Research Progress Regarding Interfacial Characteristics and the Strengthening Mechanisms of Titanium Alloy/Hydroxyapatite Composites
Abstract
:1. Introduction
2. Interfacial Characteristics of Titanium Alloy/HA Composites
2.1. The Mechanisms of Interfacial Bonding
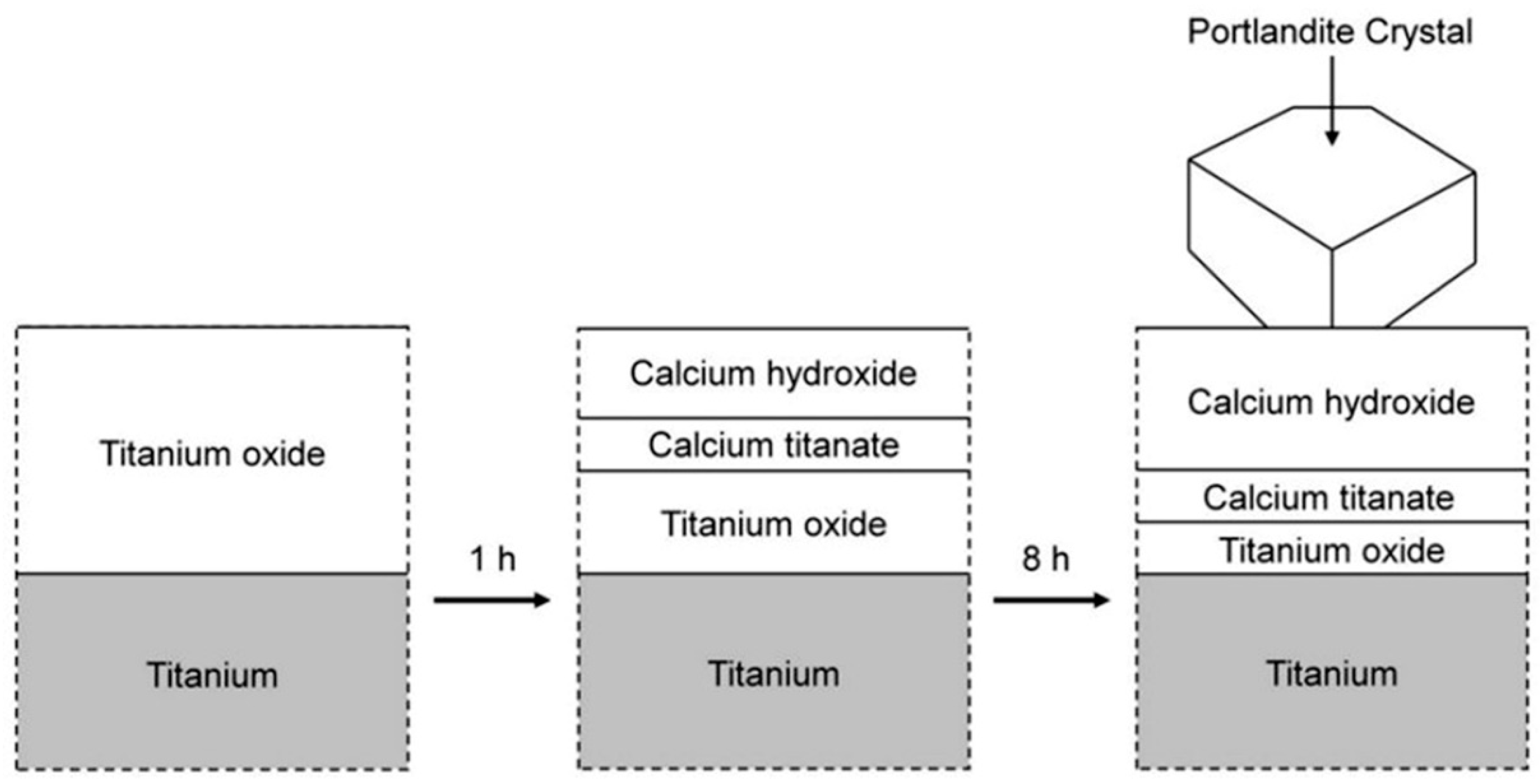
2.1.1. Interfacial Chemical Reactions in Composite Coating
2.1.2. Interfacial Chemical Reactions in Uncoated Composites
2.1.3. Interfacial Mechanical Effect
2.2. Microstructure and Formation Mechanism
3. Strengthening and Toughening Mechanisms of Titanium Alloy/HA Composites
3.1. Interfacial Bonding Strength between Coating and Substrate
3.1.1. Physical Enhancement
3.1.2. Chemical Enhancement
3.2. Strengthening and Toughening Mechanisms of Composites
3.2.1. Grain Refinement Strengthening
3.2.2. Second Phase Strengthening
3.2.3. Solution Strengthening
3.2.4. Cracks and Pulling Out Mechanism
3.2.5. Integrated Mechanisms
4. Performance Evaluation of Titanium Alloy/HA Composites
4.1. Fracture Behavior Evaluation of TitaniumAlloy/HA Composites
4.2. Biological Evaluation of Titanium Alloy/HA Composites
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Arifin, A.; Sulong, A.B.; Muhamad, N.; Syarif, J.; Ramli, M.I. Material processing of Hydroxyapatite and Titanium alloy (HA/Ti) composite as implant materials using powder metallurgy: A review. Mater. Des. 2014, 55, 165–175. [Google Scholar] [CrossRef]
- Li, Y.; Yang, C.; Zhao, H.; Qu, S.; Li, X.; Li, Y. New developments of Ti-based alloys for biomedical applications. Materials 2014, 7, 1709–1800. [Google Scholar] [CrossRef] [PubMed]
- Tjong, S.C. Recent progress in the development and properties of novel metal matrix nanocomposites reinforced with carbon nanotubes and graphene nanosheets. Mater. Sci. Eng. R 2013, 74, 281–350. [Google Scholar] [CrossRef]
- Han, C.J.; Qian, W.; Bo, S.; Li, W.; Wei, Q.S.; Wen, S.F.; Liu, J.; Shi, Y.S.; Mech, J. Microstructure and property evolutions of Titanium/nano-Hydroxyapatite composites in-situ prepared by selective laser melting. Behav. Biomed. 2017, 71, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Comin, R.; Reyna, L.A.; Cid, M.P.; Oldani, C.R.; Salvatierra, N.A. Cytotoxicity of Hydroxyapatite and morphology in composites with Ti. IEEE Lat. Am. Trans. 2013, 11, 97–100. [Google Scholar] [CrossRef]
- Zhang, L.; He, Z.Y.; Zhang, Y.Q.; Jiang, Y.H.; Zhou, R. Enhanced in vitro bioactivity of porous NiTi-HA composites with interconnected pore characteristics prepared by spark plasma sintering. Mater. Des. 2016, 101, 170–180. [Google Scholar] [CrossRef]
- Doi, K.; Abe, Y.; Kobatake, R.; Okazaki, Y.; Oki, Y.; Naito, Y.; Prananingrum, W.; Tsuga, K. Novel development of phosphate treated porous Hydroxyapatite. Materials 2017, 10, 1405. [Google Scholar] [CrossRef] [PubMed]
- Ramírez Fernández, M.P.; Gehrke, S.A.; Mazón, P.; Calvoguirado, J.L.; De Aza, P.N. Implant stability of biological Hydroxyapatites used in dentistry. Materials 2017, 10, 644. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, S.; Golestani-Fard, F.; Rezaie, H.R.; Mirhosseini, S.M.M.; Ziaee, A. MAO-derived Hydroxyapatite-TiO2 nanostructured bio-ceramic films on Titanium. Mater. Res. Bull. 2012, 47, 3407–3412. [Google Scholar] [CrossRef]
- Bovand, D.; Yousefpour, M.; Rasouli, S.; Bagherifard, S.; Bovand, N.; Tamayol, A. Characterization of Ti-HA composite fabricated by mechanical alloying. Mater. Des. 2015, 65, 447–453. [Google Scholar] [CrossRef]
- Zhao, G.; Xia, L.; Zhong, B.; Wen, G.; Song, L.; Wang, X. Effect of milling conditions on the properties of HA/Ti feedstock powders and plasma-sprayed coatings. Surf. Coat. Technol. 2014, 251, 38–47. [Google Scholar] [CrossRef]
- Chang, Q.; Ru, H.Q.; Chen, D.L.; Yang, J.L.; Hu, S.L. Effect of Iron on the sinterability and properties of HA/Ti-Fe composites. Adv. Mater. Res. 2014, 898, 271–274. [Google Scholar] [CrossRef]
- Durdu, S.; Usta, M. The tribological properties of bioceramic coatings produced on Ti6Al4V alloy by plasma electrolytic oxidation. Ceram. Int. 2014, 40, 3627–3635. [Google Scholar] [CrossRef]
- Bai, Y.; Park, I.S.; Park, H.H.; Bae, T.S.; Lee, M.H. Formation of bioceramic coatings containing Hydroxyapatite on the Titanium substrate by micro-arc oxidation coupled with electrophoretic deposition. J. Biomed. Mater. Res. B 2010, 95, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Rafieerad, A.R.; Ashra, M.R.; Mahmoodian, R.; Bushroa, A.R. Surface characterization and corrosion behavior of calcium phosphate-base composite layer on Titanium and its alloys via plasma electrolytic oxidation: A review paper. Mater. Sci. Eng. C 2015, 57, 397–413. [Google Scholar] [CrossRef] [PubMed]
- Lugovskoy, A.; Lugovskoy, S. Production of Hydroxyapatite layers on the plasma electrolytically oxidized surface of Titanium alloys. Mater. Sci. Eng. C 2014, 43, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, W.H.; Man, H.C. Laser cladding of HA/Ti composite coating on NiTi alloy. Surf. Eng. 2013, 29, 409–431. [Google Scholar] [CrossRef]
- Mohseni, E.; Zalnezhad, E.; Bushroa, A.R. Comparative investigation on the adhesion of Hydroxyapatite coating on Ti-6Al-4V implant: A review paper. Int. J. Adhes. Adhes. 2014, 48, 238–257. [Google Scholar] [CrossRef]
- Zavgorodniy, A.V.; Borrerolópez, O.; Hoffman, M.; Legeros, R.Z.; Rohanizadeh, R. Characterization of the chemically deposited Hydroxyapatite coating on a Titanium substrate. J. Mater. Sci. 2011, 22, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Miranda, G.; Araújo, A.; Bartolomeu, F.; Buciumeanu, M.; Carvalho, O.; Souza, J.C.M.; Silva, F.S.; Henriques, B. Design of Ti6Al4V-HA composites produced by hot pressing for biomedical applications. Mater. Des. 2016, 108, 488–493. [Google Scholar] [CrossRef]
- Park, S.H.; Woo, K.D.; Kim, J.Y.; Kim, S.M. Fabrication and characteristics of Ti-Nb-Mo-cpp composite fabricated by high energy mechanical milling and spark plasma sintering. Korean J. Met. Mater. 2012, 50, 469–475. [Google Scholar] [CrossRef]
- Marcu, T.; Todea, M.; Maines, L.; Leordean, D.; Berce, P.; Popa, C. Metallurgical and mechanical characterisation of Titanium based materials for endosseous applications obtained by selective laser melting. Powder Metall. 2012, 55, 309–314. [Google Scholar] [CrossRef]
- He, Y.H.; Zhang, Y.Q.; Jiang, Y.H.; Zhou, R. Effect of HA (Hydroxyapatite) content on the microstructure, mechanical and corrosion properties of (Ti,13Nb,13Zr)-xHA biocomposites synthesized by sparkle plasma sintering. Vacuum 2016, 131, 176–180. [Google Scholar] [CrossRef]
- Park, S.H.; Woo, K.D.; Kim, S.H.; Lee, S.M.; Kim, J.Y.; Ko, H.R.; Kim, S.M. Mechanical properties and bio-compatibility of Ti-Nb-Zr-HA biomaterial fabricated by rapid sintering using HEMM powders. Korean J. Mater. Res. 2011, 21, 384–390. [Google Scholar] [CrossRef]
- Anaee, R.A. Behavior of Ti/HA in saliva at different temperatures as restorative materials. J. Bio- Tribo-Corros. 2016, 2, 1–9. [Google Scholar] [CrossRef]
- Omran, A.M.; Woo, K.D.; Kang, D.S.; Abdel-Gaber, G.T.; Fouad, H.; Abdo, H.S.; Khalil, K.L. Fabrication and evaluation of porous Ti–HA bio-nanomaterial by leaching process. Arab. J. Chem. 2015, 8, 372–379. [Google Scholar] [CrossRef]
- Qian, C.; Zhang, F.; Sun, J. Fabrication of Ti/HA composite and functionally graded implant by three-dimensional printing. Bio-Med. Mater. Eng. 2015, 25, 127–136. [Google Scholar] [CrossRef]
- Arifin, A.; Sulong, A.B.; Muhamad, N.; Syarif, J. Characterization of Hydroxyapatite/Ti6Al4V composite powder under various sintering temperature. J. Appl. Sci. 2015, 75, 27–31. [Google Scholar] [CrossRef]
- Woo, K.D.; Kim, S.H.; Kang, D.S.; Kim, D.G. Microstructure and biocompatibility of Ti-Nb-Si-HA composites fabricated by rapid sintering using HEMM powders. Korean J. Mater. Res. 2013, 23, 353–358. [Google Scholar] [CrossRef]
- Zhang, L.; He, Z.Y.; Zhang, Y.Q.; Jiang, Y.H.; Zhou, R. Rapidly sintering of interconnected porous Ti-HA biocomposite with high strength and enhanced bioactivity. Mater. Sci. Eng. C 2016, 67, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Biswas, K.; Basu, B. On the toughness enhancement in Hydroxyapatite-based composites. Acta Mater. 2013, 61, 5198–5215. [Google Scholar] [CrossRef]
- Li, F.; Jiang, X.S.; Shao, Z.Y.; Zhu, D.G.; Zhu, M.H. Microstructure and mechanical properties of graphene-reinforced Titaniummatrix/nano-Hydroxyapatite nanocomposites. Materials 2018, 11, 608. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Tong, G.; Nian, Q.; Xu, R.; Saei, M.; Chen, F.; Chen, C.J.; Zhang, M.; Guo, H.F.; Xu, J.L. Laser sintered single layer graphene oxide reinforced Titanium matrix nanocomposites. Compos. Part B Eng. 2016, 93, 352–359. [Google Scholar] [CrossRef]
- Song, Y.; Chen, Y.; Liu, W.W.; Li, W.L.; Wang, Y.G.; Zhao, D.; Liu, X.B. Microscopic mechanical properties of Titanium composites containing multi-layer graphene nanofillers. Mater. Des. 2016, 109, 256–263. [Google Scholar] [CrossRef]
- Zancanela, D.C.; Simão, A.M.S.; Francisco, C.G.; Faria, A.N.D.; Ramos, A.P.; Gonçalves, R.R.; Matsubara, E.Y.; Rosolen, J.M.; Ciancaglini, P. Graphene oxide and Titanium: Synergistic effects on the biomineralization ability of osteoblast cultures. J. Mater. Sci. 2016, 27, 71. [Google Scholar] [CrossRef] [PubMed]
- Chetibi, L.; Achour, A.; Peszke, J.; Hamana, D.; Achour, S. Hydroxyapatite growth on multiwall carbon nanotubes grown on Titanium fibers from a Titanium sheet. J. Mater. Sci. 2014, 49, 621–632. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Ai, H.J.; Qi, M. Osteoblast growth on the surface of porous Zn-containing HA/TiO2, hybrid coatings on Ti substrate by MAO plus sol-gel methods. Surf. Coat. Technol. 2013, 228, 202–205. [Google Scholar] [CrossRef]
- Chang, Q.; Ru, H.Q.; Chen, D.L. Microstructure in pressureless-sintered Iron-containing Hydroxyapatite/Titanium composites. Adv. Mater. Res. 2011, 160–162, 1582–1587. [Google Scholar] [CrossRef]
- Singh, A.; Singh, G.; Chawla, V. Characterization of vacuum plasma sprayed reinforced Hydroxyapatite coatings on Ti-6Al-4V alloy. Trans. Indian Met. 2017, 20, 1–20. [Google Scholar] [CrossRef]
- Huang, J.W.; Fan, X.M.; Xiong, D.S.; Li, J.L.; Zhu, H.G.; Huang, M. Characterization and one-step synthesis of Hydroxyapatite-Ti(C,N)-TiO2composite coating by cathodic plasma electrolytic saturation and accompanying electrochemical deposition on Titanium alloy. Surf. Coat. Technol. 2017, 324, 463–470. [Google Scholar] [CrossRef]
- Liang, A.L.; Jiang, X.S.; Hong, X.; Jiang, Y.X.; Shao, Z.Y.; Zhu, D.G. Recent developments concerning the dispersion methods and mechanisms of graphene. Coatings 2018, 8, 33. [Google Scholar] [CrossRef]
- Yang, W.Z.; Huang, W.M.; Wang, Z.F.; Shang, F.J.; Huang, W.; Zhang, B.Y. Thermal and mechanical properties of graphene-Titanium composites synthesized by microwave sintering. Acta Metall. Sin.-Engl. 2016, 29, 707–713. [Google Scholar] [CrossRef]
- Kossenko, A.; Lugovskoy, S.; Astashina, N.; Lugovskoy, A.; Zinigrad, M. Effect of time on the formation of Hydroxyapatite in PEO process with hydrothermal treatment of the Ti-6Al-4V alloy. Glass Phys. Chem. 2013, 39, 639–642. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, H.F.; Geng, X.; Wang, J.J.; Xiao, J.K.; Zhu, P.Z. Effect of spray distance on microstructure and tribological performance of suspension plasma-sprayed Hydroxyapatite-Titania composite coatings. J. Therm. Spray Technol. 2016, 25, 1255–1263. [Google Scholar] [CrossRef]
- Durdu, S.; Deniz, Ö.F.; Kutbay, I.; Usta, M. Characterization and formation of Hydroxyapatite on Ti6Al4V coated by plasma electrolytic oxidation. J. Alloys Compd. 2013, 551, 422–429. [Google Scholar] [CrossRef]
- Zeng, Y.X.; Pei, X.B.; Yang, S.Y.; Qin, H.; Cai, H.; Hu, S.S.; Sui, L.; Wan, Q.B. Graphene oxide/Hydroxyapatite composite coatings fabricated by electrochemical deposition. Surf. Coat. Technol. 2016, 286, 72–79. [Google Scholar] [CrossRef]
- Nath, S.; Tripathi, R.; Basu, B. Understanding phase stability, microstructure development and biocompatibility in calcium phosphate–Titania composites, synthesized from Hydroxyapatite and Titanium powder mix. Mater. Sci. Eng. C 2009, 29, 97–107. [Google Scholar] [CrossRef]
- Ye, H.; Liu, X.Y.; Hong, H. Characterization of sintered Titanium/Hydroxyapatite biocomposite using FTIR spectroscopy. J. Mater. Sci. 2009, 20, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Wang, X.F.; Yu, C.L.; Zhao, Y.Q. Synthesis of rod-like Titanium doped Hydroxyapatite nanopowder. J. Ceram. Process. Res. 2013, 14, 700–702. [Google Scholar]
- Niespodziana, K.; Jurczyk, K.; Jurczyk, M. Mechanical and corrosion properties of Titanium-Hydroxyapatite nanocomposites. Solid State Phenom. 2009, 151, 217–221. [Google Scholar] [CrossRef]
- Akmal, M.; Raza, A.; Khan, M.M.; Khan, M.I.; Hussain, M.A. Effect of nano-Hydroxyapatite reinforcement in mechanically alloyed NiTi composites for biomedical implant. Mater. Sci. Eng. C 2016, 68, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Dom, A.H.M.; Jauhari, I.; Yazdanparast, S.; Khalid, H.M. Embedment of HA/Ti composite on superplastic Titanium alloy (Ti–6Al–4V). Mater. Sci. Eng. A 2010, 527, 5831–5836. [Google Scholar] [CrossRef]
- Chang, Q.; Ru, H.Q.; Chen, D.L.; Zhang, C.P.; Yang, J.L.; Hu, S.L. Interfacial reactions in Ti-Fe particles reinforced Hydroxyapatite matrix composites. Mater. Lett. 2014, 128, 245–247. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, J.; Niinomi, M.; Hua, L. Inhibited grain growth in Hydroxyapatite-graphene nanocomposites during high temperature treatment and their enhanced mechanical properties. Ceram. Int. 2016, 42, 11248–11255. [Google Scholar] [CrossRef]
- Zakharov, N.A.; Tkachev, A.G.; Demina, L.I.; Kiselev, M.R.; Kalinnikov, V.T. The effect of graphene oxide (GO) on biomineralization and solubility of calcium Hydroxyapatite (HA). Prot. Met. Phys. Chem. 2016, 52, 665–676. [Google Scholar] [CrossRef]
- Ji, X.; Lou, W.; Wang, Q.; Ma, J.; Xu, H.; Bai, Q.; Liu, C.; Liu, J. Sol-gel-derived Hydroxyapatite-carbon nanotube/Titania coatings on Titanium substrates. Int. J. Mol. Sci. 2012, 13, 5242–5253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Kwok, C.T. Hydroxyapatite-Anatase-carbon nanotube nanocomposite coatings fabricated by electrophoretic codeposition for biomedical applications. J. Mater. Sci. 2011, 22, 2249–2259. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.; Zeng, Y.; He, R.; Li, Z.J.; Tian, L.Y.; Wang, J.; Wan, Q.B.; Li, X.Y.; Bao, H. Single-walled carbon nanotubes/Hydroxyapatite coatings on Titanium obtained by electrochemical deposition. Appl. Surf. Sci. 2014, 295, 71–80. [Google Scholar] [CrossRef]
- Li, M.; Liu, Q.; Jia, Z.; Xu, X.; Cheng, Y.; Zheng, Y.F.; Xi, T.F.; Wei, S.C. Graphene oxide/Hydroxyapatite composite coatings fabricated by electrophoretic nanotechnology for biological applications. Carbon 2014, 67, 185–197. [Google Scholar] [CrossRef]
- Abbasi, S.; Golestani-Fard, F.; Mirhosseini, S.M.; Ziaee, A.; Mehrjoo, M. Effect of electrolyte concentration on microstructure and properties of micro arc oxidized Hydroxyapatite/Titania nanostructured composite. Mater. Sci. Eng. C 2013, 33, 2555–2561. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.R.; Ko, Y.G.; Dong, H.S. Effect of electrolyte on surface properties of pure Titanium coated by plasma electrolytic oxidation. J. Alloys Compd. 2011, 509, 478–481. [Google Scholar] [CrossRef]
- Kumari, R.; Majumdar, J.D. Studies on corrosion resistance and bio-activity of plasma spray deposited hydroxylapatite (HA) based TiO2 and ZrO2 dispersed composite coatings on Titanium alloy (Ti-6Al-4V) and the same after post spray heat treatment. Appl. Surf. Sci. 2017, 420, 935–943. [Google Scholar] [CrossRef]
- Chang, Q.; Chen, D.L.; Ru, H.Q.; Yue, X.Y.; Yu, L.; Zhang, C.P. Toughening mechanisms in Iron-containing Hydroxyapatite/Titanium composites. Biomaterials 2010, 31, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Dinçer, M.; Teker, D.; Sag, C.P.; Oztürk, K. Enhanced bonding of biomimetic apatite coatings on surface-modified Titanium substrates by hydrothermal pretreatment. Surf. Coat. Technol. 2013, 226, 27–33. [Google Scholar] [CrossRef]
- Hahn, B.D.; Lee, J.M.; Park, D.S.; Choi, J.J.; Ryu, J.; Yoon, W.H.; Lee, B.K.; Shin, D.S.; Kim, H.E. Mechanical and in vitro biological performances of Hydroxyapatite-carbon nanotube composite coatings deposited on Ti by aerosol deposition. Acta Biomater. 2009, 5, 3205–3214. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Han, Y.H.; Lee, D.Y. Adhesion characteristics of HA/MWCNT composite. Appl. Mech. Mater. 2015, 749, 313–315. [Google Scholar] [CrossRef]
- Zakharov, N.A.; Gusev, A.A.; Sentsov, M.Y.; Vasyukova, I.A. Synthesis, properties, and biocompatibility of a Ca(PO)(OH)/carbon nanotube nanocomposite. Inorg. Mater. 2014, 50, 707–715. [Google Scholar] [CrossRef]
- Duarte, L.T.; Biaggio, S.R.; Rocha-Filho, R.C.; Bocchi, N. Preparation and characterization of biomimetically and electrochemically deposited Hydroxyapatite coatings on micro-arc oxidized Ti–13Nb–13Zr. J. Mater. Sci. 2011, 22, 1663–1670. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.P.; Xu, L.J.; Chen, Y.Y.; Kee, D.W.; Xiao, S.L.; Kong, F.T.; Liu, Z.G. Effect of milling time on microstructure of Ti35Nb2.5Sn/10HA biocomposite fabricated by powder metallurgy and sintering. Trans. Nonferr. Metal. Soc. 2012, 22, 608–612. [Google Scholar] [CrossRef]
- Jakubowicz, J.; Jurczyk, K.; Niespodziana, K.; Jurczyk, M. Mechanoelectrochemical synthesis of porous Ti-based nanocomposite biomaterials. Electrochem. Commun. 2009, 11, 461–465. [Google Scholar] [CrossRef]
- Hu, Z.R.; Tong, G.Q.; Xu, R.; Zhao, L.R.; Chen, C.J.; Zhang, M.; Sun, Y.L.; Guo, H.F.; Xu, J.L. Laser sintered graphene reinforced Titaniummatrix nanocomposites. In Proceedings of the ASME 2016 11th International Manufacturing Science and Engineering Conference, Blacksburg, VA, USA, 27 June–1 July 2016. [Google Scholar]
- Salman, S.; Gunduz, O.; Yilmaz, S. Sintering effect on mechanical properties of composites of natural Hydroxyapatites and Titanium. Ceram. Int. 2009, 35, 2965–2971. [Google Scholar] [CrossRef]
- Kuma, A.; Dhara, S.; Biswas, K.; Basu, B. In vitro bioactivity and cytocompatibility properties of spark plasma sintered HA–Ti composites. J. Biomed. Mater. Res. B 2013, 101, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Biswas, K.; Basu, B. Hydroxyapatite-Titanium bulk composites for bone tissue engineering applications. J. Biomed. Mater. Res. A 2015, 103, 791–806. [Google Scholar] [CrossRef] [PubMed]
- Munir, K.S.; Qian, M.; Li, Y.; Oldfield, D.T.; Kingshott, P.; Zhu, D.M.; Wen, C. Quantitative analyses of MWCNT–Ti powder mixtures using Raman spectroscopy: The influence of milling parameters on nanostructural evolution. Adv. Eng. Mater. 2016, 17, 1660–1669. [Google Scholar] [CrossRef]
- Zhu, J.; Tjong, S.C.; Li, X. Spark plasma sintered Hydroxyapatite/multiwalled carbon nanotube composites with preferred crystal orientation. Adv. Eng. Mater. 2010, 12, 1161–1165. [Google Scholar] [CrossRef]
- Wei, W.; Zhu, Y.H.; Watari, F.; Liao, S.; Yokoyama, A.; Omori, M.; Ai, H.; Cui, F.Z. Carbon nanotubes/Hydroxyapatite nanocomposites fabricated by spark plasma sintering for bonegraft applications. Appl. Surf. Sci. 2012, 262, 194–199. [Google Scholar] [CrossRef]
- Nomura, N.; Sakamoto, K.; Takahashi, K.; Kato, S.; Abe, Y.; Doi, H.; Tsutsumi, Y.; Kobayashi, M.; Kobayashi, E.; Kim, W.J. Fabrication and mechanical properties of porous Ti/HA composites for bone fixation devices. Mater. Trans. 2010, 51, 1449–1454. [Google Scholar] [CrossRef]
- He, Y.H.; Zhang, Y.Q.; Jiang, Y.H.; Zhou, R. Microstructure evolution and enhanced bioactivity of Ti-Nb-Zr alloy by bioactive Hydroxyapatite fabricated via spark plasma sintering. RSC Adv. 2016, 6, 100939–100953. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Xu, L.; Liu, Z.; Woo, K.D. Effects of Sn content on the microstructure, mechanical properties and biocompatibility of Ti–Nb–Sn/Hydroxyapatite biocomposites synthesized by powder metallurgy. Mater. Des. 2013, 49, 511–519. [Google Scholar] [CrossRef]
- Chang, C.; Zhang, C.P.; Ru, H.U.; Yang, J.L.; Chen, D.L. Hydroxyapatite reinforced with Ti-Fe particle: Correlation between composition, microstructure and mechanical properties. Adv. Appl. Ceram. 2014, 113, 108–113. [Google Scholar] [CrossRef]
- Kumar, A.; Biswas, K.; Basu, B. Fretting wear behaviour of Hydroxyapatite-Titanium composites in simulated body fluid, supplemented with 5 g L−1 bovine serum albumin. J. Phys. D Appl. Phys. 2013, 46, 404004. [Google Scholar] [CrossRef]
- Lei, T.; Wang, L.; Ouyang, C.; Li, N.F.; Zhou, L.S. In situ preparation and enhanced mechanical properties of carbon nanotube/Hydroxyapatite composites. Int. J. Appl. Ceram. Technol. 2011, 8, 532–539. [Google Scholar] [CrossRef]
- Lu, Z.H.; Sun, K.N.; Zhao, D.M. Reinforcement of Hydroxyapatite with multi-walled carbon nanotubes. Adv. Mater. Res. 2011, 306–307, 72–75. [Google Scholar] [CrossRef]
- Buciumeanu, M.; Araujo, A.; Carvalho, O.; Miranda, G.; Souza, J.C.M.; Silva, F.S.; Henriques, B. Study of the tribocorrosion behaviour of Ti6Al4V-HA biocomposites. Tribol. Int. 2017, 107, 77–84. [Google Scholar] [CrossRef]
- Moonchaleanporn, P.; Poolthong, N.; Tongsri, R. Sintered Titanium-Hydroxyapatite composites as artificial bones. Key Eng. Mater. 2015, 659, 35–39. [Google Scholar] [CrossRef]
- Zhu, S.L.; Yang, X.J.; Cui, Z.D. Effect of Hydroxyapatite content on the microstructure, thermal and mechanical properties of Ti-based glassy alloy/Hydroxyapatite composite prepared by spark plasma sintering. Intermetallics 2011, 19, 572–576. [Google Scholar] [CrossRef]
- Chang, Q.; Ru, H.Q.; Chen, D.L.; Yue, X.Y.; Yu, L.; Zhang, C.P. An in-vitro investigation of Iron-containing Hydroxyapatite/Titanium composites. J. Mater. Sci. Technol. 2011, 27, 546–552. [Google Scholar] [CrossRef]
- Balbinotti, P.; Gemelli, E.; Buerger, G.; Lima, S.A.; De Jesus, J.; De Camargo, N.H.A.; Henriques, V.A.R.; Soares, G.D.A. Microstructure development on sintered Ti/HA biocomposites produced by powder metallurgy. Mater. Res. 2011, 14, 384–393. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Webster, T.J.; Biswas, K.; Basu, B. Flow cytometry analysis of human fetal osteoblast fate processes on spark plasma sintered Hydroxyapatite-Titanium biocomposites. J. Biomed. Mater. Res. A 2013, 101, 2925–2938. [Google Scholar] [CrossRef] [PubMed]
- Anawati; Tanigawa, H.; Asoh, H.; Ohno, T.; Kubota, M.; Ono, S. Electrochemical corrosion and bioactivity of Titanium-Hydroxyapatite composites prepared by spark plasma sintering. Corros. Sci. 2013, 70, 212–220. [Google Scholar] [CrossRef]
- Wang, X.B.; Kong, F.T.; Han, B.Q.; Chen, Y.Y. Electrochemical corrosion and bioactivity of Ti-Nb-Sn-Hydroxyapatite composites fabricated by pulse current activated sintering. J. Mech. Behav. Biomed. 2017, 75, 222–227. [Google Scholar] [CrossRef]
- Luo, R.; Liu, Z.D.; Yan, F.X.; Kong, Y.; Zhang, Y.T. The biocompatibility of Hydroxyapatite film deposition on micro-arc oxidation Ti6Al4V alloy. Appl. Surf. Sci. 2013, 266, 57–61. [Google Scholar] [CrossRef]
- Ning, C.Q.; Zhou, Y. Correlations between the in vitro and in vivo bioactivity of the Ti/HA composites fabricated by a powder metallurgy method. Acta Biomater. 2008, 4, 1944–1952. [Google Scholar] [CrossRef] [PubMed]
- Alzubaydi, T.L.; Alameer, S.S.; Ismaeel, T.; Al Hijazi, A.Y.; Geetha, M. In vivo studies of the ceramic coated Titanium alloy for enhanced osseointegration in dental applications. J. Mater. Sci. 2009, 20, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, N.; Tu, R.; Uzuka, R.; Anada, T.; Narushima, T.; Goto, T.; Niinomi, M.; Sasaki, K.; Suzuki, O. Biomechanical evaluation of amorphous calcium phosphate coated TNTZ implants prepared using a radiofrequency magnetron sputtering system. Mater. Trans. 2012, 53, 1343–1348. [Google Scholar] [CrossRef]













| Process | Materials | Young’s Modulus | Vicker Hardness | Source |
|---|---|---|---|---|
| - | cp-Ti | 100 GPa | - | [1] |
| Hot-pressing | Ti-20% HA | 102.6 GPa | 3.41 GPa | [1] |
| Hot-pressing | HA-20% Ti | 75.91 GPa | 3.13 GPa | [1] |
| Hot-pressing | cp-Ti | - | 217 ± 1.19 | [10] |
| Hot-pressing | Ti-30% nHA | - | 383.8 ± 1.13 | [10] |
| Plasma-sprayed coatings | HA/Ti | 57.4 ± 5.3 GPa | 3.9 ± 0.4 GPa | [11] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Jiang, X.; Shao, Z.; Zhu, D.; Luo, Z. Research Progress Regarding Interfacial Characteristics and the Strengthening Mechanisms of Titanium Alloy/Hydroxyapatite Composites. Materials 2018, 11, 1391. https://doi.org/10.3390/ma11081391
Li F, Jiang X, Shao Z, Zhu D, Luo Z. Research Progress Regarding Interfacial Characteristics and the Strengthening Mechanisms of Titanium Alloy/Hydroxyapatite Composites. Materials. 2018; 11(8):1391. https://doi.org/10.3390/ma11081391
Chicago/Turabian StyleLi, Feng, Xiaosong Jiang, Zhenyi Shao, Degui Zhu, and Zhiping Luo. 2018. "Research Progress Regarding Interfacial Characteristics and the Strengthening Mechanisms of Titanium Alloy/Hydroxyapatite Composites" Materials 11, no. 8: 1391. https://doi.org/10.3390/ma11081391
APA StyleLi, F., Jiang, X., Shao, Z., Zhu, D., & Luo, Z. (2018). Research Progress Regarding Interfacial Characteristics and the Strengthening Mechanisms of Titanium Alloy/Hydroxyapatite Composites. Materials, 11(8), 1391. https://doi.org/10.3390/ma11081391






