Large Area Synthesis of Vertical Aligned Metal Oxide Nanosheets by Thermal Oxidation of Stainless Steel Mesh and Foil
Abstract
:1. Introduction
2. Experimental Details
2.1. Growth of Vertical-Oriented Metal Oxide Nanosheets on Stainless Steel
2.2. Materials Characterization
3. Results and Discussion
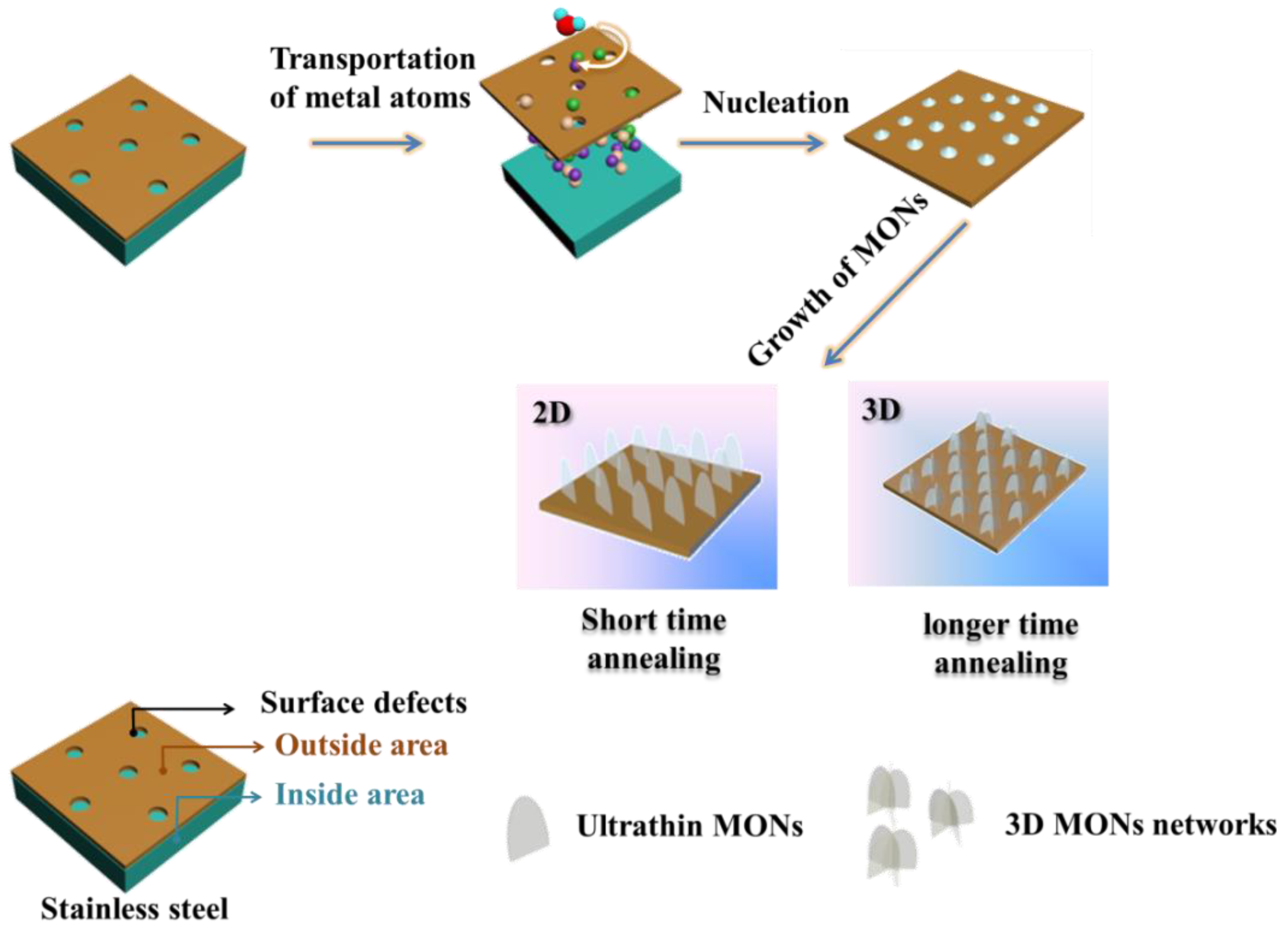
3.1. Effect of Temperature on the Synthesis of MONs
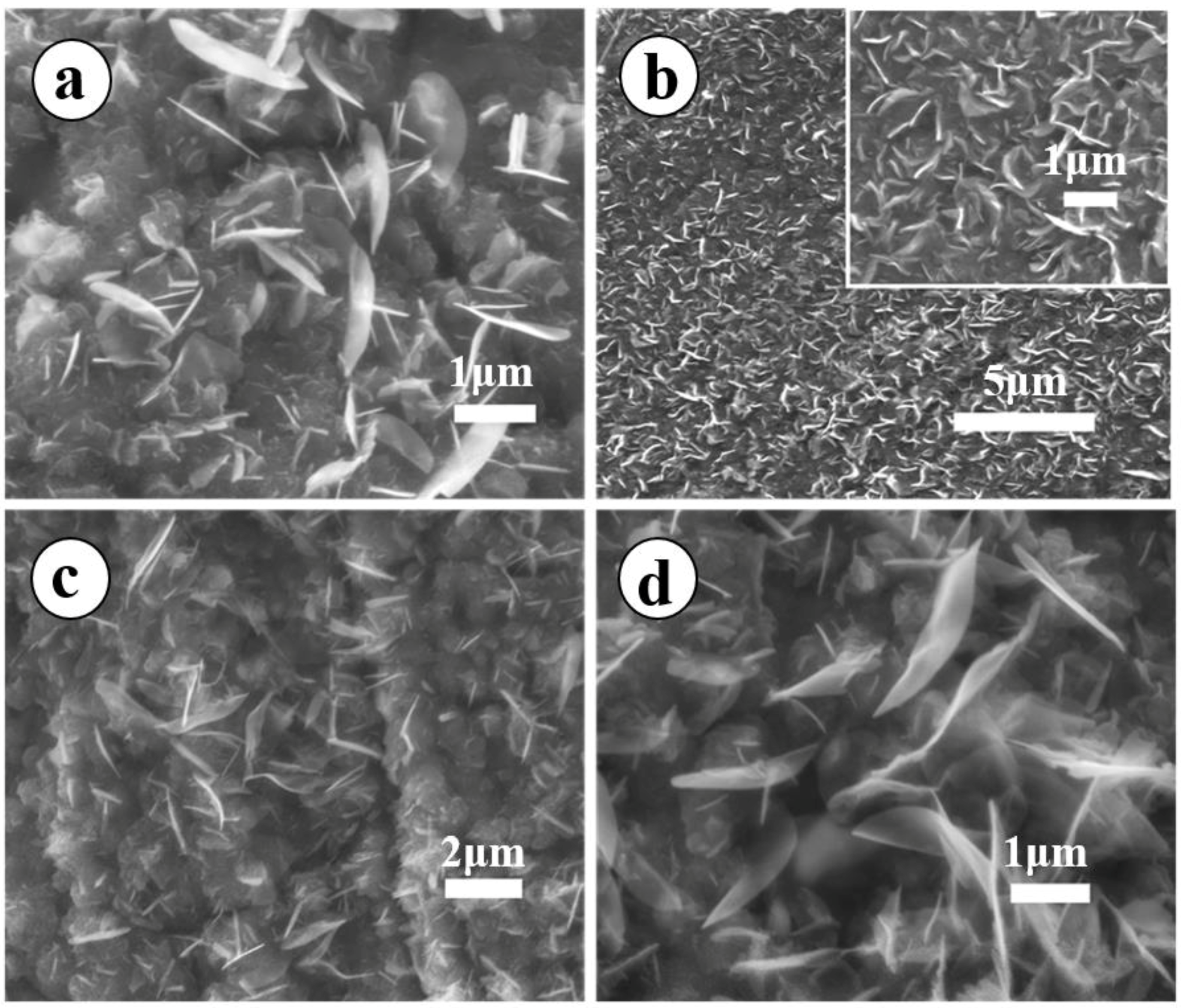
3.2. Dependence of MONs Morphology on the Reaction Time
3.3. Microstructure Analysis of the Samples
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Emeline, A.; Kataeva, G.; Panasuk, A.; Ryabchuk, V.; Sheremetyeva, N.; Serpone, N. Effect of surface photoreactions on the photocoloration of a wide band gap metal oxide: Probing whether surface reactions are photocatalytic. J. Phys. Chem. B 2005, 109, 5175–5185. [Google Scholar] [CrossRef] [PubMed]
- Kröger, M.; Hamwi, S.; Meyer, J.; Riedl, T.; Kowalsky, W.; Kahn, A. P-type doping of organic wide band gap materials by transition metal oxides: A case-study on Molybdenum trioxide. Org. Electron. 2009, 10, 932–938. [Google Scholar] [CrossRef]
- Chen, K.; Bell, A.T.; Iglesia, E. The relationship between the electronic and redox properties of dispersed metal oxides and their turnover rates in oxidative dehydrogenation reactions. J. Catal. 2002, 209, 35–42. [Google Scholar] [CrossRef]
- Sysoev, V.V.; Button, B.K.; Wepsiec, K.; Dmitriev, S.; Kolmakov, A. Toward the nanoscopic “electronic nose”: Hydrogen vs carbon monoxide discrimination with an array of individual metal oxide nano-and mesowire sensors. Nano Lett. 2006, 6, 1584–1588. [Google Scholar] [CrossRef] [PubMed]
- Mavrou, G.; Galata, S.; Tsipas, P.; Sotiropoulos, A.; Panayiotatos, Y.; Dimoulas, A.; Evangelou, E.; Seo, J.W.; Dieker, C. Electrical properties of La2O3 and HfO2/La2O3 gate dielectrics for germanium metal-oxide-semiconductor devices. J. Appl. Phys. 2008, 103, 014506. [Google Scholar] [CrossRef]
- Lee, M.J.; Han, S.; Jeon, S.H.; Park, B.H.; Kang, B.S.; Ahn, S.E.; Kim, K.H.; Lee, C.B.; Kim, C.J.; Yoo, I.K. Electrical manipulation of nanofilaments in transition-metal oxides for resistance-based memory. Nano Lett. 2009, 9, 1476–1481. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Zhang, Z.; Zhu, M. Melting and optical properties of ZnO nanorods. Appl. Phys. Lett. 2006, 88, 061913. [Google Scholar] [CrossRef]
- Dev, A.; Richters, J.; Sartor, J.; Kalt, H.; Gutowski, J.; Voss, T. Enhancement of the near-band-edge photoluminescence of ZnO nanowires: Important role of hydrogen incorporation versus plasmon resonances. Appl. Phys. Lett. 2011, 98, 131111. [Google Scholar] [CrossRef]
- Wu, M.S.; Lee, R.H. Electrochemical growth of iron Oxide thin films with nanorods and nanosheets for capacitors. J. Electrochem. Soc. 2009, 156, A737–A743. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Baruwati, B.; Varma, R.S. Self-assembly of metal oxides into three-dimensional nanostructures: Synthesis and application in catalysis. ACS Nano 2009, 3, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.F.; Liu, S.B.; Meng, F.L.; Liu, J.Y.; Jin, Z.; Kong, L.T.; Liu, J.H. Metal oxide nanostructures and their gas sensing properties: A review. Sensors 2012, 12, 2610–2631. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Wu, H.B.; Xie, Y.; Lou, X.W. Mixed transition-metal oxides: Design, synthesis, and energy-related applications. Angew. Chem. Int. Ed. 2014, 53, 1488–1504. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Huang, X.; Tan, C.; Zhang, H. Thin metal nanostructures: Synthesis, properties and applications. Chem. Sci. 2015, 6, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Wiley, B.; Sun, Y.; Chen, J.; Cang, H.; Li, Z.Y.; Li, X.; Xia, Y. Shape-controlled synthesis of silver and gold nanostructures. Mrs Bull. 2005, 30, 356–361. [Google Scholar] [CrossRef]
- Ling, T.; Wang, J.J.; Zhang, H.; Song, S.T.; Zhou, Y.Z.; Zhao, J.; Du, X.W. Freestanding ultrathin metallic nanosheets: Materials, synthesis, and applications. Adv. Mater. 2015, 27, 5396–5402. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, H.; Lu, K. Strain-induced ultrahard and ultrastable nanolaminated structure in nickel. Science 2013, 342, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Summers, C.J.; Wang, Z.L. Large-scale hexagonal-patterned growth of aligned ZnO nanorods for nano-optoelectronics and nanosensor arrays. Nano Lett. 2004, 4, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.R.; Lin, C.M.; Yeh, C.L.; Huang, R.T. Synthesis and characterization of one-dimensional WO2 nanorods. J. Vac. Sci. Technol. B 2005, 23, 2141–2145. [Google Scholar] [CrossRef]
- Wang, Z.L.; Song, J. Piezoelectric nanogenerators based on zinc oxide nanowire arrays. Science 2006, 312, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ding, Y.; Deng, S.Z.; Gong, L.; Xu, N.S.; Wang, Z.L. Three-dimensional tungsten oxide nanowire networks. Adv. Mater. 2005, 17, 2107–2110. [Google Scholar] [CrossRef]
- Park, C.J.; Choi, D.K.; Yoo, J.; Yi, G.C.; Lee, C.J. Enhanced field emission properties from well-aligned zinc oxide nanoneedles grown on the Au/Ti/n-Si substrate. Appl. Phys. Lett. 2007, 90. [Google Scholar] [CrossRef]
- Ma, H.; Qin, Z.; Wang, Z.; Ahmad, M.; Sun, H. Enhanced field emission of ZnO nanoneedle arrays via solution etching at room temperature. Mater. Lett. 2017, 206, 162–165. [Google Scholar] [CrossRef]
- Liu, S.; Gan, L.; Liu, L.; Zhang, W.; Zeng, H. Synthesis of single-crystalline TiO2 nanotubes. Chem. Mater. 2002, 14, 1391–1397. [Google Scholar] [CrossRef]
- Liu, X.C.; Piao, J.Y.; Bin, D.S.; Zhang, T.Q.; Duan, S.Y.; Wu, Z.X.; Cao, A.M.; Wan, L.J. Controlled formation of uniform nanoshells of manganese oxide and their potential in lithium ion batteries. Chem. Commun. 2017, 53, 2846–2849. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Li, J.; Hou, L.; Zhang, X.; Shen, L.; Lou, X.W. Ultrathin mesoporous NiCo2O4 nanosheets supported on Ni foam as advanced electrodes for supercapacitors. Adv. Funct. Mater. 2012, 22, 4592–4597. [Google Scholar] [CrossRef]
- Liu, H.L.; Nosheen, F.; Wang, X. Noble metal alloy complex nanostructures: Controllable synthesis and their electrochemical property. Chem. Soc. Rev. 2015, 44, 3056–3078. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Yan, N.; Yu, R.; Chang, C.R.; Zhou, G.; Hu, H.S.; Rong, H.; Niu, Z.; Mao, J.; Asakura, H. Ultrathin rhodium nanosheets. Nat. Commun. 2014, 5, 3093. [Google Scholar] [CrossRef] [PubMed]
- Zhan, L.; Chen, H.; Fang, J.; Wang, S.; Ding, L.X.; Li, Z.; Ashman, P.J.; Wang, H. Coaxial Co3O4@ polypyrrole core-shell nanowire arrays for high performance lithium ion batteries. Electrochim. Acta 2016, 209, 192–200. [Google Scholar] [CrossRef]
- Zhu, S.; Li, J.; Deng, X.; He, C.; Liu, E.; He, F.; Shi, C.; Zhao, N. Ultrathin-nanosheet-induced synthesis of 3D transition metal oxides networks for lithium ion battery anodes. Adv. Funct. Mater. 2017, 27, 1605017. [Google Scholar] [CrossRef]
- Wu, M.S.; Ou, Y.H.; Lin, Y.P. Iron oxide nanosheets and nanoparticles synthesized by a facile single-step coprecipitation method for lithium-ion batteries. J. Electrochem. Soc. 2011, 158, A231–A236. [Google Scholar] [CrossRef]
- Zhu, Y.; Cao, C.; Tao, S.; Chu, W.; Wu, Z.; Li, Y. Ultrathin nickel hydroxide and oxide nanosheets: Synthesis, characterizations and excellent supercapacitor performances. Sci. Rep. 2014, 4, 5787. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.M.; Zheng, X. Rapid catalyst-free flame synthesis of dense, aligned α-Fe2O3 nanoflake and CuO nanoneedle arrays. Nano Lett. 2009, 9, 3001–3006. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Deng, B.; Sassman, S.A.; Hu, Y.; Suslov, S.; Cheng, G.J. Magnetic field assisted growth of highly dense α-Fe2O3 single crystal nanosheets and their application in water treatment. RSC Adv. 2014, 4, 18621–18626. [Google Scholar] [CrossRef]
- Deshmukh, P.R.; Sohn, Y.; Shin, W.G. Flexible solid-state symmetric supercapacitor based on (Fe,Cr)2O3 oxide layer developed on the stainless steel mesh. ACS Sustain. Chem. Eng. 2017, 6, 300–310. [Google Scholar] [CrossRef]
- Gund, G.S.; Dubal, D.P.; Chodankar, N.R.; Cho, J.Y.; Gomez-Romero, P.; Park, C.; Lokhande, C.D. Low-cost flexible supercapacitors with high-energy density based on nanostructured MnO2 and Fe2O3 thin films directly fabricated onto stainless steel. Sci. Rep. 2015, 5, 12454. [Google Scholar] [CrossRef] [PubMed]
- Takagi, R. Growth of oxide whiskers on metals at high temperature. J. Phys. Soc. Jpn. 1957, 12, 1212–1218. [Google Scholar] [CrossRef]






| C | N | Si | Mn | S | P | Cu | Co | B | Ni | Cr | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.035 | ≤0.08 | ≤1.00 | ≤2.00 | ≤0.015 | ≤0.03 | ≤1.00 | ≤0.06 | ≤0.0018 | 9.00–10.00 | 18.5–20.00 | Bal. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, F.; Wang, C.; Wu, M.H.; Vinodgopal, K.; Dai, G.-P. Large Area Synthesis of Vertical Aligned Metal Oxide Nanosheets by Thermal Oxidation of Stainless Steel Mesh and Foil. Materials 2018, 11, 884. https://doi.org/10.3390/ma11060884
Wu F, Wang C, Wu MH, Vinodgopal K, Dai G-P. Large Area Synthesis of Vertical Aligned Metal Oxide Nanosheets by Thermal Oxidation of Stainless Steel Mesh and Foil. Materials. 2018; 11(6):884. https://doi.org/10.3390/ma11060884
Chicago/Turabian StyleWu, Fan, Chen Wang, Marvin H. Wu, Kizhanipuram Vinodgopal, and Gui-Ping Dai. 2018. "Large Area Synthesis of Vertical Aligned Metal Oxide Nanosheets by Thermal Oxidation of Stainless Steel Mesh and Foil" Materials 11, no. 6: 884. https://doi.org/10.3390/ma11060884
APA StyleWu, F., Wang, C., Wu, M. H., Vinodgopal, K., & Dai, G.-P. (2018). Large Area Synthesis of Vertical Aligned Metal Oxide Nanosheets by Thermal Oxidation of Stainless Steel Mesh and Foil. Materials, 11(6), 884. https://doi.org/10.3390/ma11060884






