Study on the Preparation of Plasma-Modified Fly Ash Catalyst and Its De–NOX Mechanism
Abstract
:1. Introduction
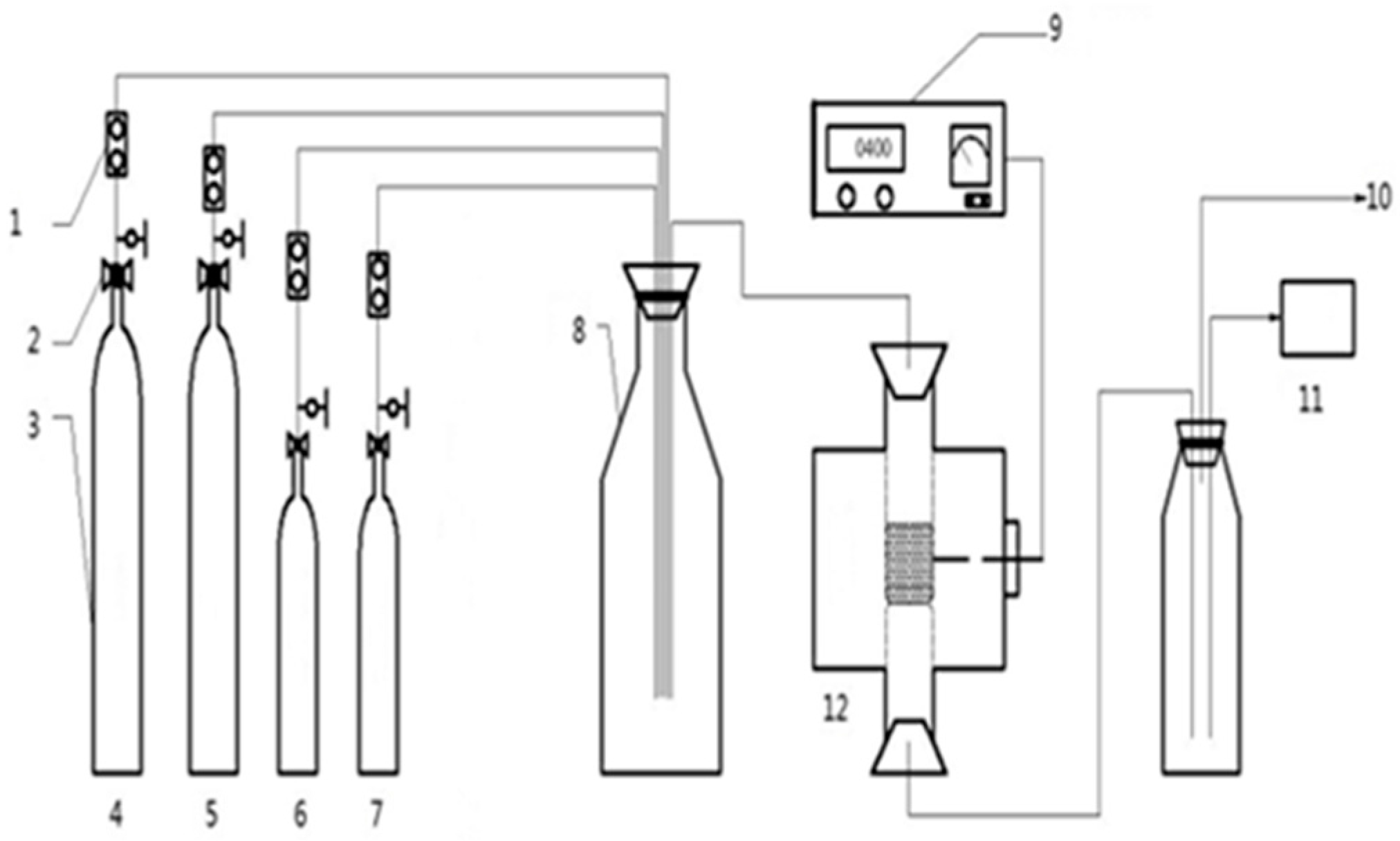
2. Materials and Methods
2.1. Materials
2.2. The Preparation of Catalysts and Activity Evaluation
3. Results
3.1. The Modification of the Fly Ash Catalyst with Different Gases
3.2. Parameters When Using Oxygen for Modification
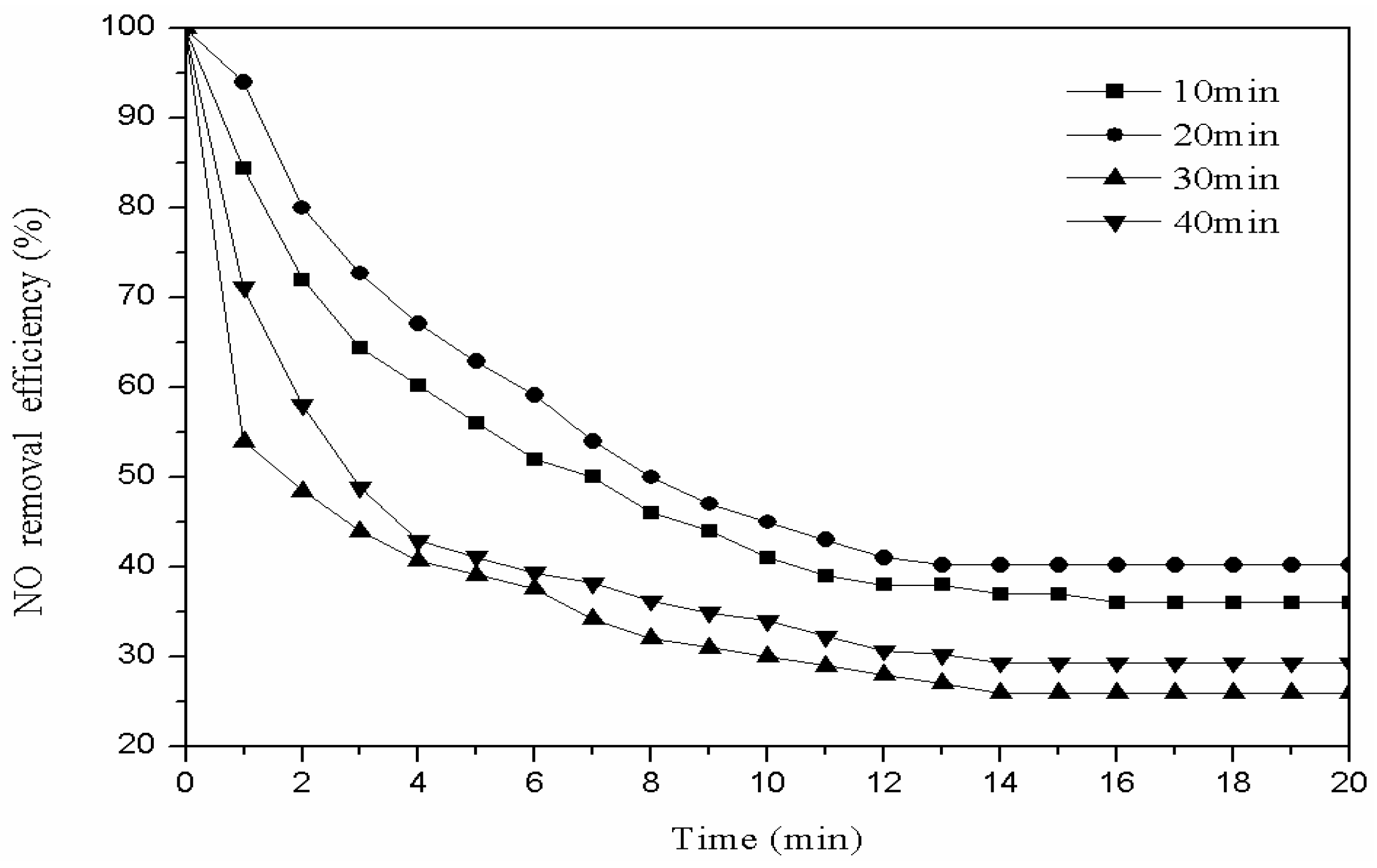
3.2.1. Oxygen Modification Time
3.2.2. Plasma Power of the Modification
3.2.3. Modification Gas Flow
3.3. Characterization
3.3.1. Characterization of Catalysts Prepared by Different Modification Time
3.3.2. Characterization of Catalysts Prepared with Different Modification Power Levels
3.3.3. Characterization of Catalysts Prepared by Different Gas Flows
4. Conclusions
- (1)
- The catalyst can be prepared by plasma modification technology, and the plasma power, time and gas flow of the modification have an effect on the denitration efficiency of the catalyst. The optimum plasma power is 60 W, the optimum modification time is 20 min, and the optimum gas flow rate is 40 mL/min.
- (2)
- The catalyst was modified by the plasma; the denitration effect of the catalyst modified by the plasma was significantly higher than that of the unmodified catalyst.
- (3)
- The different preparation conditions can make different functional groups and alter the efficiency of denitration. The effect of basic functional groups N=O on denitration was significant, followed by C=O. The effect of phenolic hydroxyl was not obvious, and may be synergistic with other functional groups.
Author Contributions
Funding
Conflicts of Interest
References
- Chen, Y.; Sun, L.; Yu, Z.; Wang, L.; Xiang, G.; Wan, S. Synergistic degradation performance and mechanism of 17β-estradiol by dielectric barrier discharge non-thermal plasma combined with Pt–TiO2 by dielectric barrier discharge non-thermal plasma combined with Pt–TiO2. Sep. Purif. Technol. 2015, 152, 46–54. [Google Scholar] [CrossRef]
- Lee, H.; Lim, T.H.; Kim, D.H. Complementary effect of plasma–catalysis hybrid system on methane complete oxidation over non-PGM catalysts. Catal. Commun. 2015, 69, 223–227. [Google Scholar] [CrossRef]
- Jõgia, I.; Stamate, E.; Irimiea, C.; Schmidt, M.; Brandenburg, R.; Hołub, M.; Bonisławski, M.; Jakubowski, T.; Kääriäinen, M.L.; Cameron, D.C. Comparison of direct and indirect plasma oxidation of NO combined with oxidation by catalyst. Fuel 2015, 144, 137–144. [Google Scholar] [CrossRef]
- Oda, T.; Kato, T.; Takahashi, T.; Shimizu, K. Nitric oxide decomposition in air by using nonthermal plasma processing with additives and catalyst. J. Electrost. 1997, 42, 151–157. [Google Scholar] [CrossRef]
- Park, S.Y.; Deshwal, B.R.; Moon, S.H. NOX removal from the flue gas of oil-fired boiler using multistage plasma-catalyst hybrid system. Fuel Process. Technol. 2008, 89, 540–548. [Google Scholar] [CrossRef]
- Zhao, Y. Research on mineralogical properties of fly ash. Clean Coal Technol. 2015, 21, 112–116. [Google Scholar] [CrossRef]
- Wang, M.; Xu, L.; Liu, F.; Wang, Y.; Ye, Q.; Yang, S.; Liu, T. Investigation on the effect of denitrified fly ash on the properties of cement. New Build. Mater. 2011, 38, 12–14. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Wei, Z.; Kollarb, M.; Gao, F.; Wang, Y.; Szanyi, J.; Peden, C.H. A comparative study of N2O formation during the selective catalytic reduction of NOX with NH3 on zeolite supported Cu catalysts. J. Catal. 2015, 329, 490–498. [Google Scholar] [CrossRef]
- Jiang, L.; Zeng, Z.; Tang, X. Preparation of Fly Ash Loading Calcium-based Sorbent. Guangzhou Chem. Ind. 2009, 37, 86–88. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, Y.L.; Wang, S.M. Experimental study and application of fly ash-desulfurization gypsum double-blended mortar. J. Changsha Univ. Sci. Technol. (Nat. Sci.) 2010, 7, 48–52. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, B.; Zhuo, Y.; Chen, C.; Xu, X. Effect of fly ash on the denitrification performance of NH3 at moderate temperatures. J. Tsinghua Univ (Sci. Technol.) 2011, 51, 667–671. [Google Scholar] [CrossRef]
- Nie, W.J.; Sha, X.L.; Zhang, L.; Wang, Y.S.; Wen, X.; Li, Y.H. Research on the denitration mechanism of fly ash catalysts modified by low temperature plasma technology. AIP Adv. 2017, 7, 085206. [Google Scholar] [CrossRef]
- Chen, F.; Liu, S.; Liu, J.Y.; Huang, S.; Xia, G.; Song, J.; Xu, W.; Sun, J.; Liu, X. Surface modification of tube inner wall by transferred atmospheric pressure plasma. Appl. Surf. Sci. 2016, 389, 967–976. [Google Scholar] [CrossRef]
- Zhang, Y.-P.; Wang, X.L. WO3 modification on MnOx/TiO2 low-temperature De-NOx SCR catalyst. J. Fuel Chem. Technol. 2011, 39, 782–786. [Google Scholar] [CrossRef]
- Li, Z.; Niu, Y.Y.; Fu, T.J.; Li-Hua, Q.F. Influence of Modification of Activated Carbon Surface on Cu Species and Catalytic Activity of Cu/AC Catalyst. Chin. J. Inorg. Chem. 2011, 27, 1277–1284. [Google Scholar]
- Jiang, Y.; Gao, X.; Wu, W.; Zhang, Y. Review of the Deactivation of Selective Catalytic Reduction DeNOx Catalysts. Proc. CSEE 2013, 33, 18–31. [Google Scholar] [CrossRef]
- Hegde, M.S.; Bera, P. Noble metal ion substituted CeO2 catalysts: Electronic interaction between noble metal ions and CeO2 lattice. Catal. Today 2015, 253, 40–50. [Google Scholar] [CrossRef]
- Zhang, F.; Ouyang, P.; Zhang, X.M. Progress of research on modification and adsorption of fly ash. Appl. Chem. Ind. 2016, 45, 747–750. [Google Scholar] [CrossRef]
- Duan, A.; Li, T.; Niu, H.; Yang, X.; Wang, Z.; Zhao, Z.; Jiang, G.; Liu, J.; Wei, Y.; Pan, H. Synthesis of a novel zeolite W and application in the catalyst for FCC gasoline hydro-upgrading. Catal. Today 2015, 245, 163–171. [Google Scholar] [CrossRef]
- Li, F.F.; Liu, D.R.; Gao, G.M.; Xue, B.; Jiang, Y.S. Improved visible-light photo catalytic activity of NaTaO3 with perovskite-like structure via sulfur anion doping. Appl. Catal. B Environ. 2015, 166–167, 104–111. [Google Scholar] [CrossRef]
- Shin, H.S.; Jo, C.; Ko, S.H.; Ryoo, R. Mesoporous titania with anatase framework synthesized using polyphenolic structure-directing agent: Synthesis domain and catalytic metal loading. Microporous Mesoporous Mater. 2015, 212, 117–124. [Google Scholar] [CrossRef]
- Li, K.; Liu, G.; Gao, T.; Lu, F.; Tang, L.; Liu, S.; Ning, P. Surface modification of Fe/MCSAC catalysts with coaxial cylinder dielectric barrier discharge plasma for low-temperature catalytic hydrolysis of CS2. Appl. Catal. A Gen. 2016, 527, 171–181. [Google Scholar] [CrossRef]
- Rezaei, F.; Shokri, B.; Sharifian, M. Atmospheric-pressure DBD plasma-assisted surface modification of polymethyl methacrylate: A study on cell growth/proliferation and antibacterial properties. Appl. Surf. Sci. 2016, 360, 641–651. [Google Scholar] [CrossRef]






| Metal Type | Sample Mass | Nitrogen Content/% | Carbon Content/% | Hydrogen Content/% | C/N |
|---|---|---|---|---|---|
| Fly ash | 3.726 | 0.04 | 2.191 | 0.14 | 54.37 |
| bentonite | 3.769 | 0.016 | 0.174 | 1.678 | 10.93 |
| Metal Type | >5 ppm | 1~5 ppm | 0.5~1 ppm | 0.1~0.5 ppm | <0.1 ppm | Metal Number |
|---|---|---|---|---|---|---|
| Fly ash | K Mg | Ba Mn Sr | Cr | Cu Nb Ni Ru U V Zn | Be Cd and other 19 kinds | 32 |
| bentonite | K Mg | none | Ba Sr Zn | Ce Cr Cu Mn | Pb Ru Be Dy and other 20 kinds | 31 |
| Sample | Acid Functional Groups | Basic Functional Groups | Phenolic Hydroxyl Groups | Carboxyl |
|---|---|---|---|---|
| 10 min | 3.6000 | 0.0000 | 0.0000 | 0.0172 |
| 20 min | 0.4500 | 2.8000 | 0.0035 | 0.0033 |
| 30 min | 3.6500 | 0.0500 | 0.0082 | 0.0075 |
| 40 min | 3.8000 | 0.0000 | 0.0107 | 0.0078 |
| Sample | Acid Functional Groups | Basic Functional Groups | Phenolic Hydroxyl Groups | Carboxyl |
|---|---|---|---|---|
| 30 W | 0.1700 | 2.3800 | 0.0054 | 0.0076 |
| 60 W | 0.4500 | 2.8000 | 0.0035 | 0.0033 |
| 90 W | 0.1000 | 2.4500 | 0.0000 | 0.0170 |
| Sample | Acid Functional Groups | Basic Functional Groups | Phenolic Hydroxyl Groups | Carboxyl |
|---|---|---|---|---|
| 20 mL/min | 4.3000 | 0.0000 | 0.0055 | 0.0072 |
| 40 mL/min | 0.4500 | 2.8000 | 0.0035 | 0.0033 |
| 60 mL/min | 4.4500 | 0.0000 | 0.0100 | 0.0087 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Wen, X.; Zhang, L.; Sha, X.; Wang, Y.; Chen, J.; Luo, M.; Li, Y. Study on the Preparation of Plasma-Modified Fly Ash Catalyst and Its De–NOX Mechanism. Materials 2018, 11, 1047. https://doi.org/10.3390/ma11061047
Zhang L, Wen X, Zhang L, Sha X, Wang Y, Chen J, Luo M, Li Y. Study on the Preparation of Plasma-Modified Fly Ash Catalyst and Its De–NOX Mechanism. Materials. 2018; 11(6):1047. https://doi.org/10.3390/ma11061047
Chicago/Turabian StyleZhang, Lei, Xin Wen, Lei Zhang, Xiangling Sha, Yusu Wang, Jihao Chen, Min Luo, and Yonghui Li. 2018. "Study on the Preparation of Plasma-Modified Fly Ash Catalyst and Its De–NOX Mechanism" Materials 11, no. 6: 1047. https://doi.org/10.3390/ma11061047
APA StyleZhang, L., Wen, X., Zhang, L., Sha, X., Wang, Y., Chen, J., Luo, M., & Li, Y. (2018). Study on the Preparation of Plasma-Modified Fly Ash Catalyst and Its De–NOX Mechanism. Materials, 11(6), 1047. https://doi.org/10.3390/ma11061047




