POSS Nanofiller-Induced Enhancement of the Thermomechanical Properties in a Fluoroelastomer Terpolymer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Compounding and Vulcanization Procedure
2.3. Characterization
3. Results and Discussion
3.1. Composition and Fabrication of Composite Films
3.2. Evaluation of the Dispersion State
3.3. Thermal Characterization
3.3.1. DSC
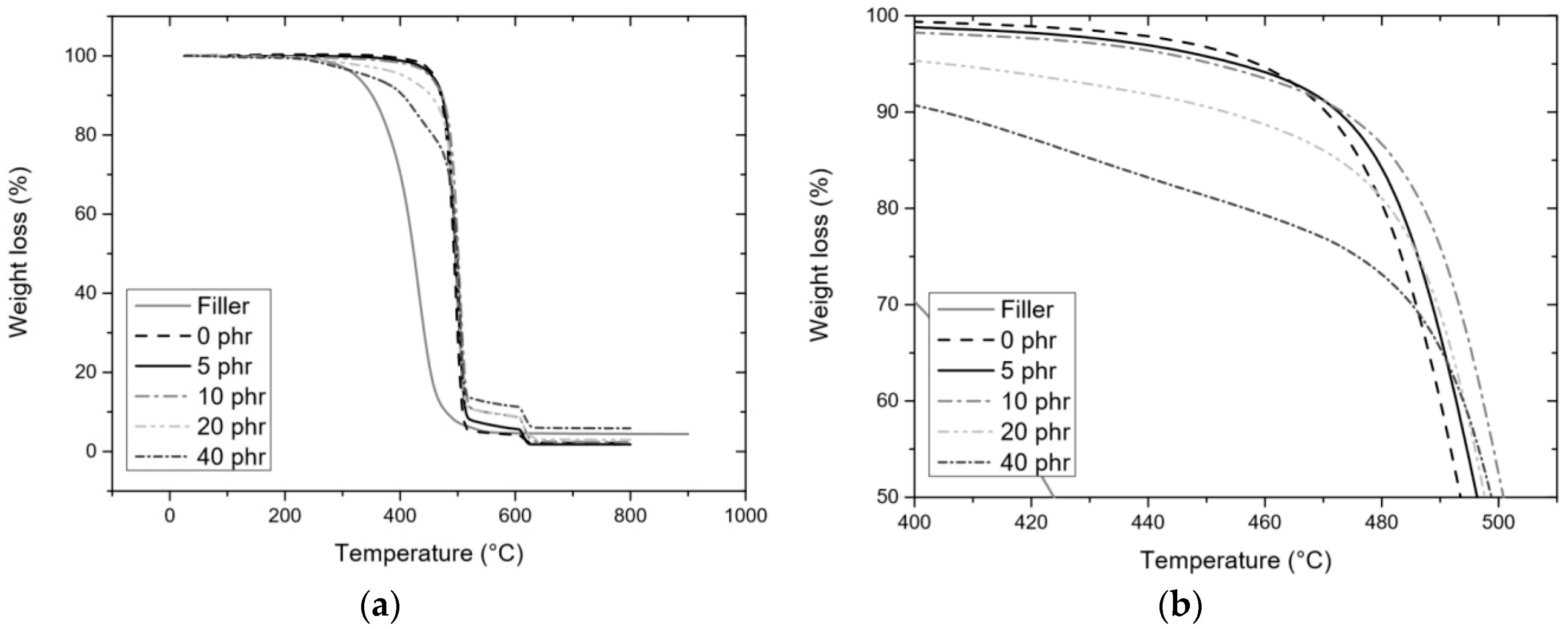
3.3.2. TGA
3.4. Mechanical Characterization
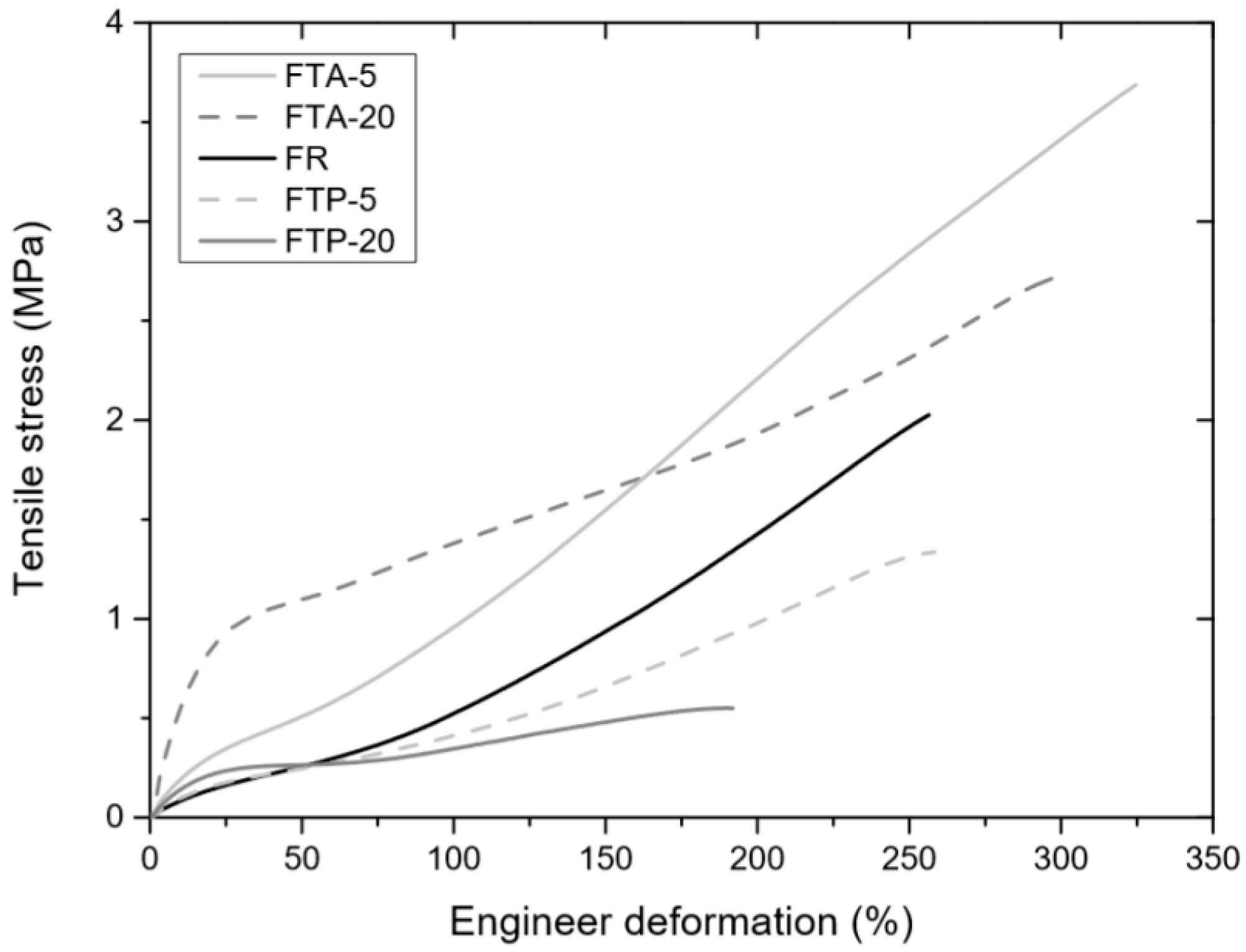
3.4.1. Tensile Tests
3.4.2. DMA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Améduri, B.; Boutevin, B.; Kostov, G. Fluoroelastomers: Synthesis, properties and applications. Prog. Polym. Sci. 2001, 26, 105–187. [Google Scholar] [CrossRef]
- Moore, A.L. Fluoroelastomers Handbook: The Definitive User’s Guide; William Andrew Publishing: Norwich, UK, 2006; pp. 25–125. [Google Scholar]
- Rhein, R.A. Thermally stable elastomers: A review, California. 1983. Available online: http://www.dtic.mil/docs/citations/ADA137914 (accessed on 15 September 2015).
- Ameduri, B.; Boutevin, B. Update on fluoroelastomers: From perfluoroelastomers to fluorosilicones and fluorophosphazenes. J. Fluor. Chem. 2005, 126, 221–229. [Google Scholar] [CrossRef]
- Drobny, J.G. Fluoropolymers in automotive applications. Polym. Adv. Technol. 2007, 18, 117–121. [Google Scholar] [CrossRef]
- Sugama, T.; Pyatina, T.; Redline, E.; McElhanon, J.; Blankenship, D. Degradation of different elastomeric polymers in simulated geothermal environments at 300 °C. Polym. Degrad. Stab. 2015, 120, 328–339. [Google Scholar] [CrossRef] [Green Version]
- Mitra, S.; Ghanbari-Siahkali, A.; Kingshott, P.; Almdal, K.; Kem Rehmeier, H.; Christensen, A.G. Chemical degradation of fluoroelastomer in an alkaline environment. Polym. Degrad. Stab. 2004, 83, 195–206. [Google Scholar] [CrossRef]
- Koo, J.H. Processing, Characterisation and Applications. In Polymer Nanocomposites; McGraw-Hill: New York, NY, USA, 2006; pp. 1–239. ISBN 9780071458214. [Google Scholar]
- Zimmermann, H.; Schuster, R.H. Advanced dispersion strategies and property design for CNT/Rubber Nanocomposites. World J. Eng. 2011, 1369–1370. [Google Scholar]
- Hassar, M. Influence des Nano-Charges de Noir de Carbone sur le Comportement Mécanique de Matériaux Composites: Application au Blindage Electromagnétique. Ph.D. Thesis, Technology University of Compiègne, Compiègne, France, 2013. [Google Scholar]
- Arroyo, M.; López-Manchado, M.; Herrero, B. Organo-montmorillonite as substitute of carbon black in natural rubber compounds. Polymer 2003, 44, 2447–2453. [Google Scholar] [CrossRef]
- Feher, F.J.; Wyndham, K.D.; Soulivong, D.; Nguyen, F. Syntheses of highly functionalized cube-octameric polyhedral oligosilsesquioxanes. J. Chem. Soc. Dalt. Trans. 1999, 0, 1491–1498. [Google Scholar] [CrossRef]
- Scott, D.W. Thermal rearrangement of branched-chain methylpolysiloxanes 1. J. Am. Chem. Soc. 1946, 68, 356–358. [Google Scholar] [CrossRef]
- Li, F.; Wang, J.; Xie, T.; Wang, D.; Tang, J.; Tang, X. Synthesis and study of a new polyorganophosphazene. J. Appl. Polym. Sci. 2001, 80, 1446–1451. [Google Scholar] [CrossRef]
- Wu, J.; Mather, P.T. POSS polymers: Physical properties and biomaterials applications. Polym. Rev. 2009, 49, 25–63. [Google Scholar] [CrossRef]
- Deng, Y. Study on RAFT Polymerization and Nano-Structured Hybrid System of POSS Macromers. Ph.D. Thesis, INSA (Institut national des science appliqué), University of Lyon, Lyon, France, 2012. [Google Scholar]
- Laik, S. Investigation of Polyhedral Oligomeric Silsesquioxanes for Improved Fire Retardancy of Hybrid Epoxy-Based Polymer Systems. Ph.D. Thesis, INSA (Institut national des science appliqué), University of Lyon, Lyon, France, 2014. [Google Scholar]
- Blanco, I.; Bottino, F.A.; Cicala, G.; Latteri, A.; Recca, A. A kinetic study of the thermal and thermal oxidative degradations of new bridged POSS/PS nanocomposites. Polym. Degrad. Stab. 2013, 98, 2564–2570. [Google Scholar] [CrossRef]
- Li, H.; Yu, D.; Zhang, J. A novel and facile method for direct synthesis of cross-linked polysiloxanes by anionic ring-opening copolymerization with Ph12-POSS/D4/Ph8D4. Polymer 2005, 46, 5317–5323. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, Y.; Zhang, D.; Li, J.; Huang, G. Preparation and thermal degradation behavior of room temperature vulcanized silicone rubber-g-polyhedral oligomeric silsesquioxanes. Polymer 2013, 54, 6140–6149. [Google Scholar] [CrossRef]
- Pan, G.; Mark, J.E.; Schaefer, D.W. Synthesis and characterization of fillers of controlled structure based on polyhedral oligomeric silsesquioxane cages and their use in reinforcing siloxane elastomers. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 3314–3323. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, W.; Yao, R.; Jiang, B. Thermal stability enhancement mechanism of poly (dimethylsiloxane) composite by incorporating octavinyl polyhedral oligomeric silsesquioxanes. Polym. Degrad. Stab. 2013, 98, 109–114. [Google Scholar] [CrossRef]
- Chen, D.; Liu, Y.; Zhang, H.; Zhou, Y.; Huang, C.; Xiong, C. Influence of polyhedral oligomeric silsesquioxanes (POSS) on thermal and mechanical properties of polydimethylsiloxane (PDMS) composites filled with fumed silica. J. Inorg. Organomet. Polym. Mater. 2013, 23, 1375–1382. [Google Scholar] [CrossRef]
- Zhang, Y.; Mao, Y.; Chen, D.; Wu, W.; Yi, S.; Mo, S.; Huang, C. Synthesis and characterization of addition-type silicone rubbers (ASR) using a novel cross linking agent PH prepared by vinyl-POSS and PMHS. Polym. Degrad. Stab. 2013, 98, 916–925. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, X.; Zhang, X.; Hou, L. Toughened elastomer/polyhedral oligomeric silsesquioxane (POSS)-intercalated rectorite nanocomposites: Preparation, microstructure, and mechanical properties. Polym. Compos. 2017, 38, E443–E450. [Google Scholar] [CrossRef]
- Sahoo, S.; Bhowmick, A.K. Polyhedral oligomeric silsesquioxane (POSS) nanoparticles as new crosslinking agent for functionalized rubber. Rubber Chem. Technol. 2007, 80, 826–837. [Google Scholar] [CrossRef]
- Seurer, B.; Coughlin, E.B. Fluoroelastomer copolymers incorporating polyhedral oligomeric silsesquioxane. Macromol. Chem. Phys. 2008, 209, 2040–2048. [Google Scholar] [CrossRef]
- Asim, N.; Ahmadi, S.; Alghoul, M.A.; Hammadi, F.Y.; Saeedfar, K.; Sopian, K. Research and development aspects on chemical preparation techniques of photoanodes for dye sensitized solar cells. Int. J. Photoenergy 2014, 2014, 1–21. [Google Scholar] [CrossRef]
- Wu, J.; Haddad, T.S.; Mather, P.T. Vertex group effects in entangled polystyrene−polyhedral oligosilsesquioxane (POSS) copolymers. Macromolecules 2009, 42, 1142–1152. [Google Scholar] [CrossRef]
- Ayandele, E.; Sarkar, B.; Alexandridis, P. Polyhedral oligomeric silsesquioxane (POSS)-containing polymer nanocomposites. Nanomaterials 2012, 2, 445–475. [Google Scholar] [CrossRef] [PubMed]
- Wagner, M. Termal Analysis in Practice, METTLER TOLEDO Collected Applications Handbook; Mettler-Tolledo: Schwerzenbach, Switzerland, 2013; pp. 30–326. [Google Scholar]
- Banik, I.; Bhowmick, A.K.; Raghavan, S.V.; Majali, A.B.; Tikku, V.K. Thermal degradation studies of electron beam cured terpolymeric fluorocarbon rubber. Polym. Degrad. Stab. 1999, 63, 413–421. [Google Scholar] [CrossRef]
- Cordes, D.B.; Lickiss, P.D.; Rataboul, F. Recent developments in the chemistry of cubic polyhedral oligosilsesquioxanes. Chem. Rev. 2010, 110, 2081–2173. [Google Scholar] [CrossRef] [PubMed]
- Fina, A.; Tabuani, D.; Carniato, F.; Frache, A.; Boccaleri, E.; Camino, G. Polyhedral oligomeric silsesquioxanes (POSS) thermal degradation. Thermochim. Acta 2006, 440, 36–42. [Google Scholar] [CrossRef]
- Blanco, I.; Bottino, F.A.; Cicala, G.; Latteri, A.; Recca, A. Synthesis and characterization of differently substituted phenyl hepta isobutyl-polyhedral oligomeric silsesquioxane/polystyrene nanocomposites. Polym. Compos. 2014, 35, 151–157. [Google Scholar] [CrossRef]
- Zheng, L.; Farris, R.J.; Coughlin, E.B. Novel polyolefin nanocomposites: Synthesis and characterizations of metallocene-catalyzed polyolefin polyhedral oligomeric silsesquioxane copolymers. Macromolecules 2001, 34, 8034–8039. [Google Scholar] [CrossRef]
- Diani, J.; Fayolle, B.; Gilormini, P. A review on the Mullins effect. Eur. Polym. J. 2009, 45, 601–612. [Google Scholar] [CrossRef]
- Wu, J.; Huang, G.; Wang, X.; He, X.; Zheng, J. Detecting different modes of molecular motion in polyisobutylene and chlorinated butyl rubber by using dielectric probes. Soft Matter. 2011, 7, 9224–9230. [Google Scholar] [CrossRef]
- Chang, M.C.O.; Thomas, D.A.; Sperling, L.H. Characterization of the area under loss modulus and tan δ–temperature curves: Acrylic polymers and their sequential interpenetrating polymer networks. J. Appl. Polym. Sci. 1987, 34, 409–422. [Google Scholar] [CrossRef]
- Epoxy Adhesive Application Guide, Billerica, n.d. Available online: http://www.epotek.com/site/files/brochures/pdfs/adhesive_application_guide.pdf (accessed on 14 September 2017).
- Fu, B.X.; Lee, A.; Haddad, T.S. Styrene−butadiene−styrene triblock copolymers modified with polyhedral oligomeric silsesquioxanes. Macromolecules 2004, 37, 5211–5218. [Google Scholar] [CrossRef]
- Berthier, D.; Deffarges, M.P.; Lacroix, F.; Berton, N.; Schmaltz, B.; Tendron, Y.; Pestel, E.; Tran Van, F.; Meo, S. Nanoparticles effects on the thermomechanical properties of a fluoroelastomer. In Proceedings of the 10th European Conference on Constitutive Models for Rubber (ECCMR X), Munich, Germany, 28–31 August 2017; Lion & Joh, Taylor & Francis Group: London, UK, 2017. [Google Scholar]












| FR | phr | FTA | FTP | FTF | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Raw | Vulcanized | - | Raw | Vulcanized | Raw | Vulcanized | Raw | Vulcanized | ||||
| Tg | ΔH | Tg | Tg | ΔH | Tg | Tg | ΔH | Tg | Tg | ΔH | Tg | |
| −16.8 | −24.6 | −16.2 | 5 | −18.8 | −30.8 | −15.6 | −18.8 | −20.5 | −16.2 | −16.4 | −21.7 | −14.4 |
| 10 | −21.0 | −22.7 | −16.5 | −17.4 | −24.1 | −16.7 | −22.6 | −18.2 | −14.6 | |||
| 15 | −19.7 | −23.7 | −14.8 | −17.1 | −15.6 | −16.4 | −20.5 | −22.3 | −15.5 | |||
| 20 | −16.1 | −23.3 | −16.5 | −18.1 | −13.7 | −15.8 | −19.7 | −22.9 | −15.9 | |||
| phr | POSS-A | FR | FTA | |||||
| 0 | 5 | 10 | 20 | 40 | ||||
| Atmosphere | Nitrogen | Tonset (°C) | 371 ± 4 | 467 ± 2 | 469 ± 2 | 474 ± 3 | 478 ± 3 | 481 ± 1 |
| T5% (°C) | 321 ± 3 | 458 ± 1 | 450 ± 4 | 449 ± 2 | 419 ± 2 | 328 ± 4 | ||
| T50% (°C) | 432 ± 1 | 497 ± 1 | 497 ± 1 | 501 ± 1 | 502 ± 2 | 502 ± 1 | ||
| Ash (%) | 3.8 ± 0.8 | 1.8 ± 0.2 | 2.2 ± 0.3 | 2.3 ± 0.02 | 3.8 ± 0.9 | 5.7 ± 0.9 | ||
| Theoretical ash (%) | 0.2 | 0.3 | 0.6 | 1.1 | ||||
| Air | Tonset (°C) | 258 ± 6 | 456 ± 4 | 465 ± 1 | 467 ± 1 | 470 ± 2 | 474 ± 2 | |
| T5% (°C) | 300 ± 1 | 430 ± 1 | 426 ± 1 | 421 ± 2 | 399 ± 1 | 336 ± 2 | ||
| T50% (°C) | 502 ± 1 | 488 ± 1 | 493 ± 1 | 493 ± 2 | 492 ± 2 | 493 ± 3 | ||
| Ash (%) | 47.4 ± 0.9 | 2.0 ± 0.1 | 2.2 ± 0.08 | 2.4 ± 0.2 | 4.7 ± 0.4 | 12.3 ± 0.8 | ||
| Theoretical ash (%) | 2.3 | 4.3 | 7.9 | 13.5 | ||||
| phr | POSS-P | 1 | FTP | |||||
| 5 | 10 | 20 | 40 | |||||
| Atmosphere | Nitrogen | Tonset (°C) | 543 ± 6 | 465 ± 3 | 468 ± 3 | 468 ± 3 | 469 ± 1 | |
| T5% (°C) | 518 ± 1 | 450 ± 3 | 452 ± 4 | 455 ± 3 | 452 ± 1 | |||
| T50% (°C) | 606 ± 1 | 495 ± 1 | 499 ± 1 | 501 ± 1 | 499 ± 2 | |||
| Ash (%) | 74.7 ± 0.2 | 2.0 ± 0.1 | 2.1 ± 0.01 | 3.02 ± 0.9 | 3.5 ± 0.5 | |||
| Theoretical ash (%) | 3.6 | 6.8 | 12.5 | 21.3 | ||||
| Air | Tonset (°C) | 518 ± 3 | 460 ± 2 | 459 ± 1 | 466 ± 2 | 463 ± 2 | ||
| T5% (°C) | 538 ± 1 | 428 ± 1 | 429 ± 3 | 428 ± 2 | 434 ± 1 | |||
| T50% (°C) | 583 ± 1 | 493 ± 1 | 495 ± 1 | 496 ± 1 | 494 ± 1 | |||
| Ash (%) | 45.3 ± 0.2 | 1.5 ± 0.06 | 1.6 ± 0.1 | 2.4 ± 0.1 | 2.5 ± 0.2 | |||
| Theoretical ash (%) | 2.2 | 4.1 | 7.6 | 12.9 | ||||
| phr | POSS-F | FTF | ||||||
| 10 | 20 | |||||||
| Atmosphere | Nitrogen | Tonset (°C) | 361 ± 6 | 467 ± 1 | 470 ± 1 | |||
| T5% (°C) | 331 ± 2 | 354 ± 1 | 317 ± 2 | |||||
| T50% (°C) | 405 ± 6 | 497 ± 1 | 497 ± 1 | |||||
| Ash (%) | 0.9 ± 0.1 | 1.7 ± 0.2 | 1.2 ± 0.3 | |||||
| Theoretical ash (%) | 0.1 | 0.2 | ||||||
| Air | Tonset (°C) | 346 ± 6 | ||||||
| T5% (°C) | 319 ± 1 | |||||||
| T50% (°C) | 396 ± 6 | |||||||
| Ash (%) | 0.06 ± 0.3 | |||||||
| Theoretical ash (%) | ||||||||
| Sample | FR | FTA | FTP | ||
|---|---|---|---|---|---|
| phr | 0 | 5 | 20 | 5 | 20 |
| Modulus at 10% elongation (MPa) | 0.07 ± 0.03 | 0.2 ± 0.04 | 0.6 ± 0.1 | 0.07 ± 0.02 | 0.2 ± 0.03 |
| Modulus at 100% elongation (MPa) | 0.6 ± 0.05 | 0.9 ± 0.08 | 1.3 ± 0.09 | 0.4 ± 0.06 | 0.3 ± 0.02 |
| Tensile at break (MPa) | 2.4 ± 0.7 | 3.23 ± 0.4 | 2.6 ± 0.2 | 1.2 ± 0.1 | 0.5 ± 0.07 |
| Elongation at break (%) | 278.3 ± 45 | 286.0 ± 37 | 279.2 ± 25 | 243.4 ± 15 | 180.4 ± 14 |
| Sample | Temperature at the Peak of Tan δ Tg (°C) | Tan δ at Temperature of Tg (T = Tg) Tan δ | Storage Modulus at 100 °C E’100 (MPa) | Storage Modulus at 200 °C E’200 (MPa) |
|---|---|---|---|---|
| FR | −2.8 ± 0.2 | 1.6 ± 0.09 | 3.05 ± 0.3 | 1.3 ± 0.4 |
| FTA-05 | 1.4 ± 0.2 | 1.5 ± 0.07 | 4.0 ± 0.1 | 2.1 ± 0.07 |
| FTA-10 | 1.5 ± 0.1 | 1.4 ± 0.2 | 4.8 ± 0.8 | 2.7 ± 0.6 |
| FTA-20 | 0.2 ± 0.07 | 0.9 ± 0.01 | 9.2 ± 1.6 | 4.0 ± 0.6 |
| FTP-05 | −1.8 ± 0.09 | 1.5 ± 0.2 | 3.9 ± 0.1 | 1.8 ± 0.4 |
| FTP-10 | −0.9 ± 0.02 | 1.3 ± 0.07 | 4.4 ± 0.2 | 2.0 ± 0.7 |
| FTP-20 | 1.1 ± 0.1 | 1.05 ± 0.03 | 5.6 ± 0.4 | 2.4 ± 0.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berthier, D.; Deffarges, M.-P.; Berton, N.; Venin, M.; Lacroix, F.; Schmaltz, B.; Tendron, Y.; Pestel, E.; Tran-Van, F.; Méo, S. POSS Nanofiller-Induced Enhancement of the Thermomechanical Properties in a Fluoroelastomer Terpolymer. Materials 2018, 11, 1358. https://doi.org/10.3390/ma11081358
Berthier D, Deffarges M-P, Berton N, Venin M, Lacroix F, Schmaltz B, Tendron Y, Pestel E, Tran-Van F, Méo S. POSS Nanofiller-Induced Enhancement of the Thermomechanical Properties in a Fluoroelastomer Terpolymer. Materials. 2018; 11(8):1358. https://doi.org/10.3390/ma11081358
Chicago/Turabian StyleBerthier, Daphné, Marie-Pierre Deffarges, Nicolas Berton, Mathieu Venin, Florian Lacroix, Bruno Schmaltz, Yohan Tendron, Eric Pestel, François Tran-Van, and Stéphane Méo. 2018. "POSS Nanofiller-Induced Enhancement of the Thermomechanical Properties in a Fluoroelastomer Terpolymer" Materials 11, no. 8: 1358. https://doi.org/10.3390/ma11081358
APA StyleBerthier, D., Deffarges, M.-P., Berton, N., Venin, M., Lacroix, F., Schmaltz, B., Tendron, Y., Pestel, E., Tran-Van, F., & Méo, S. (2018). POSS Nanofiller-Induced Enhancement of the Thermomechanical Properties in a Fluoroelastomer Terpolymer. Materials, 11(8), 1358. https://doi.org/10.3390/ma11081358






