Filtration of Sub-3.3 nm Tungsten Oxide Particles Using Nanofibrous Filters
Abstract
:1. Introduction
2. Materials and Experimental Methods
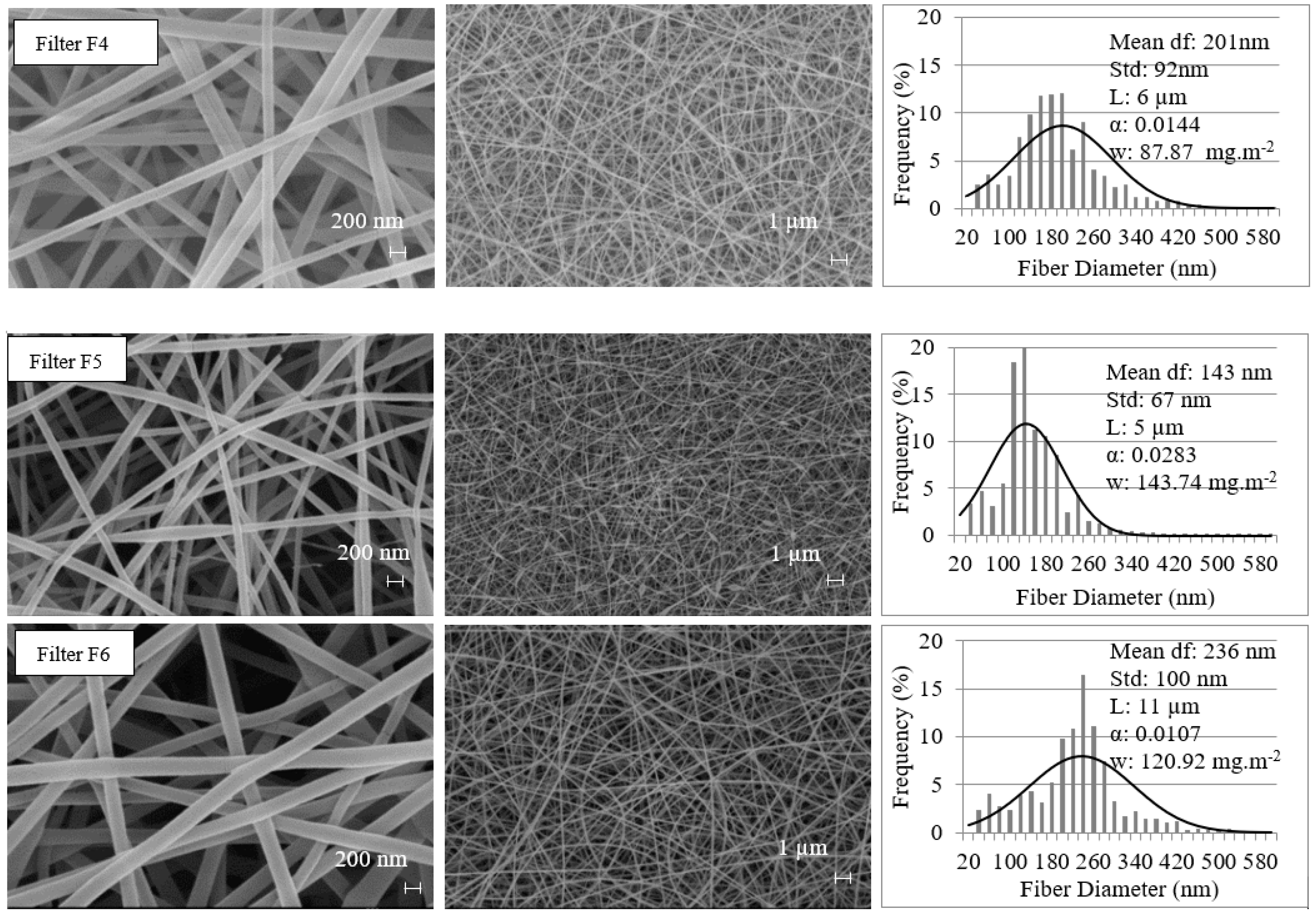
2.1. Materials
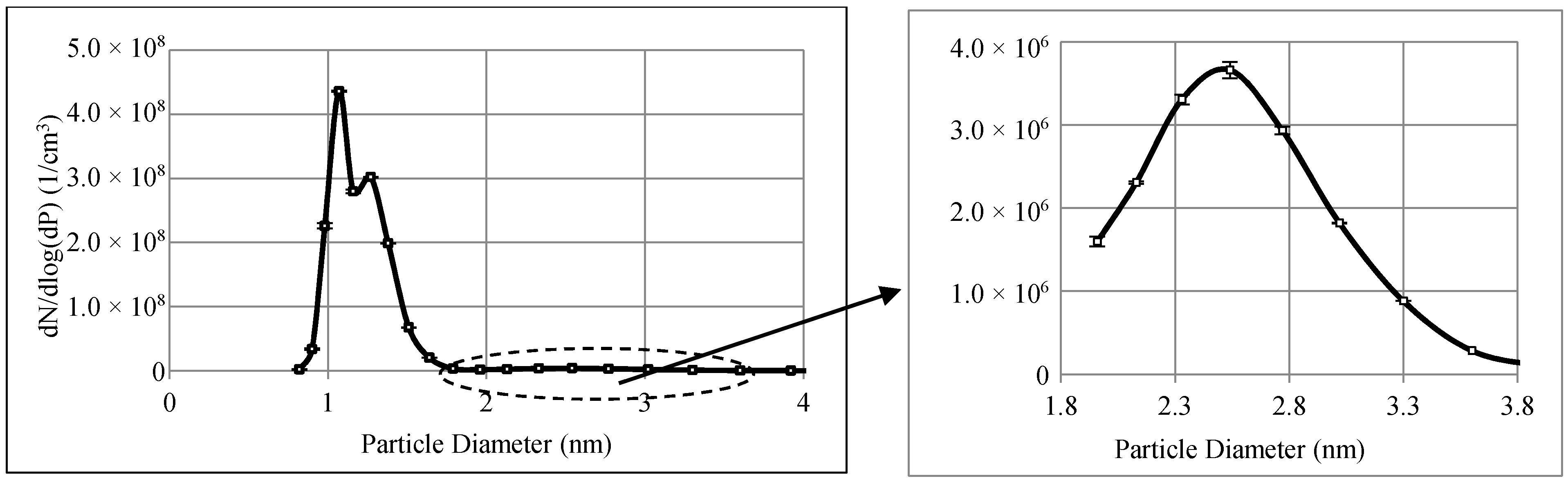
2.2. Experimental Methods
3. Results and Discussion
3.1. Filtration Efficiencies of Tested Filters for Sub-3.3 nm Particles
3.2. Effects of Particle Concentration on the Filtration of Nanoparticles
3.3. Discussion on Thermal Rebound
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Castranova, V. The Nanotoxicology Research Program in NIOSH. J. Nanopart. Res. 2009, 11, 5–13. [Google Scholar] [CrossRef]
- Ferreira, A.; Cemlyn-Jones, J.; Cordeiro, C.R. Nanoparticles, nanotechnology and pulmonary nanotoxicology. Rev. Port. Pneumol. 2013, 19, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Kreyling, W.G.; Semmler-Behnke, M.; Möller, W. Ultrafine particle-lung interactions: Does size matter? J. Aerosol Med. 2006, 19, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Givehchi, R.; Tan, Z. An Overview of Airborne Nanoparticle Filtration and Thermal Rebound Theory. Aerosol Air Qual. Res. 2014, 14, 45–63. [Google Scholar] [CrossRef]
- Givehchi, R.; Tan, Z. The effect of capillary force on airborne nanoparticle filtration. J. Aerosol Sci. 2015, 83, 12–24. [Google Scholar] [CrossRef]
- Wang, J. Effects of particle size and morphology on filtration of airborne nanoparticles. KONA Powder Part. J. 2012, 30, 256–266. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Tafreshi, H.V. On the importance of fibers’ cross-sectional shape for air filters operating in the slip flow regime. Powder Technol. 2011, 212, 425–431. [Google Scholar] [CrossRef]
- Givehchi, R.; Li, Q.; Tan, Z. The effect of electrostatic forces on filtration efficiency of granular filters. Powder Technol. 2015, 277, 135–140. [Google Scholar] [CrossRef]
- Wang, Q.; Lin, X.; Chen, D. Effect of dust loading rate on the loading characteristics of high efficiency filter media. Powder Technol. 2016, 287, 20–28. [Google Scholar] [CrossRef]
- Heim, M.; Mullins, B.J.; Wild, M.; Meyer, J.; Kasper, G. Filtration efficiency of aerosol particles below 20 nanometers. Aerosol Sci. Technol. 2005, 39, 782–789. [Google Scholar] [CrossRef]
- Kim, C.S.; Bao, L.; Okuyama, K.; Shimada, M.; Niinuma, H. Filtration efficiency of a fibrous filter for nanoparticles. J. Nanopart. Res. 2006, 8, 215–221. [Google Scholar] [CrossRef]
- Boskovic, L.; Agranovski, I.E.; Altman, I.S.; Braddock, R.D. Filter efficiency as a function of nanoparticle velocity and shape. J. Aerosol Sci. 2008, 39, 635–644. [Google Scholar] [CrossRef]
- Shaffer, R.E.; Rengasamy, S. Respiratory protection against airborne nanoparticles: A review. J. Nanopart. Res. 2009, 11, 1661–1672. [Google Scholar] [CrossRef]
- Mostofi, R.; Wang, B.; Haghighat, F.; Bahloul, A.; Jaime, L. Performance of mechanical filters and respirators for capturing nanoparticles—Limitations and future direction. Ind. Health 2010, 48, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Otani, Y. Removal of nanoparticles from gas streams by fibrous filters: A review. Ind. Eng. Chem. Res. 2013, 52, 5–17. [Google Scholar] [CrossRef]
- Wang, J.; Tronville, P. Toward standardized test methods to determine the effectiveness of filtration media against airborne nanoparticles. J. Nanopart. Res. 2014, 16, 2417. [Google Scholar] [CrossRef]
- Junninen, H.; Ehn, M.; Petäjä, T.; Luosujärvi, L.; Kotiaho, T.; Kostiainen, R.; Rohner, U.; Gonin, M.; Fuhrer, K.; Kulmala, M. A high-resolution mass spectrometer to measure atmospheric ion composition. Atmos. Meas. Tech. 2010, 3, 1039–1053. [Google Scholar] [CrossRef] [Green Version]
- Ardkapan, S.R.; Johnson, M.S.; Yazdi, S.; Afshari, A.; Bergsøe, N.C. Filtration efficiency of an electrostatic fibrous filter: Studying filtration dependency on ultrafine particle exposure and composition. J. Aerosol Sci. 2014, 72, 14–20. [Google Scholar] [CrossRef]
- Givehchi, R.; Li, Q.; Tan, Z. Quality factors of PVA nanofibrous filters for airborne particles in the size range of 10–125 nm. Fuel 2016, 181, 1273–1280. [Google Scholar] [CrossRef]
- Davies, C. The seperation of airborne dust and mist particles. Proc. Inst. Mech. Eng. 1952, 1, 185–213. [Google Scholar] [CrossRef]
- Erickson, K.; Singh, M.; Osmondson, B. Measuring Nanoparticle Size Distributions in Real-Time: Key Factors for Accuracy; NSTI-Nanotech: Danville, CA, USA, 2007. [Google Scholar]
- Kangasluoma, J.; Attoui, M.; Junninen, H.; Lehtipalo, K.; Samodurov, A.; Korhonen, F.; Sarnela, N.; Schmidt-Ott, A.; Worsnop, D.; Kulmala, M. Sizing of neutral sub 3nm tungsten oxide clusters using Airmodus Particle Size Magnifier. J. Aerosol Sci. 2015, 87, 53–62. [Google Scholar] [CrossRef]
- Kousaka, Y.; Okuyama, K.; Adachi, M.; Mimura, T. Effect of Brownian diffusion on electrical classification of ultrafine aerosol particles in differential mobility analyzer. J. Chem. Eng. Jpn. 1986, 19, 401–407. [Google Scholar] [CrossRef]
- Heim, M.; Mullins, B.J.; Kasper, G. Comment on: Penetration of ultrafine particles and ion clusters through wire screens by ichitsubo et al. Aerosol Sci. Technol. 2006, 40, 144–145. [Google Scholar] [CrossRef]
- Alonso, M.; Kousaka, Y.; Hashimoto, T.; Hashimoto, N. Penetration of nanometer-sized aerosol particles through wire screen and laminar flow tube. Aerosol Sci. Technol. 1997, 27, 471–480. [Google Scholar] [CrossRef]
- Otani, Y.; Emi, H.; Cho, S.J.; Namiki, N. Generation of nanometer size particles and their removal from air. Adv. Powder Technol. 1995, 6, 271–281. [Google Scholar] [CrossRef]
- Ichitsubo, H.; Hashimoto, T.; Alonso, M.; Kousaka, Y. Penetration of ultrafine particles and ion clusters through wire screens. Aerosol Sci. Technol. 1996, 24, 119–127. [Google Scholar] [CrossRef]
- Ankilov, A.; Baklanov, A.; Colhoun, M.; Enderle, K.; Gras, J.; Julanov, Y.; Kaller, D.; Lindner, A.; Lushnikov, A.A.; Mavliev, R.; et al. Particle size dependent response of aerosol counters. Atmos. Res. 2002, 62, 209–237. [Google Scholar] [CrossRef]
- Wimmer, D.; Lehtipalo, K.; Franchin, A.; Kangasluoma, J.; Kreissl, F.; Kürten, A.; Kupc, A.; Metzger, A.; Mikkilä, J.; Petäjä, T. Performance of diethylene glycol-based particle counters in the sub-3 nm size range. Atmos. Meas. Tech. 2013, 6, 1793–1804. [Google Scholar] [CrossRef] [Green Version]
- Heim, M.; Attoui, M.; Kasper, G. The efficiency of diffusional particle collection onto wire grids in the mobility equivalent size range of 1.2–8 nm. J. Aerosol Sci. 2010, 41, 207–222. [Google Scholar] [CrossRef]
- Sem, G.J. Design and performance characteristics of three continuous-flow condensation particle counters: A summary. Atmos. Res. 2002, 62, 267–294. [Google Scholar] [CrossRef]
- Kangasluoma, J.; Junninen, H.; Lehtipalo, K.; Mikkilä, J.; Vanhanen, J.; Attoui, M.; Sipilä, M.; Worsnop, D.; Kulmala, M.; Petäjä, T. Remarks on ion generation for CPC detection efficiency studies in sub-3-nm size range. Aerosol Sci. Technol. 2013, 47, 556–563. [Google Scholar] [CrossRef]
- Kulmala, M.; Kontkanen, J.; Junninen, H.; Lehtipalo, K.; Manninen, H.E.; Nieminen, T.; Petaja, T.; Sipila, M.; Schobesberger, S.; Rantala, P.; et al. Direct observations of atmospheric aerosol nucleation. Science 2013, 339, 943–946. [Google Scholar] [CrossRef] [PubMed]
- Keck, L.; Spielvogel, J.; Grimm, H. From nanoparticles to large aerosols: Ultrafast measurement methods for size and concentration. In Proceedings of the Nanosafe 2008: International Conference on Safe Production and Use of Nanomaterials, Grenoble, France, 3–7 November 2008. [Google Scholar]
- Boskovic, L.; Altman, I.S.; Agranovski, I.E.; Braddock, R.D.; Myojo, T.; Choi, M. Influence of particle shape on filtration processes. Aerosol Sci. Technol. 2005, 39, 1184–1190. [Google Scholar] [CrossRef]
- Chen, S.; Wang, J.; Fissan, H.; Pui, D.Y.H. Use of Nuclepore filters for ambient and workplace nanoparticle exposure assessment—Spherical particles. Atmos. Environ. 2013, 77, 385–393. [Google Scholar] [CrossRef]
- Kim, S.C.; Harrington, M.S.; Pui, D.Y.H. Experimental study of nanoparticles penetration through commercial filter media. J. Nanopart. Res. 2007, 9, 117–125. [Google Scholar] [CrossRef]
- Yang, S.; Lee, G.W.M. Filtration characteristics of a fibrous filter pretreated with anionic surfactants for monodisperse solid aerosols. J. Aerosol Sci. 2005, 36, 419–437. [Google Scholar] [CrossRef]
- Thomas, D.; Mouret, G.; Cadavid-Rodriguez, M.C.; Chazelet, S.; Bémer, D. An improved model for the penetration of charged and neutral aerosols in the 4 to 80 nm range through stainless steel and dielectric meshes. J. Aerosol Sci. 2013, 57, 32–44. [Google Scholar] [CrossRef]
- Furuuchi, M.; Eryu, K.; Nagura, M.; Hata, M.; Kato, T.; Tajima, N.; Sekiguchi, K.; Ehara, K.; Seto, T.; Otani, Y. Development and performance evaluation of air sampler with inertial filter for nanoparticle sampling. Aerosol Air Qual. Res. 2010, 10, 185–192. [Google Scholar] [CrossRef]
- Balazy, A.; Toivola, M.; Reponen, T.; Podgorski, A.; Zimmer, A.; Grinshpun, S.A. Manikin-based performance evaluation of N95 filtering-facepiece respirators challenged with nanoparticles. Ann. Occup. Hyg. 2006, 50, 259–269. [Google Scholar] [PubMed]
- Bałazy, A.; Toivola, M.; Adhikari, A.; Sivasubramani, S.K.; Reponen, T.; Grinshpun, S.A. Do N95 respirators provide 95% protection level against airborne viruses, and how adequate are surgical masks? Am. J. Infect. Control 2006, 34, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Brochot, C.; Mouret, G.; Michielsen, N.; Chazelet, S.; Thomas, D. Penetration of nanoparticles in 5 nm to 400 nm size range through two selected fibrous media. J. Phys. Conf. Ser. 2011, 304, 012068. [Google Scholar] [CrossRef]
- Buha, J.; Fissan, H.; Wang, J. Filtration behavior of silver nanoparticle agglomerates and effects of the agglomerate model in data analysis. J. Nanopart. Res. 2013, 15, 359–369. [Google Scholar] [CrossRef]
- Cyrs, W.D.; Boysen, D.A.; Casuccio, G.; Lersch, T.; Peters, T.M. Nanoparticle collection efficiency of capillary pore membrane filters. J. Aerosol Sci. 2010, 41, 655–664. [Google Scholar] [CrossRef]
- Eninger, R.M.; Honda, T.; Adhikari, A.; Heinonen-Tanski, H.; Reponen, T.; Grinshpun, S.A. Filter performance of N99 and N95 facepiece respirators against viruses and ultrafine particles. Ann. Occup. Hyg. 2008, 52, 385–396. [Google Scholar] [PubMed]
- Golanski, L.; Guiot, A.; Rouillon, F.; Pocachard, J.; Tardif, F. Experimental evaluation of personal protection devices against graphite nanoaerosols: Fibrous filter media, masks, protective clothing, and gloves. Hum. Exp. Toxicol. 2009, 28, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Lore, M.B.; Sambol, A.R.; Japuntich, D.A.; Franklin, L.M.; Hinrichs, S.H. Inter-laboratory performance between two nanoparticle air filtration systems using scanning mobility particle analyzers. J. Nanopart. Res. 2011, 13, 1581–1591. [Google Scholar] [CrossRef]
- Otani, Y.; Eryu, K.; Furuuchi, M.; Tajima, N.; Tekasakul, P. Inertial classification of nanoparticles with fibrous filters. Aerosol Air Qual. Res. 2007, 7, 343–352. [Google Scholar] [CrossRef]
- Rengasamy, S.; Eimer, B.C.; Shaffer, R.E. Comparison of nanoparticle filtration performance of NIOSH-approved and CE-marked particulate filtering facepiece respirators. Ann. Occup. Hyg. 2009, 53, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Golshahi, L.; Abedi, J.; Tan, Z. Granular filtration for airborne particles: Correlation between experiments and models. Can. J. Chem. Eng. 2009, 87, 726–731. [Google Scholar] [CrossRef]
- Huang, S.; Chen, C.; Chang, C.; Lai, C.; Chen, C. Penetration of 4.5 nm to 10 μm aerosol particles through fibrous filters. J. Aerosol Sci. 2007, 38, 719–727. [Google Scholar] [CrossRef]
- Leskinen, J.; Joutsensaari, J.; Lyyränen, J.; Koivisto, J.; Ruusunen, J.; Järvelä, M.; Tuomi, T.; Hämeri, K.; Auvinen, A.; Jokiniemi, J. Comparison of nanoparticle measurement instruments for occupational health applications. J. Nanopart. Res. 2012, 14, 718. [Google Scholar] [CrossRef]
- Japuntich, D.A.; Franklin, L.M.; Pui, D.Y.; Kuehn, T.H.; Kim, S.C.; Viner, A.S. A comparison of two nano-sized particle air filtration tests in the diameter range of 10 to 400 nanometers. J. Nanopart. Res. 2007, 9, 93–107. [Google Scholar] [CrossRef]
- Li, L.; Zuo, Z.; Japuntich, D.A.; Pui, D.Y.H. Evaluation of filter media for particle number, surface area and mass penetrations. Ann. Occup. Hyg. 2012, 56, 581–594. [Google Scholar] [PubMed]
- Hinds, W.C. Aerosol Technology Properties, Behevior, and Measurement of Airborne Particles; Wiley—Interscience: Hoboken, NJ, USA, 1999. [Google Scholar]
- Shi, B.; Ekberg, L. Ionizer Assisted Air Filtration for Collection of Submicron and Ultrafine Particles—Evaluation of Long-Term Performance and Influencing Factors. Environ. Sci. Technol. 2015, 49, 6891–6898. [Google Scholar] [CrossRef] [PubMed]
- Van Gulijk, C.; Bal, E.; Schmidt-Ott, A. Experimental evidence of reduced sticking of nanoparticles on a metal grid. J. Aerosol Sci. 2009, 40, 362–369. [Google Scholar] [CrossRef] [Green Version]
- Rennecke, S.; Weber, A.P. On the pressure dependence of thermal rebound. J. Aerosol Sci. 2013, 58, 129–134. [Google Scholar] [CrossRef]
- Van Osdell, D.W.; Liu, B.Y.H.; Rubow, K.L.; Pui, D.Y.H. Experimental study of sub-micrometer and ultrafine particle penetration and pressure drop for high efficiency filters. Aerosol Sci. Technol. 1990, 12, 911–925. [Google Scholar] [CrossRef]
- Peineke, C.; Schmidt-Ott, A. Explanation of charged nanoparticle production from hot surfaces. J. Aerosol Sci. 2008, 39, 244–252. [Google Scholar] [CrossRef]
- Bateman, A.P.; Belassein, H.; Martin, S.T. Impactor apparatus for the study of particle rebound: Relative humidity and capillary forces. Aerosol Sci. Technol. 2014, 48, 42–52. [Google Scholar] [CrossRef]
- Stein, S.W.; Turpin, B.J.; Cai, X.; Huang, P.; Mcmurry, P.H. Measurements of relative humidity-dependent bounce and density for atmospheric particles using the DMA-impactor technique. Atmos. Environ. 1994, 28, 1739–1746. [Google Scholar] [CrossRef]
- Chen, S.; Tsai, C.; Chen, H.; Huang, C.; Roam, G. The influence of relative humidity on nanoparticle concentration and particle mass distribution measurements by the MOUDI. Aerosol Sci. Technol. 2011, 45, 596–603. [Google Scholar] [CrossRef]
- Pakarinen, O.; Foster, A.; Paajanen, M.; Kalinainen, T.; Katainen, J.; Makkonen, I.; Lahtinen, J.; Nieminen, R. Towards an accurate description of the capillary force in nanoparticle-surface interactions. Model. Simul. Mater. Sci. Eng. 2005, 13, 1175. [Google Scholar] [CrossRef]
- Tan, Z. Air Pollution and Greenhouse Gases: From Basic Concepts to Engineering Applications for Air Emission Control; Springer: Berlin, Germany, 2014. [Google Scholar]
- Namasivayam, C.; Muniasamy, N.; Gayatri, K.; Rani, M.; Ranganathan, K. Removal of dyes from aqueous solutions by cellulosic waste orange peel. Bioresour. Technol. 1996, 57, 37–43. [Google Scholar] [CrossRef]
- Boor, B.E.; Siegel, J.A.; Novoselac, A. Monolayer and multilayer particle deposits on hard surfaces: Literature review and implications for particle resuspension in the indoor environment. Aerosol Sci. Technol. 2013, 47, 831–847. [Google Scholar] [CrossRef]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Givehchi, R.; Li, Q.; Tan, Z. Filtration of Sub-3.3 nm Tungsten Oxide Particles Using Nanofibrous Filters. Materials 2018, 11, 1277. https://doi.org/10.3390/ma11081277
Givehchi R, Li Q, Tan Z. Filtration of Sub-3.3 nm Tungsten Oxide Particles Using Nanofibrous Filters. Materials. 2018; 11(8):1277. https://doi.org/10.3390/ma11081277
Chicago/Turabian StyleGivehchi, Raheleh, Qinghai Li, and Zhongchao Tan. 2018. "Filtration of Sub-3.3 nm Tungsten Oxide Particles Using Nanofibrous Filters" Materials 11, no. 8: 1277. https://doi.org/10.3390/ma11081277
APA StyleGivehchi, R., Li, Q., & Tan, Z. (2018). Filtration of Sub-3.3 nm Tungsten Oxide Particles Using Nanofibrous Filters. Materials, 11(8), 1277. https://doi.org/10.3390/ma11081277






