Fabrication of Superhydrophobic Mg/Al Layered Double Hydroxide (LDH) Coatings on Medium Density Fiberboards (MDFs) with Flame Retardancy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Medium Density Fiberboards
2.3. Preparation of FDTS-Modified Mg/Al LDH
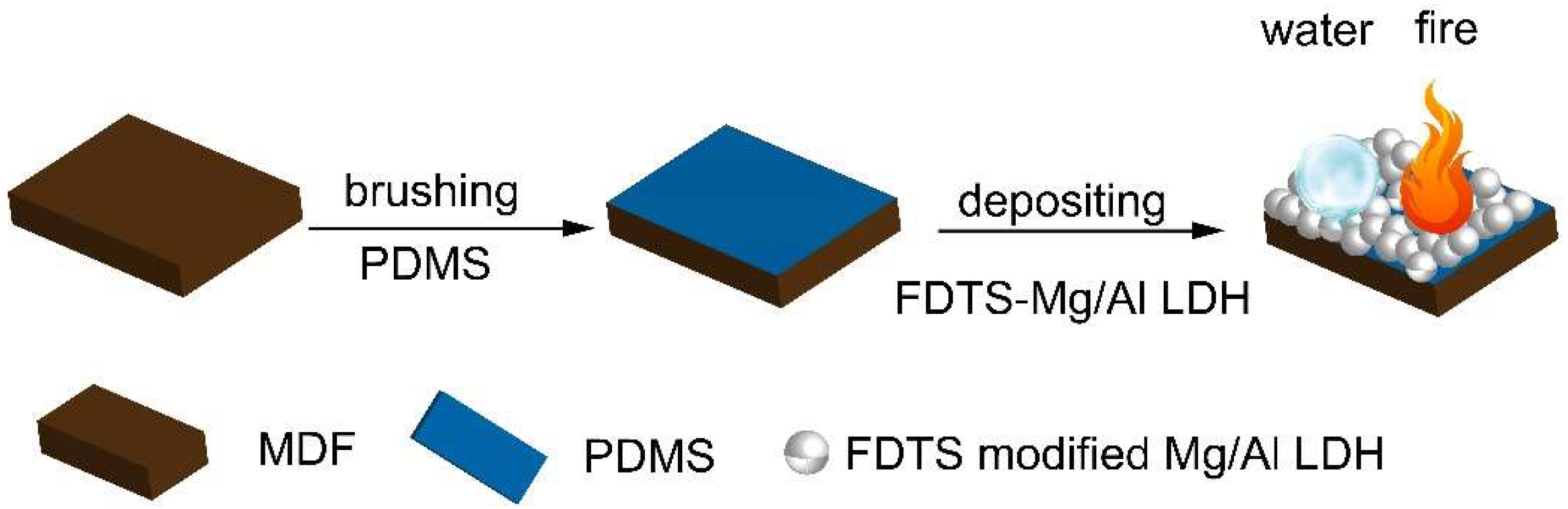
2.4. Preparation of PDMS@FDTS-Mg/Al LDH Coating on MDF
2.5. Characterization
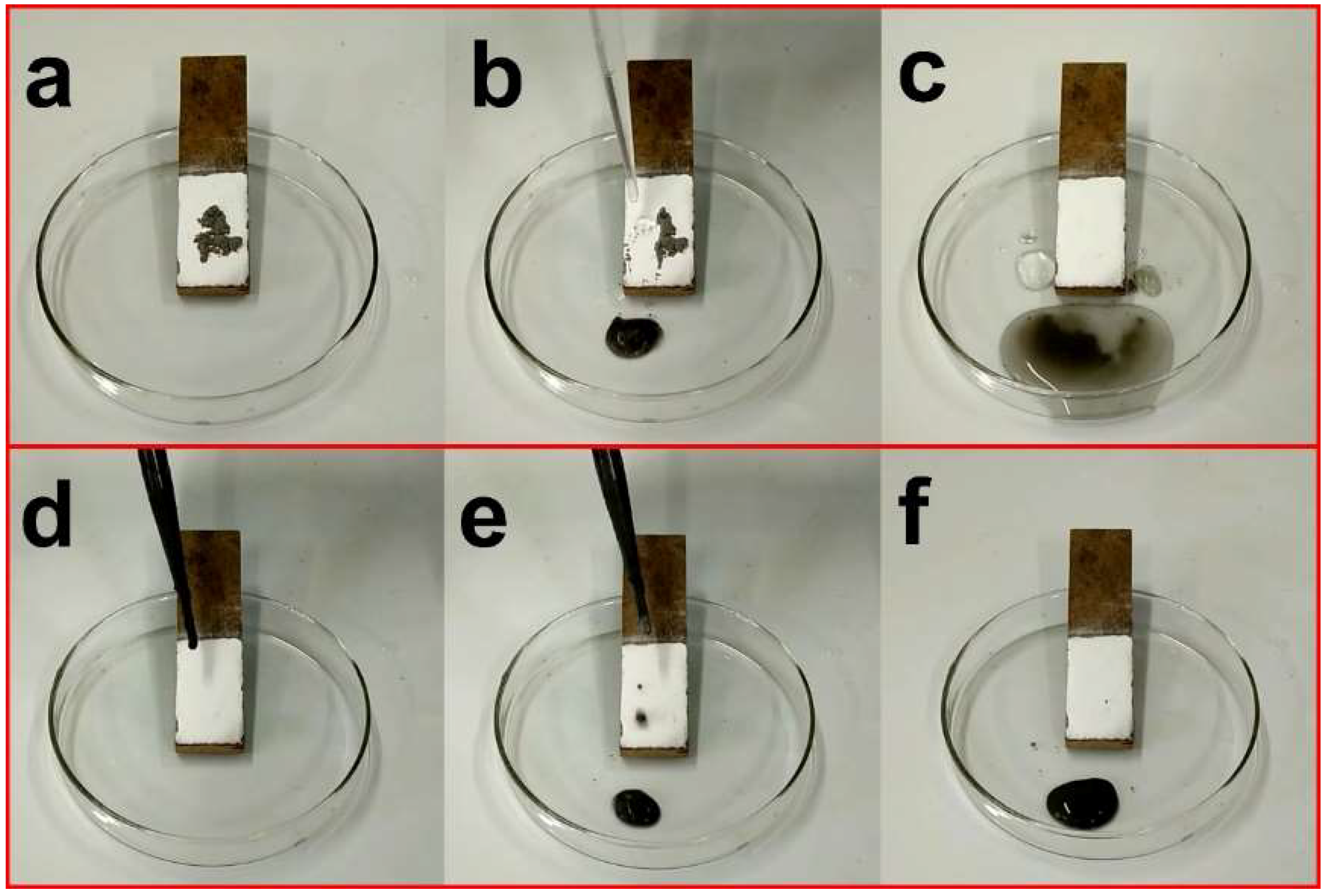
3. Results and Discussion
3.1. XPS Analysis
3.2. FTIR and XRD Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ji, X.; Li, B.; Yuan, B.; Guo, M. Preparation and characterizations of a chitosan-based medium-density fiberboard adhesive with high bonding strength and water resistance. Carbohydr. Polym. 2017, 176, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Li, D.L.; Ge, S.B.; Peng, W.X.; Wu, Q.D.; Wu, J.G. Chemical structure characteristics of wood/lignin composites during mold pressing. Polym. Compos. 2017, 38, 955–965. [Google Scholar] [CrossRef]
- Ji, X.; Guo, M. Facile surface hydrophobization of medium-density fiberboard (MDF) by silver deposition. Holzforschung 2017, 71, 337–340. [Google Scholar] [CrossRef]
- Hashim, R.; How, L.S.; Kumar, R.N.; Sulaiman, O. Some of the properties of flame retardant medium density fiberboard made from rubberwood and recycled containers containing aluminum trihydroxide. Bioresour. Technol. 2005, 96, 1826–1831. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yan, Y.; Shen, X.; Qian, T.; Wang, J.; Sun, Q.; Jin, C. Lignocellulose-chitosan-multiwalled carbon nanotube composites with improved mechanical strength, dimensional stability and fire retardancy. Polymers 2018, 10, 341. [Google Scholar] [CrossRef]
- Grigsby, W.J.; Thumm, A. Resin and wax distribution and mobility during medium density fibreboard manufacture. Eur. J. Wood Wood Prod. 2011, 70, 337–348. [Google Scholar] [CrossRef]
- Roffael, E.; Schneider, T.; Dix, B.; Buchholz, T. On paraffin sizing of medium density fiberboards (MDF). Part 1: Influence of the chemical composition of paraffin and type of emulsifier on the hydrophobic properties of MDF. Holz als Roh- und Werkstoff 2005, 63, 192–203. [Google Scholar] [CrossRef]
- Xu, X.; Yao, F.; Wu, Q.; Zhou, D. The influence of wax-sizing on dimension stability and mechanical properties of bagasse particleboard. Ind. Crops Prod. 2009, 29, 80–85. [Google Scholar] [CrossRef]
- Grigsby, W.; Thumm, A. The interactions between wax and UF resin in medium density fibreboard. Eur. J. Wood Wood Prod. 2011, 70, 507–517. [Google Scholar] [CrossRef]
- Wu, Y.; Jia, S.; Wang, S.; Qing, Y.; Yan, N.; Wang, Q.; Meng, T. A facile and novel emulsion for efficient and convenient fabrication of durable superhydrophobic materials. Chem. Eng. J. 2017, 328, 186–196. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, F.; Qian, H.; Wang, H.; Mu, L.; Zhu, J. A biomimetic spherical cactus superhydrophobic coating with durable and multiple anti-corrosion effects. Chem. Eng. J. 2018, 338, 670–679. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Z.; Li, Y.; Men, X. An eco-friendly one-step method to fabricate superhydrophobic nanoparticles with hierarchical architectures. Chem. Eng. J. 2017, 327, 530–538. [Google Scholar] [CrossRef]
- Wu, Y.; Jia, S.; Qing, Y.; Luo, S.; Liu, M. A versatile and efficient method to fabricate durable superhydrophobic surfaces on wood, lignocellulosic fiber, glass, and metal substrates. J. Mater. Chem. A 2016, 4, 14111–14121. [Google Scholar] [CrossRef]
- Wang, K.; Dong, Y.; Yan, Y.; Zhang, S.; Li, J. Mussel-inspired chemistry for preparation of superhydrophobic surfaces on porous substrates. RSC Adv. 2017, 7, 29149–29158. [Google Scholar] [CrossRef] [Green Version]
- Long, M.; Peng, S.; Deng, W.; Miao, X.; Wen, N.; Zhou, Q.; Yang, X.; Deng, W. A robust superhydrophobic PDMS@ZnSn(OH)6 coating with under-oil self-cleaning and flame retardancy. J. Mater. Chem. A 2017, 5, 22761–22771. [Google Scholar] [CrossRef]
- Wang, S.; Liu, C.; Liu, G.; Zhang, M.; Li, J.; Wang, C. Fabrication of superhydrophobic wood surface by a sol–gel process. Appl. Surf. Sci. 2011, 258, 806–810. [Google Scholar] [CrossRef]
- Tu, K.; Wang, X.; Kong, L.; Guan, H. Facile preparation of mechanically durable, self-healing and multifunctional superhydrophobic surfaces on solid wood. Mater. Des. 2018, 140, 30–36. [Google Scholar] [CrossRef]
- Kong, L.; Tu, K.; Guan, H.; Wang, X. Growth of high-density ZnO nanorods on wood with enhanced photostability, flame retardancy and water repellency. Appl. Surf. Sci. 2017, 407, 479–484. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, H.; Yao, Q.; Fan, B.; Wang, C.; Xiong, Y.; Jin, C.; Sun, Q. Biomimetic taro leaf-like films decorated on wood surfaces using soft lithography for superparamagnetic and superhydrophobic performance. J. Mater. Sci. 2017, 52, 7428–7438. [Google Scholar] [CrossRef]
- Fu, Q.; Medina, L.; Li, Y.; Carosio, F.; Hajian, A.; Berglund, L.A. Nanostructured Wood Hybrids for Fire-Retardancy Prepared by Clay Impregnation into the Cell Wall. ACS Appl. Mater. Interfaces 2017, 41, 36154–36163. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Xie, Y.; Cai, L.; Zhuang, B.; Wang, X.A.; Wu, Z.; Niu, M.; Lin, M. Mesoporous aluminosilicate material with hierarchical porosity for ultralow density wood fiber composite (ULD_WFC). ACS Sustain. Chem. Eng. 2016, 4, 3888–3896. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, X.; Yan, Y.; Qian, T.; Wang, J.; Sun, Q.; Jin, C. Facile fabrication of a PDMS @ stearic acid-Al(OH)3 coating on lignocellulose composite with superhydrophobicity and flame retardancy. Appl. Surf. Sci. 2018, 450, 387–395. [Google Scholar] [CrossRef]
- Carosio, F.; Cuttica, F.; Medina, L.; Berglund, L.A. Clay nanopaper as multifunctional brick and mortar fire protection coating—Wood case study. Mater. Des. 2016, 93, 357–363. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Q.; O’Hare, D.; Sun, L. Preparation of two dimensional layered double hydroxide nanosheets and their applications. Chem. Soc. Rev. 2017, 46, 5950–5974. [Google Scholar] [CrossRef] [PubMed]
- Miyata, S. Synthesis of #ydrotalcite-like compounds and their physico-chemical properties—The systems Mg2+-Al3+-SO42−and Mg2+-Al3+-CrO42−. Clay Clay Miner. 1977, 25, 14–18. [Google Scholar]
- Evans, D.G.; Slade, R.C. Structural Aspects of Layered Double Hydroxides. In Layered Double Hydroxides; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1–87. [Google Scholar]
- Kalali, E.N.; Wang, X.; Wang, D.Y. Functionalized layered double hydroxide-based epoxy nanocomposites with improved flame retardancy and mechanical properties. J. Mater. Chem. A 2015, 3, 6819–6826. [Google Scholar] [CrossRef]
- Li, C.; Wan, J.; Pan, Y.T.; Zhao, P.C.; Fan, H.; Wang, D.Y. Sustainable, biobased silicone with layered double hydroxide hybrid and their application in natural-fiber reinforced phenolic composites with enhanced performance. ACS Sustain. Chem. Eng. 2016, 4, 3113–3121. [Google Scholar] [CrossRef]
- Wang, D.Y.; Das, A.; Costa, F.R.; Leuteritz, A.; Wang, Y.Z.; Wagenknecht, U.; Heinrich, G. Synthesis of organo cobalt–aluminum layered double hydroxide via a novel single-step self-assembling method and its use as flame retardant nanofiller in PP. Langmuir 2010, 26, 14162–14169. [Google Scholar] [CrossRef] [PubMed]
- Kuila, T.; Srivastava, S.K.; Bhowmick, A.K. Rubber/LDH nanocomposites by solution blending. J. Appl. Polym. Sci. 2009, 111, 635–641. [Google Scholar] [CrossRef]
- Guo, B.; Liu, Y.; Zhang, Q.; Wang, F.; Wang, Q.; Liu, Y.; Li, J.; Yu, H. Efficient flame-retardant and smoke-suppression properties of Mg-Al-layered double-hydroxide nanostructures on wood substrate. ACS Appl. Mater. Interfaces 2017, 9, 23039–23047. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Ma, X.; He, J.; Feng, J.; Liu, S.; Yao, Y.; Hou, L.; Liu, X. Facile Selective and Diverse Fabrication of Superhydrophobic, Superoleophobic-Superhydrophilic and Superamphiphobic Materials from Kaolin. ACS Appl. Mater. Interfaces 2017, 9, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Yin, P.; Liang, B.; Wang, H.; Guo, L. Bioinspired design and assembly of layered double hydroxide/poly (vinyl alcohol) film with high mechanical performance. ACS Appl. Mater. Interfaces 2014, 6, 15154–15161. [Google Scholar] [CrossRef] [PubMed]
- Barik, S.; Khandual, A.; Behera, L.; Badamali, S.K.; Luximon, A. Nano-Mg-Al-layered double hydroxide application to cotton for enhancing mechanical, UV protection and flame retardancy at low cytotoxicity level. Cellulose 2016, 24, 1107–1120. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, W.; Cao, J. Preparation of sodium ligninsulfonate-layered double hydroxide and its effects on wood flour/polypropylene composites during accelerated UV weathering. Polym. Compos. 2016. [Google Scholar] [CrossRef]
- Zhao, J.; Huang, Q.; Liu, M.; Dai, Y.; Chen, J.; Huang, H.; Wen, Y.; Zhu, X.; Zhang, X.; Wei, Y. Synthesis of functionalized MgAl-layered double hydroxides via modified mussel inspired chemistry and their application in organic dye adsorption. J. Colloid Interface Sci. 2017, 505, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Peng, Y.; Qiu, H.; Liu, X.; Ge, L. Anti-fouling membranes by manipulating surface wettability and their anti-fouling mechanism. Desalination 2017, 413, 127–135. [Google Scholar] [CrossRef]
- Devaprakasam, D.; Sampath, S.; Biswas, S.K. Thermal stability of perfluoroalkyl silane self-assembled on a polycrystalline aluminum surface. Langmuir 2004, 20, 1329–1334. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Shahsavan, H.; Zhang, W.; Yang, F.K.; Zhao, B. Superhydro-oleophobic bio-inspired polydimethylsiloxane micropillared surface via FDTS coating/blending approaches. Appl. Surf. Sci. 2015, 324, 612–620. [Google Scholar] [CrossRef]
- Zhitova, E.S.; Krivovichev, S.V.; Pekov, I.V.; Yakovenchuk, V.N.; Pakhomovsky, Y.A. Correlation between the d-value and the M2+:M3+ cation ratio in Mg–Al–CO3 layered double hydroxides. Appl. Clay Sci. 2016, 130, 2–11. [Google Scholar] [CrossRef]
- Bellotto, M.; Rebours, B.; Clause, O.; Lynch, J.; Bazin, D.; Elkaïm, E. A reexamination of hydrotalcite crystal chemistry. J. Phys. Chem. 1996, 100, 8527–8534. [Google Scholar] [CrossRef]





| Samples | 003 | 006 | 012 | 015 | 018 | 110 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2θ (°) | d (Å) | 2θ (°) | d (Å) | 2θ (°) | d (Å) | 2θ (°) | d (Å) | 2θ (°) | d (Å) | 2θ (°) | d (Å) | |
| A a | 11.76 | 7.52 | 23.58 | 3.77 | 34.76 | 2.58 | 39.56 | 2.28 | 47.24 | 1.92 | 60.94 | 1.52 |
| B b | 11.48 | 7.70 | 23.16 | 3.84 | 34.76 | 2.58 | 39.18 | 2.30 | 46.64 | 1.95 | 60.72 | 1.53 |
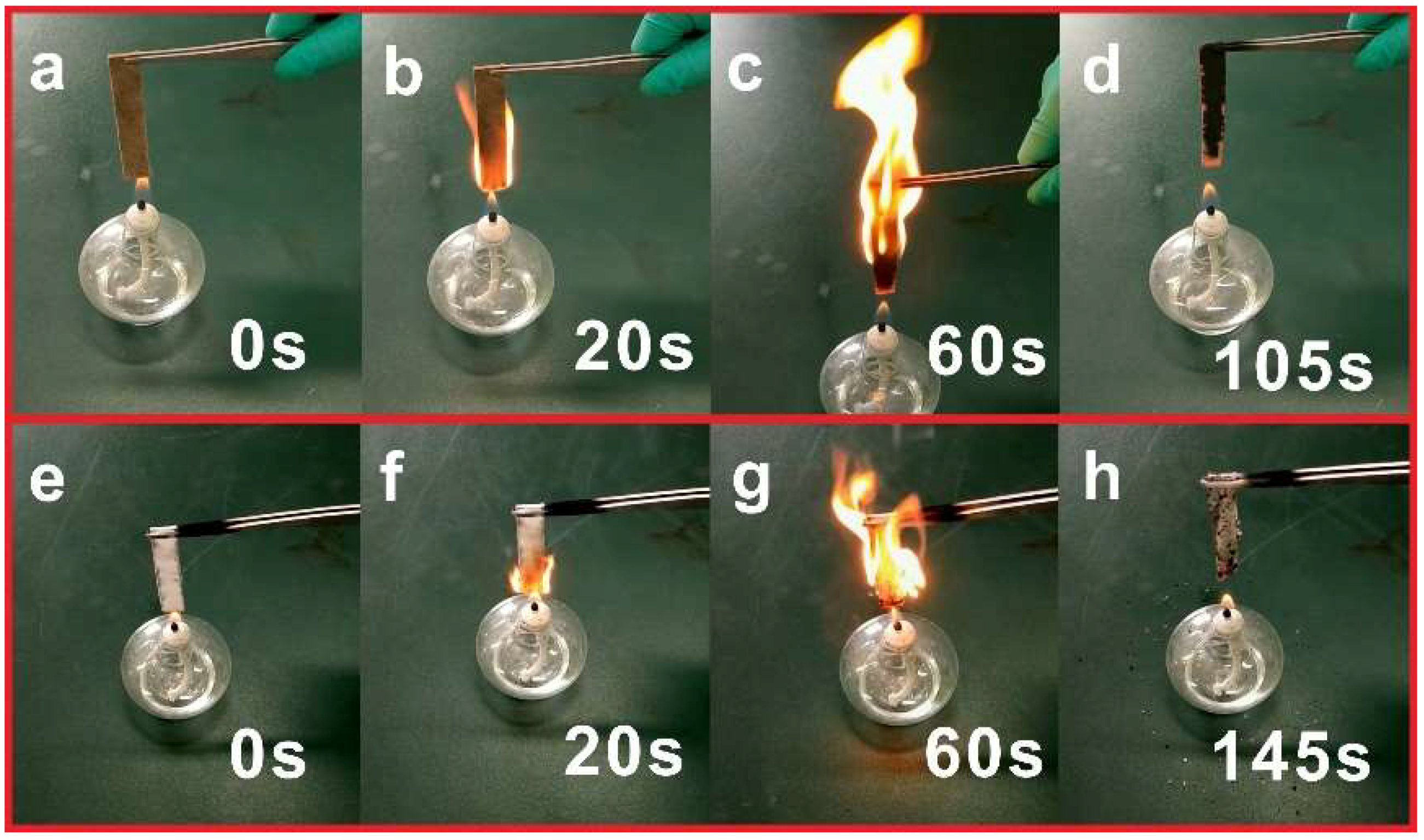
| Samples | TTI a (s) | PHRR a (kW/m2) | TPHRR a (s) | FIGRA a (kW/m2 s) | THR a (MJ/m2) |
|---|---|---|---|---|---|
| A b | 21 | 298.8 | 239 | 1.3 | 57.1 |
| B c | 42 | 224.9 | 279 | 0.8 | 50.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Shen, X.; Qian, T.; Xu, K.; Sun, Q.; Jin, C. Fabrication of Superhydrophobic Mg/Al Layered Double Hydroxide (LDH) Coatings on Medium Density Fiberboards (MDFs) with Flame Retardancy. Materials 2018, 11, 1113. https://doi.org/10.3390/ma11071113
Wang Z, Shen X, Qian T, Xu K, Sun Q, Jin C. Fabrication of Superhydrophobic Mg/Al Layered Double Hydroxide (LDH) Coatings on Medium Density Fiberboards (MDFs) with Flame Retardancy. Materials. 2018; 11(7):1113. https://doi.org/10.3390/ma11071113
Chicago/Turabian StyleWang, Zhe, Xiaoping Shen, Temeng Qian, Kang Xu, Qingfeng Sun, and Chunde Jin. 2018. "Fabrication of Superhydrophobic Mg/Al Layered Double Hydroxide (LDH) Coatings on Medium Density Fiberboards (MDFs) with Flame Retardancy" Materials 11, no. 7: 1113. https://doi.org/10.3390/ma11071113
APA StyleWang, Z., Shen, X., Qian, T., Xu, K., Sun, Q., & Jin, C. (2018). Fabrication of Superhydrophobic Mg/Al Layered Double Hydroxide (LDH) Coatings on Medium Density Fiberboards (MDFs) with Flame Retardancy. Materials, 11(7), 1113. https://doi.org/10.3390/ma11071113




