Effect of POSS Particles and Synergism Action of POSS and Poly-(Melamine Phosphate) on the Thermal Properties and Flame Retardance of Silicone Rubber Composites
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Methods
Preparation of Rubber Blends
2.3. Experimental Techniques
2.3.1. FTIR Analysis
2.3.2. EDS Techniques
2.3.3. Thermal Properties
2.3.4. Flammability
3. Results
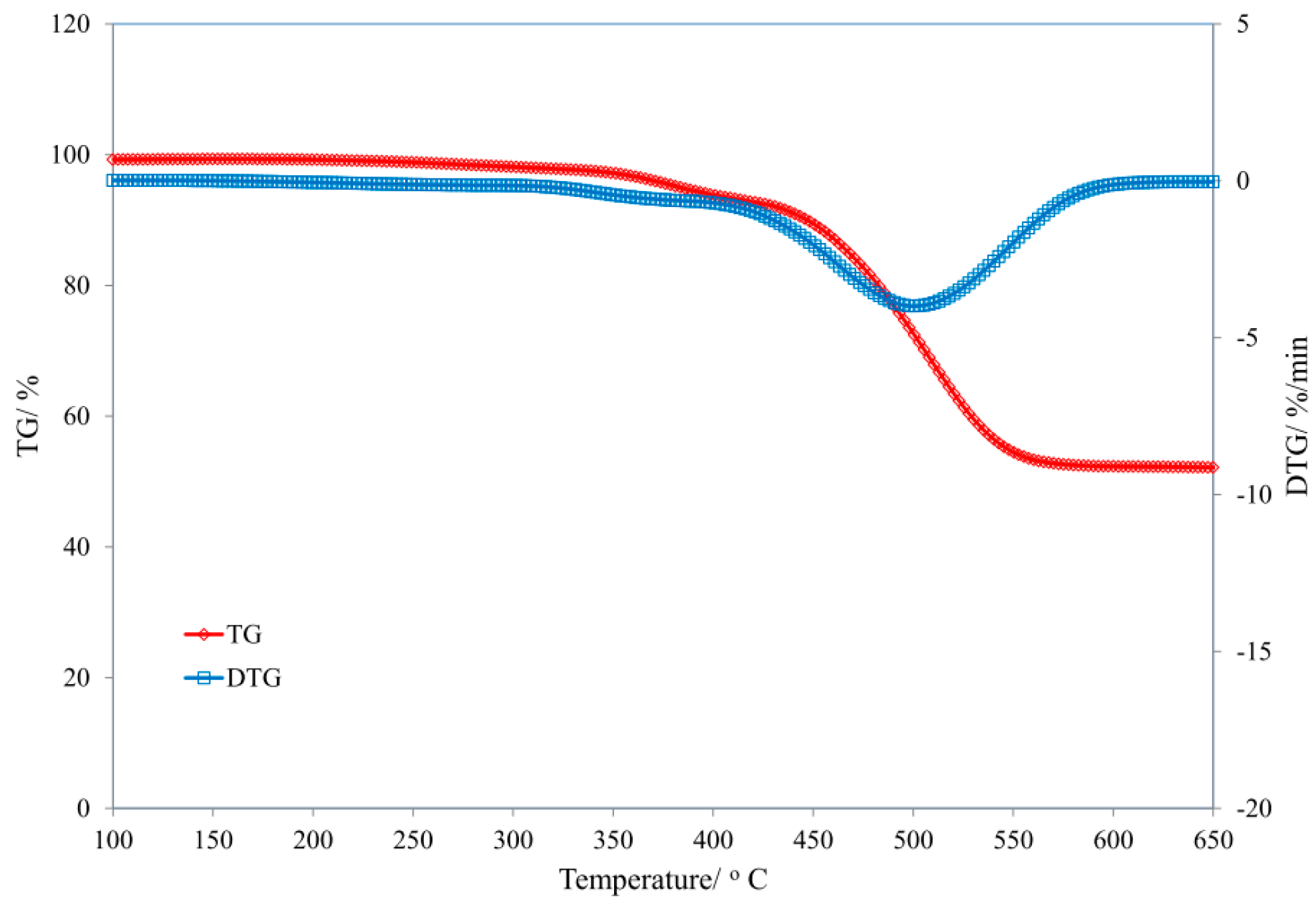
3.1. Thermal Properties of Uncured and Cured Silicone Rubber
3.2. POSS Characteristic
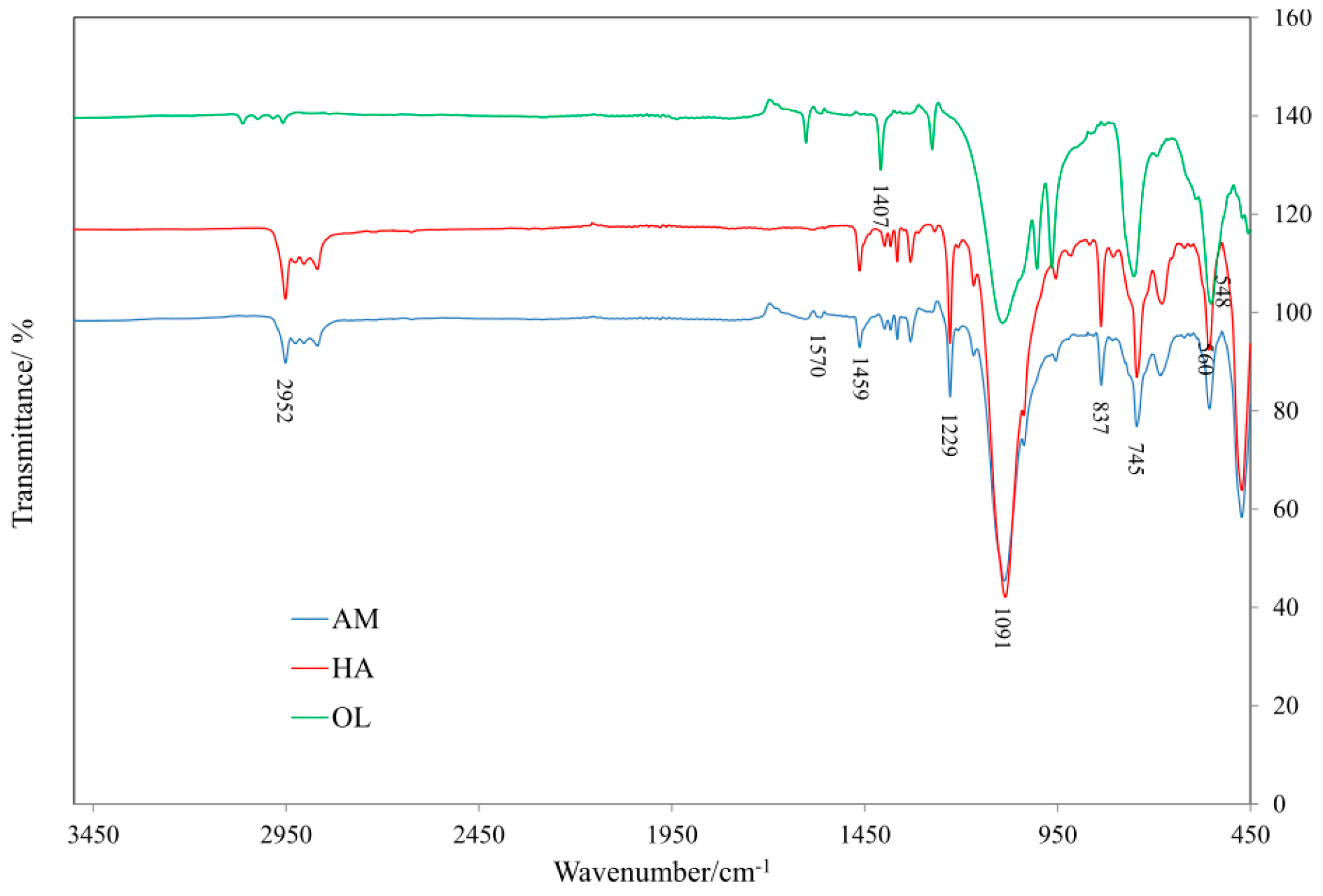
3.3. IR Analysis of SR Composites
3.4. Thermal Properties of SR Composites
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhu, C.; Deng, C.; Cao, J.-Y.; Wang, Y.-Z. An efficient flame retardant for silicone rubber: Preparation and application. Polym. Degrad. Stab. 2015, 121, 42–50. [Google Scholar] [CrossRef]
- Rybiński, P.; Żukowski, W.; Bradło, D. Effect of cenospheric fillers on the flammability and fire hazard of silicone rubber composites. J. Therm. Anal. Calorim. 2016, 125, 1373–1386. [Google Scholar] [CrossRef]
- Schartel, B. Phosphorous based flame retardancy mechanism old hat or a starting point for future development. Materials 2010, 3, 4710–4745. [Google Scholar] [CrossRef] [PubMed]
- Rybiński, P.; Janowska, G. Influence synergetic effect of halloysite nanotubes and halogen-free flame retardants on properties nitrile rubber composites. Thermochim. Acta 2013, 557, 24–30. [Google Scholar] [CrossRef]
- Bar, M.; Alagirusamy, R.; Das, A. Flame retardant polymer composites. Fibers Polym. 2015, 16, 705–717. [Google Scholar] [CrossRef]
- Balaram, P.; Sirohi, S.; Singh, D. Studies on the effects of various flame retardants on polypropylene. Am. J. Polym. Sci. 2013, 3, 63–69. [Google Scholar] [CrossRef]
- Rybiński, P.; Syrek, B.; Masłowski, M.; Miedzianowska, J.; Strzelec, K.; Żukowski, W.; Bradło, D. Influence of lignocellulose fillers on properties natural rubber composites. J. Polym. Environ. 2018, 26, 2489–2501. [Google Scholar] [CrossRef]
- Dong, F.; Zhao, P.; Dou, R.; Feng, S. Amine-functionalized POSS as cross-linkers of polysiloxane containing γ-chloropropyl groups for preparing heat-curable silicone rubber. Mater. Chem. Phys. 2018, 208, 19–27. [Google Scholar] [CrossRef]
- Zhang, W.; Camino, G.; Yang, R. Polymer/polyhedral oligomeric silsesquioxane (POSS) nanocomposites: An overview of fire retardance. Prog. Polym. Sci. 2017, 67, 77–125. [Google Scholar] [CrossRef]
- Kuo, S.-W.; Chang, F.-C. POSS related polymer nanocomposites. Prog. Polym. Sci. 2011, 36, 1649–1696. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Song, L.; Yang, H.; Yu, B.; Kandola, B.; Deli, D. Comparative study on the synergistic effect of POSS and graphene with melamine phosphate on the flame retardance of poly(butylene succinated). Thermochim. Acta 2012, 543, 156–164. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, Y.; Zhang, D.; Li, J.; Huang, G. Preparation and thermal degradation behavior of room temperature vulcanized silicone rubber-g-polyhedral oligomeric silsesquioxanes. Polymer 2013, 54, 6140–6149. [Google Scholar] [CrossRef]
- Turgot, G.; Dogan, M.; Tayfun, U.; Ozekoc, G. The effect of POSS particles on the flame retardancy of intumescent polypropylene composites and the structure-property relationship. Polym. Degrad. Stab. 2018, 149, 96–111. [Google Scholar] [CrossRef]
- Xuan, S.; Hu, Y.; Song, L.; Wang, X.; Yang, H.; Lu, H. Synergistic effect of polyhedral oligomeric silsesquioxane on the flame retardancy and thermal degradation of intumescent flame retardant polylactide. Combust. Sci. Technol. 2012, 184, 459–468. [Google Scholar] [CrossRef]
- Song, L.; Xuan, S.; Wang, X.; Hu, Y. Flame retardancy and thermal degradation behaviors of phosphate in combination with POSS in polylactide composites. Thermochim. Acta 2012, 527, 1–7. [Google Scholar] [CrossRef]
- Fox, D.M.; Novy, M.; Brown, K.; Murariu, M.; Harris, R.H.; Seppala, J.E.; Gilman, J.W. Flame retarded poly(lactic acid) using POSS-modified cellulose. Effects of intumescing flame retardant formulations on polymer degradation and composite physical properties. Polym. Degrad. Stab. 2014, 106, 54–65. [Google Scholar] [CrossRef]
- Chigwada, G.; Jash, P.; Jiang, D.D.; Wilkie, C.A. Fire retardancy of vinyl ester nanocomposites: Synergy with phosphorous-based fire retardants. Polym. Degrad. Stab. 2005, 89, 85–100. [Google Scholar] [CrossRef]
- Gerard, C.; Fontaine, G.; Bourbigot, S. Synergistic and antagonistic effects in flame retardancy of an intumescent epoxy resin. Polym. Adv. Technol. 2011, 22, 1085–1090. [Google Scholar] [CrossRef]
- Carosio, F.; Alongi, J. Influence of layer by layer coatings containing octapropylammonium polyhedral oligomeric silsesquioxane and ammonium polyphosphate on the thermal stability and flammability of acrylic fabrics. J. Anal. Appl. Pyrolysis 2016, 119, 114–123. [Google Scholar] [CrossRef]
- Rybiński, P.; Żukowski, W.; Bradło, D. Influence of cenosphere particles on thermal properties composites of silicone rubbers. J. Therm. Anal. Calorim. 2015, 122, 1307–1318. [Google Scholar] [CrossRef]
- Flynn, J.H.; Wall, L.A. A quick, direct method for determination of activation energy from thermogravimetric data. J. Polym. Sci. Polym. Lett. 1966, 4, 323–328. [Google Scholar] [CrossRef]
- Ozawa, T.A. New method of analyzing thermogravimetric data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Hamdai, S.; Longuest, C.; Perrin, D.; Lopez-Cuesta, J.-M.; Ganachaud, F. Flame retardancy of silicone-based materials. Polym. Degrad. Stab. 2009, 94, 465–495. [Google Scholar] [CrossRef]
- Radhakrishnan, T.S. New method for evaluation of kinetic parameters and mechanism of degradation from pyrolysis-GC studies. Thermal degradation of polydimethylsiloxanes. J. Appl. Polym. Sci. 1999, 73, 441–450. [Google Scholar] [CrossRef]
- Fang, W.; Zeng, X.; Lai, X.; Li, H.; Chen, W.; Zhang, Y. Thermal degradation mechanism of addition-cure liquid silicone rubber with urea-containing silane. Thermochim. Acta 2015, 605, 28–36. [Google Scholar] [CrossRef]
- Grassie, N.; Macfarlane, I.G. The thermal degradation of polysiloxanes-I. Poly(dimethylsiloxane). Eur. Polym. J. 1978, 14, 875–884. [Google Scholar] [CrossRef]
- Fina, A.; Tabuani, D.; Carniato, F.; Frache, A.; Boccaleri, E.; Camino, G. Polyhedral oligomeric silsesquioxanes (POSS) thermal degradation. Thermochim. Acta 2006, 440, 36–42. [Google Scholar] [CrossRef]
- Chen, D.; Liu, Y.; Huang, C. Synergistic effect between POSS and fumed silica on thermal stabilities and mechanical properties of room temperature vulcanized (RTV) silicone rubbers. Polym. Degrad. Stab. 2012, 97, 308–315. [Google Scholar] [CrossRef]
- Hshieh, F.-Y. Shielding effects of silica-ash layer on the combustion of silicones and their possible applications on the fire retardancy of organic polymers. Fire Mater. 1998, 22, 69–76. [Google Scholar] [CrossRef]
- Zhang, S.; Horrock, A.R. A review of flame retardant polypropylene fibers. Prog. Polym. Sci. 2003, 28, 1517–1538. [Google Scholar] [CrossRef]
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.-M.; Dubois, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Rybiński, P.; Syrek, B.; Bradło, D.; Żukowski, W.; Anyszka, R.; Imiela, M. Influence of cenospheric fillers on the thermal properties, ceramisation and flammability of nitrile rubber composites. J. Compos. Mater. 2018. [Google Scholar] [CrossRef]
- Thirumal, M.; Khastgir, D.; Nando, G.B.; Naik, Y.-P.; Singha, N.K. Halogen-free flame retardant PUF: Effect of melamine compounds on mechanical, thermal and flame retardant properties. Polym. Degrad. Stab. 2010, 95, 1138–1145. [Google Scholar] [CrossRef]

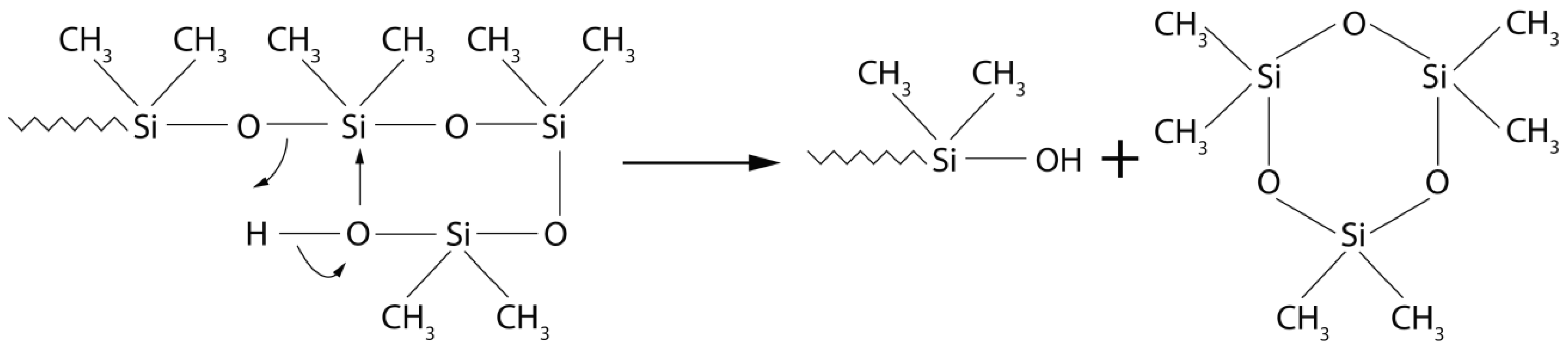
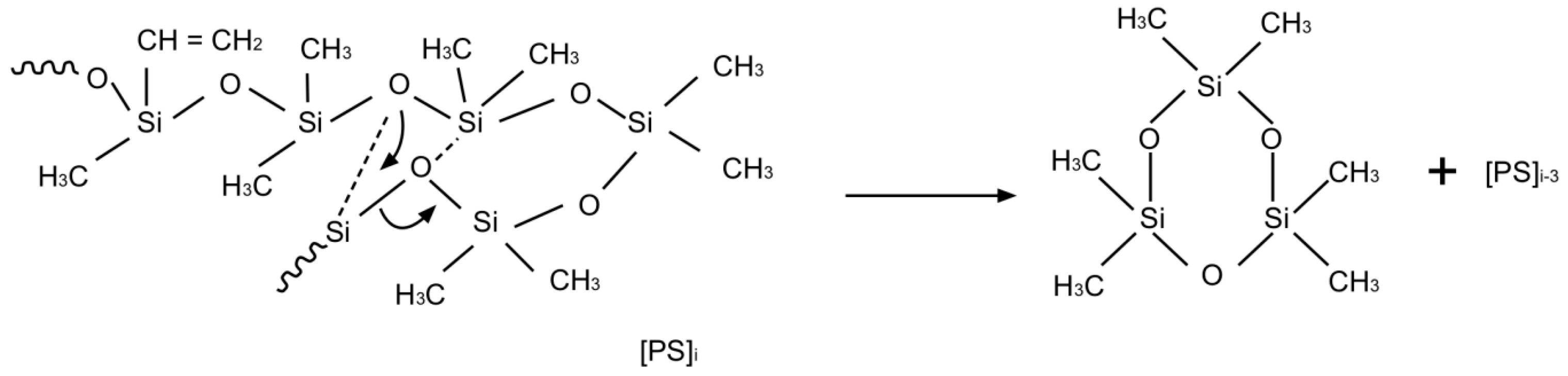


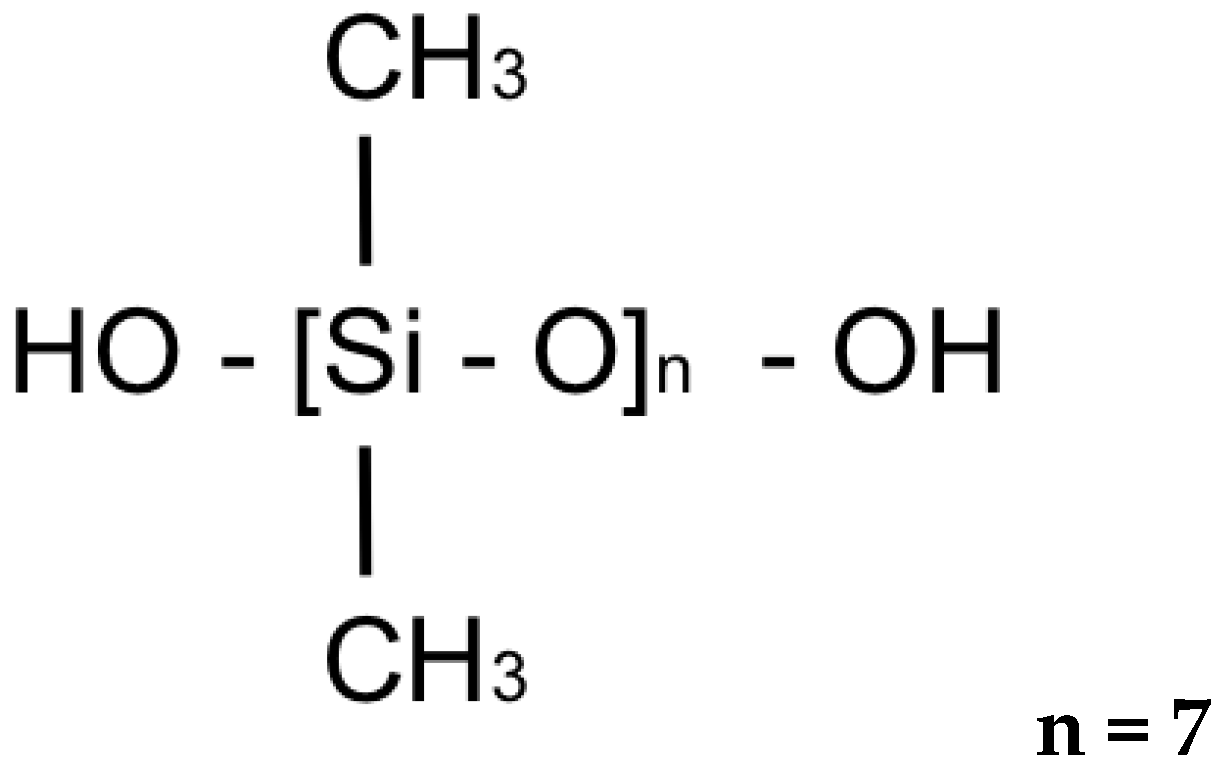





| Material | Chemical Structure |
|---|---|
| AM-POSS | 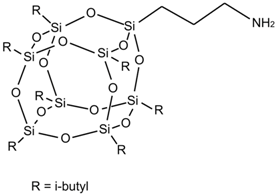 |
| HA-POSS |  |
| OL-POSS | 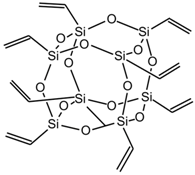 |
| Sample Description | Component (phr) | |||||
|---|---|---|---|---|---|---|
| SR | OP | POSS/AM | POSS/HA | POSS/OL | MPP | |
| R OP | 100 | 0.7 | 3 | − | − | − |
| SR/AM3 | 100 | 0.7 | − | − | − | − |
| SR/HA3 | 100 | 0.7 | − | 3 | − | − |
| SR/OL3 | 100 | 0.7 | − | − | 3 | − |
| SR/AM6 | 100 | 0.7 | 6 | − | − | − |
| SR/HA6 | 100 | 0.7 | − | 6 | − | − |
| SR/OL6 | 100 | 0.7 | − | − | 6 | − |
| SR/MPP | 100 | 0.7 | − | − | − | 20 |
| SR/AM3/MPP17 | 100 | 0.7 | 3 | − | − | 17 |
| SR/AM6/MPP14 | 100 | 0.7 | 6 | − | − | 14 |
| SR/HA3/MPP17 | 100 | 0.7 | − | 3 | − | 17 |
| SR/HA6/MPP14 | 100 | 0.7 | − | 6 | − | 14 |
| SR/OL3/MPP17 | 100 | 0.7 | − | − | 3 | 17 |
| SR/OL6/MPP14 | 100 | 0.7 | − | − | 6 | 14 |
| Sample | T5 (°C) | T50 (°C) | dm/dt (%/min) | TRMAX (°C) | Pw (%) | P650 (%) |
|---|---|---|---|---|---|---|
| SR | 184 | 520 | 5.25 | 505 | 45.3 | 44.5 |
| SR/AM3 | 320 | − | 4.74 | 520 | 49.0 | 49.5 |
| SR/HA3 | 318 | − | 5.06 | 525 | 51.4 | 51.1 |
| SR/OL3 | 384 | − | 4.87 | 506 | 51.3 | 50.2 |
| SR/AM6 | 337 | 579 | 4.59 | 524 | 53.6 | 51.5 |
| SR/HA6 | 304 | − | 5.40 | 517 | 52.5 | 52.3 |
| SR/OL6 | 378 | − | 3.91 | 514 | 52.3 | 52.4 |
| Sample | Ea (kJ/mol) |
|---|---|
| SR | 46.57 |
| SR/AM6 | 51.84 |
| SR/HA6 | 84.17 |
| SR/OL6 | 98.22 |
| Sample | T5 (°C) | T50 (°C) | dm/dt (%/min) | TRMAX (°C) | Pw (%) | P650 (%) |
|---|---|---|---|---|---|---|
| SR | 184 | 520 | 5.25 | 505 | 45.3 | 44.5 |
| SR/MPP | 315 | 420 | 17.65 | 408 | 32.4 | 28.1 |
| SR/AM3/MPP17 | 313 | 424 | 13.32 | 410 | 32.8 | 29.2 |
| SR/AM6/MPP14 | 305 | 435 | 10.71 | 415 | 35.1 | 32.9 |
| SR/HA3/MPP17 | 380 | 418 | 16.16 | 412 | 33.4 | 30.7 |
| SR/HA6/MPP14 | 328 | 435 | 10.08 | 415 | 36.2 | 33.9 |
| SR/OL3/MPP17 | 340 | 421 | 14.81 | 412 | 33.6 | 34.2 |
| SR/OL6/MPP14 | 378 | 440 | 9.89 | 415 | 35.6 | 36.1 |
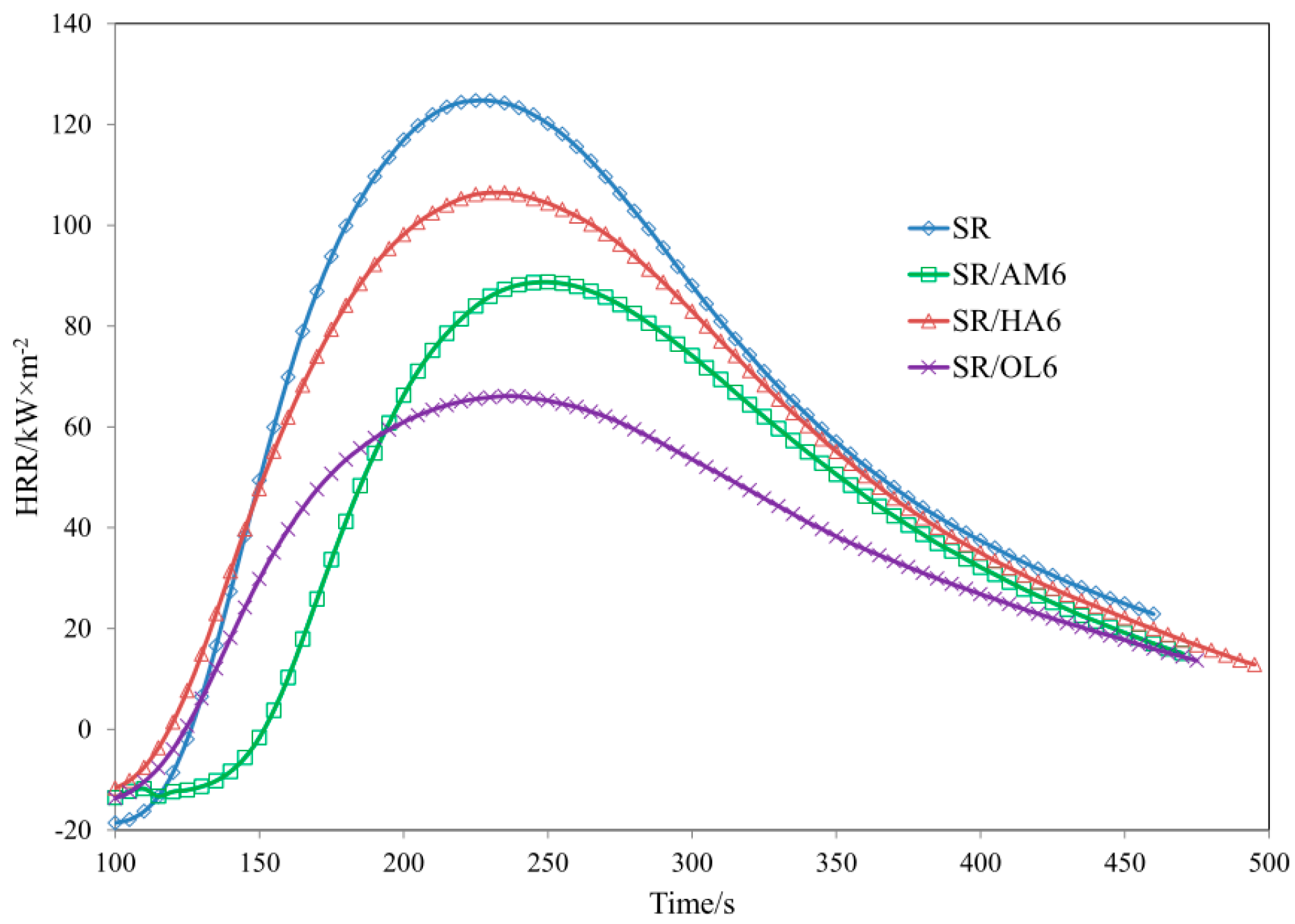
| Sample | TTI (s) ± 5 | THR (MJ/m2) ± 1 | HRR (Kw/m2) ± 2 | HRRMAX (kW/m2) ± 2 | EHC (MJ/kg) ± 2 | EHCMAX (MJ/kg) ± 1 | MLR (g·m−2·s−1) ± 0.01 |
|---|---|---|---|---|---|---|---|
| SR | 93 | 21.6 | 73.62 | 124.73 | 37.22 | 79.82 | 0.064 |
| SR/AM3 | 91 | 18.1 | 59.81 | 102.67 | 38.88 | 77.80 | 0.049 |
| SR/HA3 | 80 | 20.3 | 73.59 | 120.26 | 36.50 | 61.50 | 0.051 |
| SR/OL3 | 85 | 19.3 | 62.49 | 105.50 | 34.41 | 76.49 | 0.061 |
| SR/AM6 | 114 | 14.1 | 52.81 | 88.72 | 30.49 | 61.44 | 0.057 |
| SR/HA6 | 106 | 20.7 | 60.58 | 106.45 | 38.1 | 77.42 | 0.050 |
| SR/OL6 | 99 | 13.5 | 38.72 | 66.10 | 31.72 | 78.05 | 0.036 |
| Sample | C% | Si% | O% | P% |
|---|---|---|---|---|
| SR | −1 | 51.60 | 48.40 | − |
| SR/AM6 | −1 | 53.87 | 46.13 | − |
| SR/HA6 | −1 | 54.96 | 45.04 | − |
| SR/OL6 | −1 | 55.64 | 44.36 | − |
| SR/MPP | 10.00 | 38.27 | 49.94 | 1.79 |
| SR/AM6/MPP14 | 9.59 | 40.90 | 48.40 | 1.11 |
| SR/HA6/MPP14 | 4.48 | 42.45 | 51.01 | 2.06 |
| SR/OL6/MPP14 | 3.00 | 45.36 | 49.37 | 2.27 |
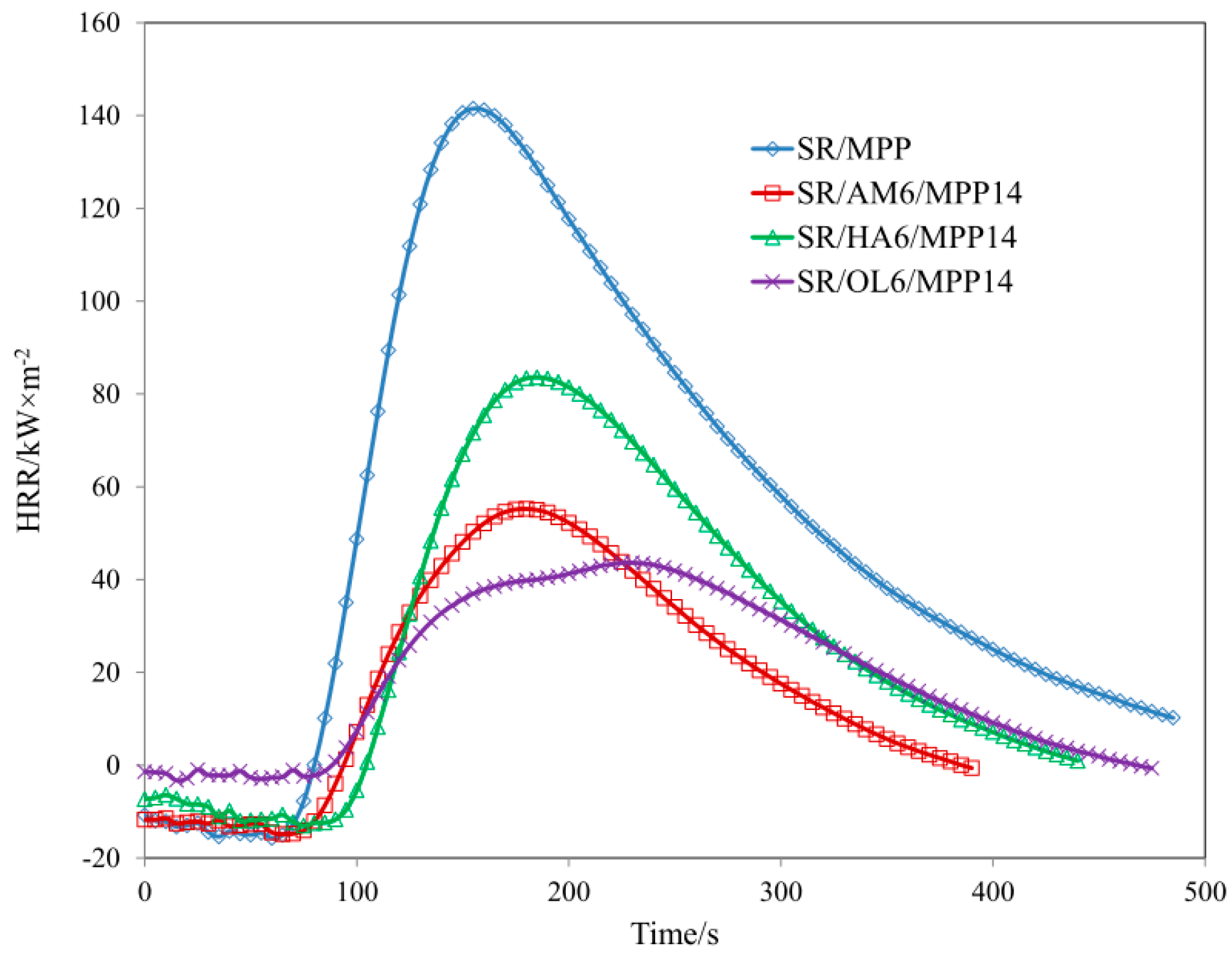
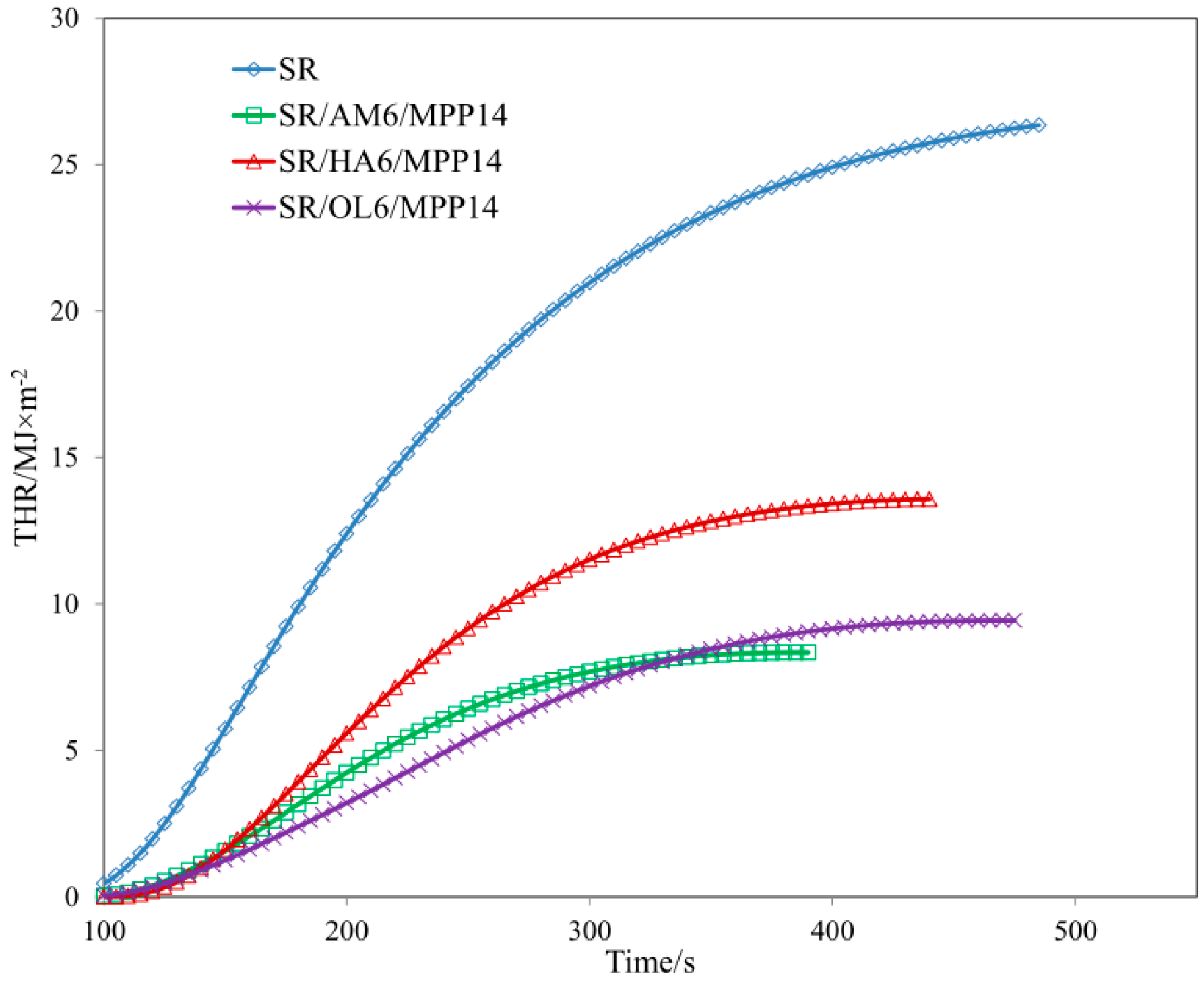
| Sample | TTI (s) | THR (MJ/m2) ± 1 | HRR (Kw/m2) ± 2 | HRR MAX (kW/m2) ± 2 | EHC (MJ/kg) ± 2 | EHC MAX (MJ/kg) ± 1 | MLR (g·m−2·s−1) ± 0.01 |
|---|---|---|---|---|---|---|---|
| SR/MPP | 56 | 24.5 | 74.04 | 141.48 | 27.95 | 79.13 | 0.120 |
| SR/AM3/MPP17 | 56 | 14.2 | 42.18 | 78.25 | 29.49 | 60.33 | 0.052 |
| SR/AM6/MPP14 | 63 | 7.9 | 30.47 | 55.22 | 13.61 | 59.06 | 0.099 |
| SR/HA3/MPP17 | 54 | 17.4 | 69.75 | 114.42 | 25.31 | 77.38 | 0.092 |
| SR/HA6/MPP14 | 83 | 12.3 | 49.95 | 83.58 | 22.46 | 75.77 | 0.085 |
| SR/OL3/MPP17 | 70 | 11.5 | 29.26 | 50.12 | 21.15 | 73.54 | 0.049 |
| SR/OL6/MPP14 | 72 | 9.2 | 27.52 | 43.63 | 14.24 | 75.85 | 0.059 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rybiński, P.; Syrek, B.; Bradło, D.; Żukowski, W. Effect of POSS Particles and Synergism Action of POSS and Poly-(Melamine Phosphate) on the Thermal Properties and Flame Retardance of Silicone Rubber Composites. Materials 2018, 11, 1298. https://doi.org/10.3390/ma11081298
Rybiński P, Syrek B, Bradło D, Żukowski W. Effect of POSS Particles and Synergism Action of POSS and Poly-(Melamine Phosphate) on the Thermal Properties and Flame Retardance of Silicone Rubber Composites. Materials. 2018; 11(8):1298. https://doi.org/10.3390/ma11081298
Chicago/Turabian StyleRybiński, Przemysław, Bartłomiej Syrek, Dariusz Bradło, and Witold Żukowski. 2018. "Effect of POSS Particles and Synergism Action of POSS and Poly-(Melamine Phosphate) on the Thermal Properties and Flame Retardance of Silicone Rubber Composites" Materials 11, no. 8: 1298. https://doi.org/10.3390/ma11081298
APA StyleRybiński, P., Syrek, B., Bradło, D., & Żukowski, W. (2018). Effect of POSS Particles and Synergism Action of POSS and Poly-(Melamine Phosphate) on the Thermal Properties and Flame Retardance of Silicone Rubber Composites. Materials, 11(8), 1298. https://doi.org/10.3390/ma11081298







