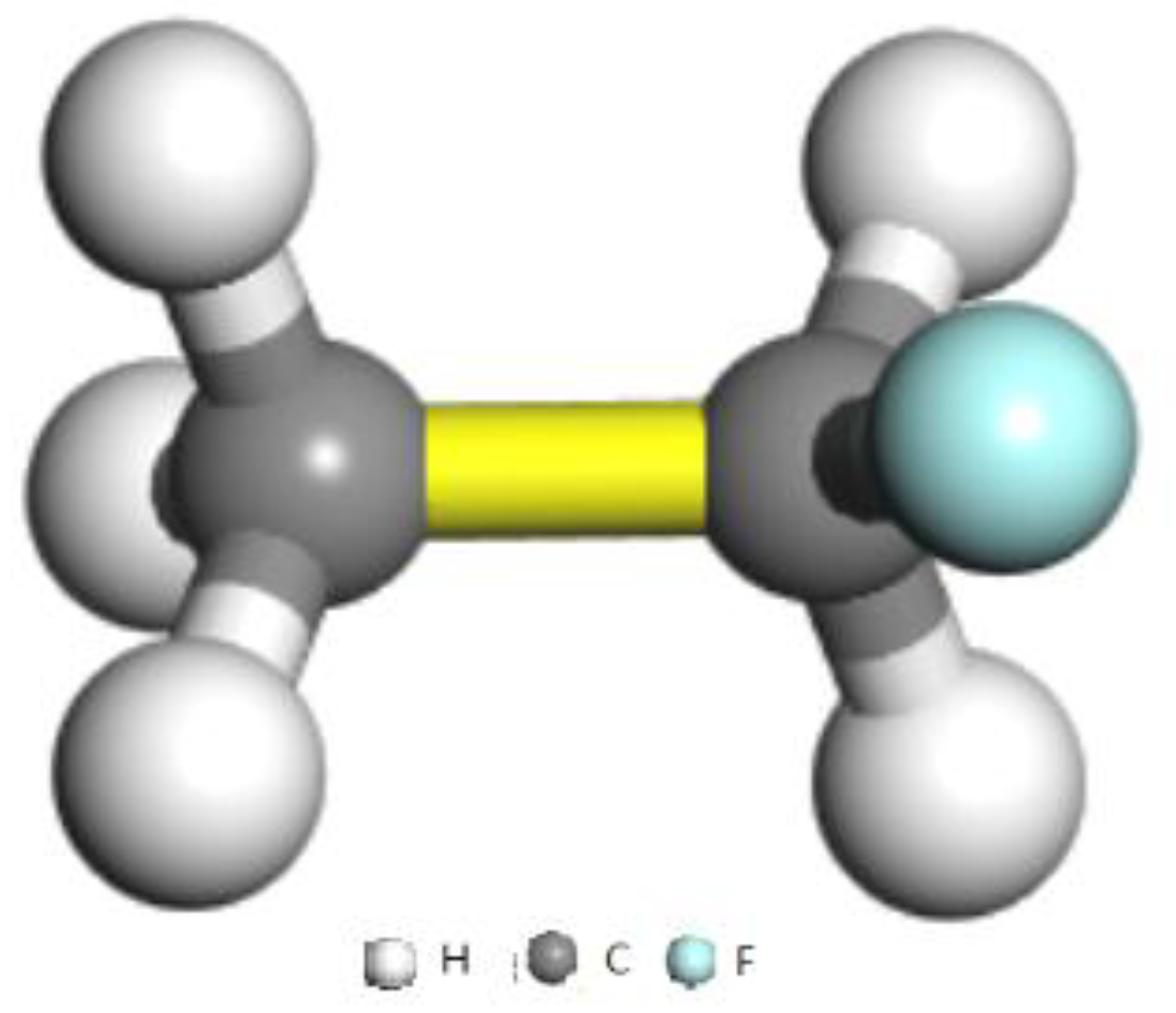




| C–C | GCMC280K | GCMC300K | GCMC320K | GCMC340K | GCMC360K | GCMC380K | GCMC400K | GCMC420K |
|---|---|---|---|---|---|---|---|---|
| 41.74 | 48.58 | 48.34 | 48.10 | 47.62 | 47.51 | 47.16 | 47.01 | 46.85 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Tang, S.; Li, L. Energy Storage Analysis of a Mixed R161/MOF-5 Nanoparticle Nanofluid Based on Molecular Simulations. Materials 2018, 11, 848. https://doi.org/10.3390/ma11050848
Wang Q, Tang S, Li L. Energy Storage Analysis of a Mixed R161/MOF-5 Nanoparticle Nanofluid Based on Molecular Simulations. Materials. 2018; 11(5):848. https://doi.org/10.3390/ma11050848
Chicago/Turabian StyleWang, Qiang, Shengli Tang, and Leilei Li. 2018. "Energy Storage Analysis of a Mixed R161/MOF-5 Nanoparticle Nanofluid Based on Molecular Simulations" Materials 11, no. 5: 848. https://doi.org/10.3390/ma11050848
APA StyleWang, Q., Tang, S., & Li, L. (2018). Energy Storage Analysis of a Mixed R161/MOF-5 Nanoparticle Nanofluid Based on Molecular Simulations. Materials, 11(5), 848. https://doi.org/10.3390/ma11050848




