Stress Corrosion Behaviors of 316LN Stainless Steel in a Simulated PWR Primary Water Environment
Abstract
:1. Introduction
2. Experimental Methods
3. Results and Discussion
4. Conclusions
- (1)
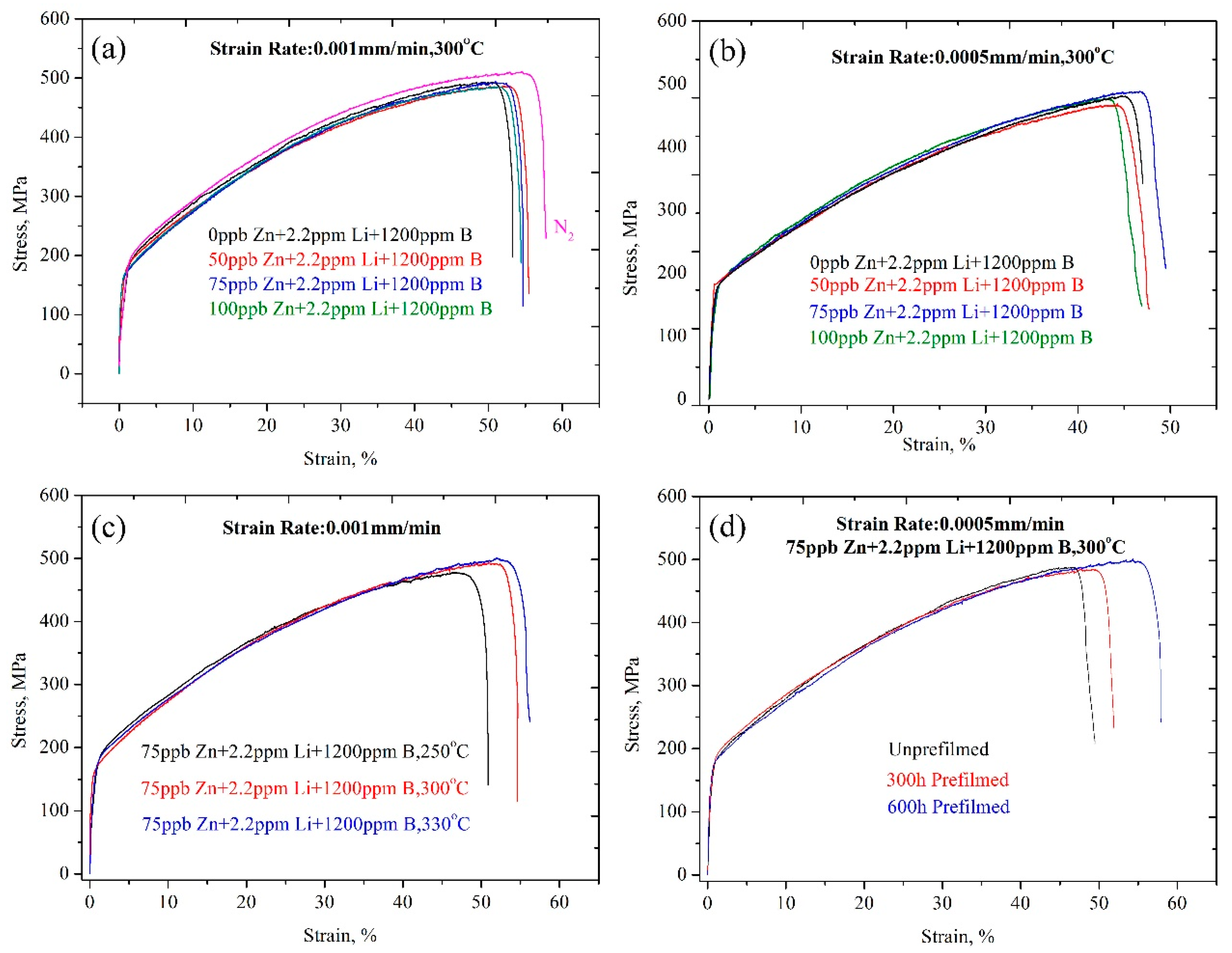
- The experimental parameters obviously affected the tensile properties. The δ and UTS of the samples tested under a nitrogen atmosphere were clearly larger than those of the samples tested in the chemical solution. However, in general, all samples showed a ductile fracture characteristic and an excellent tensile property under all experimental conditions.
- (2)
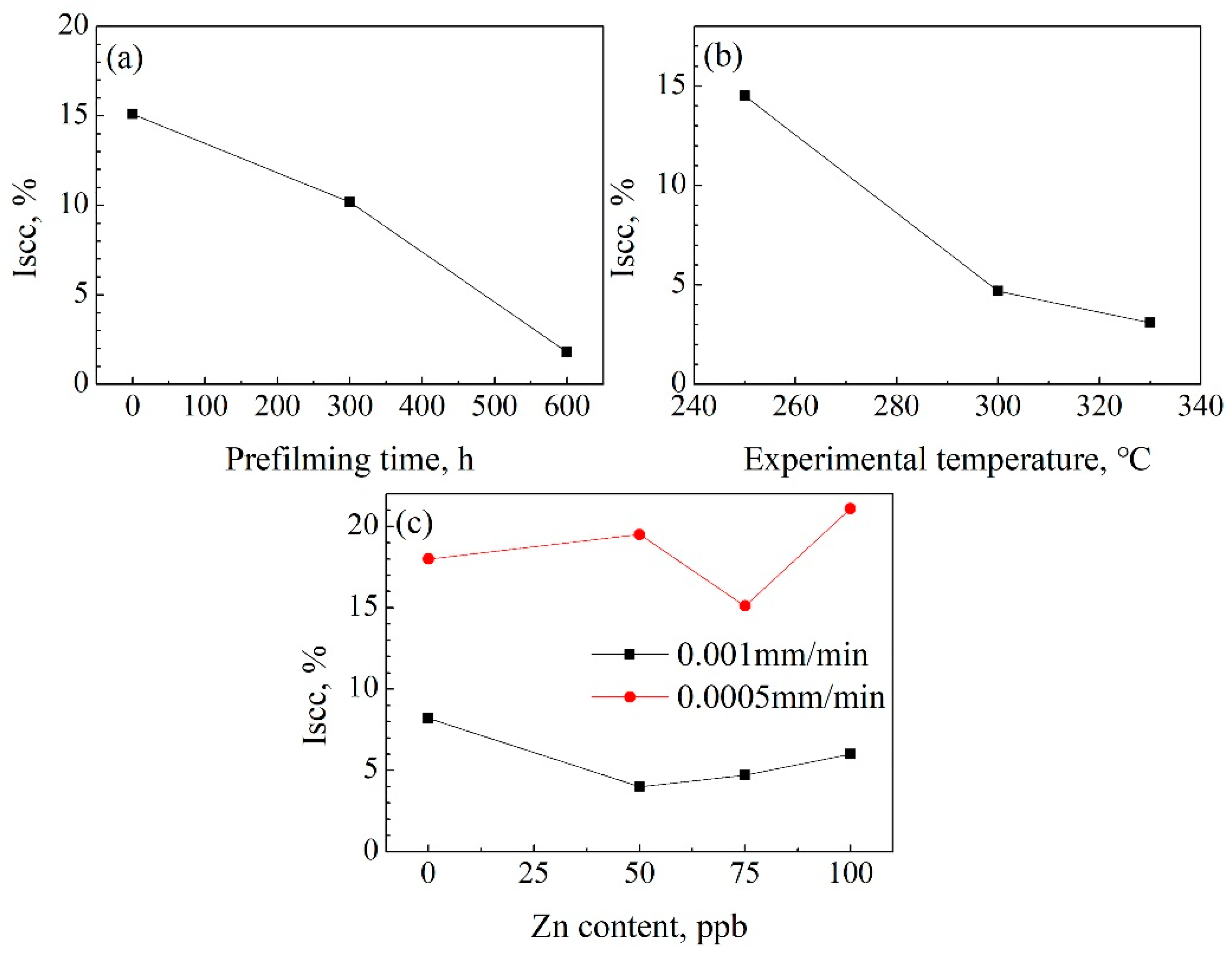
- The δ and UTS first increased with increasing Zn content, and then decreased at both a 9.26 × 10−7/s and 4.63 × 10−7/s strain rate. The δ and UTS were largest in the chemical solution with 50 ppb Zn. The difference values of the tensile properties at different strain rates showed fluctuations with increasing Zn content. The lower the strain rate was, the larger the Iscc value would be.
- (3)
- The δ and UTS increased with increasing experimental temperature. The UTS was 477.1 MPa, 491 MPa, and 496.1 MPa for temperatures of 250 °C, 300 °C, and 330 °C, respectively. Correspondingly, the δ was 47.0%, 52.4%, and 53.3%, respectively. The Iscc decreased with increasing experimental temperatures.
- (4)
- The previous corrosion before the SSRT test evidently enhanced the elongation. The elongation was 46.7%, 49.4%, and 54% at a prefilming time of 0 h, 300 h, and 600 h, respectively. However, the UTS first decreased with increasing prefilming time, and then increased. The UTS was about 496.9 MPa after previous corrosion for 600 h. Previous corrosion in the chemical solution obviously reduced the SCC susceptibility.
- (5)
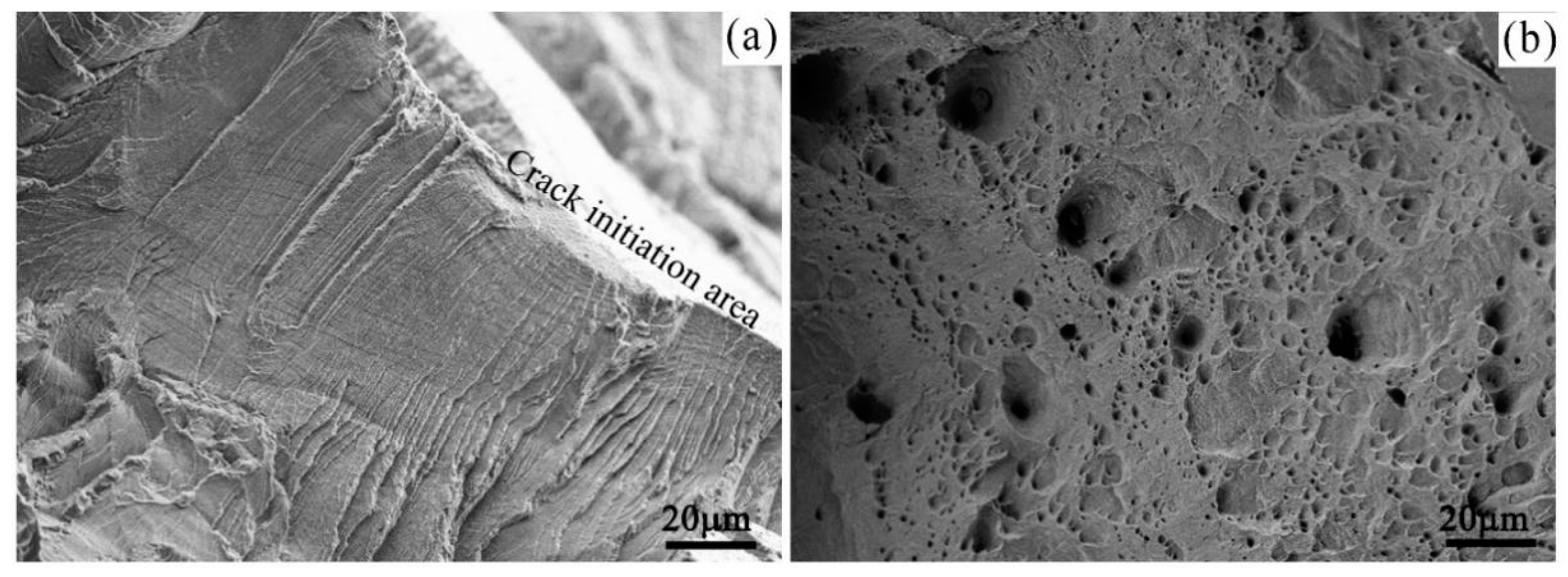
- Many particles with a polyhedron shape were formed on the sample surfaces, which was attributed to corrosion in a periodical location at the sample surface. The average length of the formed particles decreased with increasing Zn content, but increased with increasing experimental temperatures. The longer the previous corrosion time was, the larger the average length of particles would be.
Author Contributions
Funding
Conflicts of Interest
References
- Nezakat, M.; Akhiani, H.; Penttilä, S.; Sabet, S.M.; Szpunar, J. Effect of thermo-mechanical processing on oxidation of austenitic stainless steel 316L in supercritical water. Corros. Sci. 2015, 94, 197–206. [Google Scholar] [CrossRef]
- Was, G.S.; Ampornrat, P.; Gupta, G.; Teysseyre, S.; West, E.A.; Allen, T.R.; Sridharan, K.; Tan, L.; Chen, Y.; Ren, X.; et al. Corrosion and stress corrosion cracking in supercritical water. J. Nucl. Mater. 2007, 371, 176–201. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, K.; Wang, J.; Guo, X.; Du, D.; Andresen, P.L. Effects of zinc injection on stress corrosion cracking of cold worked, austenitic stainless steel in high-temperature water environments. Scr. Mater. 2017, 140, 50–54. [Google Scholar] [CrossRef]
- Terachi, T.; Yamada, T.; Miyamoto, T.; Arioka, K. SCC growth behaviors of austenitic stainless steels in simulated PWR primary water. J. Nucl. Mater. 2012, 426, 59–70. [Google Scholar] [CrossRef]
- Liu, X.; Wu, X.; Han, E.H. Influence of Zn injection on characteristics of oxide film on 304 stainless steel in borated and lithiated high temperature water. Corros. Sci. 2011, 53, 3337–3345. [Google Scholar] [CrossRef]
- Betova, I.; Bojinov, M.; Kinnunen, P.; Saario, T. Zn injection in Pressurized Water Reactors–laboratory tests, field experience and modelling. In Research Report No. VTT-R-05511-11; Technical Research Center of Finland: Espoo, Finland, 2011. [Google Scholar]
- Marks, C.; Dumouchel, M.; Reid, R.; White, G. Quantifying the benefit of chemical mitigation of PWSCC via zinc addition or hydrogen optimization. In Proceedings of the 15th International Conference on Environmental Degradation of Materials in Nuclear Power System-Water Reactors, Colorado Springs, CO, USA, 7–11 August 2011; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 1893–1906. [Google Scholar]
- Norring, K.; Engström, J. Initiation of SCC in nickel base alloys in primary PWR environment: Studies at Studsvik since mid 1980s. Energy Mater. 2008, 3, 113–118. [Google Scholar] [CrossRef]
- Piippo, J.; Saario, T.; Tegeder, V.; Stellwag, B. Influence of zinc on properties and growth of oxide layers in simulated primary coolant. In Proceedings of the 7th International Conference on the Water Chemistry of Nuclear Reactor Systems, Bournemouth, UK, 13–17 October 1996; Thomas Telford Services LTD.: London, UK, 1996; pp. 131–134. [Google Scholar]
- Ziemniak, S.E.; Hanson, M. Zinc treatment effects on corrosion behavior of Alloy 600 in high temperature, hydrogenated water. Corros. Sci. 2006, 48, 3330–3348. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Wu, X.; Han, E.H. Electrochemical and surface analytical investigation of the effects of Zn concentrations on characteristics of oxide films on 304 stainless steel in borated and lithiated high temperature water. Electrochim. Acta 2013, 108, 554–565. [Google Scholar] [CrossRef]
- Betova, I.; Bojinov, M.; Kinnunen, P.; Lundgren, K.; Saario, T. Influence of Zn on the oxide layer on AISI 316L (NG) stainless steel in simulated pressurized water reactor coolant. Electrochim. Acta 2009, 54, 1056–1069. [Google Scholar] [CrossRef]
- Huang, J.; Liu, X.; Han, E.H.; Wu, X. Influence of Zn on oxide films on Alloy 690 in borated and lithiated high temperature water. Corros. Sci. 2011, 53, 3254–3261. [Google Scholar] [CrossRef]
- Kim, Y.J.; Andresen, P.L. Transformation kinetics of oxide formed on noble metal-treated type 304 stainless steel in 288 °C water. Corrosion 2003, 56, 511–519. [Google Scholar] [CrossRef]
- Pathania, R.S.; Cheng, B.O. Evaluation of zinc addition to the primary coolant of Farley-2 PWR. Nucl. Power Plant 1998, 31, 959–971. [Google Scholar]
- Hanzawa, Y.; Hiroishi, D.; Matsuura, C.; Ishigure, K. Solubility of zinc ferrite in high-temperature oxygenated water. J. Nucl. Mater. 1998, 252, 209–215. [Google Scholar] [CrossRef]
- Liu, X.; Wu, X.; Han, E.H. Effect of Zn injection on established surface oxide films on 316 L stainless steel in borated and lithiated high temperature water. Corros. Sci. 2012, 65, 136–144. [Google Scholar] [CrossRef]
- Lu, Z.; Shoji, T.; Meng, F.; Qiu, Y.; Dan, T.; Xue, H. Effects of water chemistry and loading conditions on stress corrosion cracking of cold-rolled 316NG stainless steel in high temperature water. Corros. Sci. 2011, 53, 247–262. [Google Scholar] [CrossRef]
- Toppo, A.; Pujar, M.G.; Mallika, C.; Mudali, U.K.; Dayal, R.K. Effect of nitrogen on stress corrosion behavior of austenitic stainless steels using electrochemical noise technique. J. Mater. Eng. Perform. 2015, 24, 1140–1149. [Google Scholar] [CrossRef]
- Furutani, G.; Nakajima, N.; Konishi, T.; Kodama, M. Stress corrosion cracking on irradiated 316SS. J. Nucl. Mater. 2001, 288, 179–186. [Google Scholar] [CrossRef]
- Totsuka, N.; Nishikawa, Y.; Kaneshima, Y. Effect of strain rate on primary water stress corrosion cracking fracture mode and crack growth rate of nickel alloy and austenitic stainless steel. Corrosion 2005, 61, 219–229. [Google Scholar] [CrossRef]
- Liu, X.; Han, E.H.; Wu, X. Effects of pH value on characteristics of oxide films on 316L stainless steel in Zn-injected borated and lithiated high temperature water. Corros. Sci. 2014, 78, 200–207. [Google Scholar] [CrossRef]
- Zhang, S.; Shi, R.; Chen, Y.; Wang, M. Corrosion behavior of oxide films on AISI 316L SS formed in high temperature water with simultaneous injection of zinc and aluminum. J. Alloy. Compd. 2018, 731, 1230–1237. [Google Scholar] [CrossRef]
- Andresen, P.L.; Wilson, J.A.; Ahluwalia, K.S. Use of primary water chemistry in PWRs to mitigate PWSCC of Ni-Base alloys. Presented at the International Conference on Water Chemistry of Nuclear Reactor Systems, Jeju Island, Korea, 23–26 October 2006. [Google Scholar]
- Kawamura, H.; Hirano, H.; Shirai, S.; Takamatsu, H.; Matsunaga, T.; Yamaoka, K.; Oshinden, K.; Takiguchi, H. Inhibitory effect of zinc addition to high-Temperature hydrogenated water on mill-annealed and prefilmed Alloy 600. Corrosion 2000, 56, 623–637. [Google Scholar] [CrossRef]
- Angell, M.G.; Allan, S.J.; Airey, G.P. The effect of primary coolant zinc additions on the SCC behavior of alloy 600 and 690. In Proceedings of the 9th International Conference on Environmental Degradation of Materials in Nuclear Power System—Water Reactors, Newport Beach, USA, 1–5 August 1999; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1999; pp. 96–103. [Google Scholar]
- Maeng, W.Y.; Cho, Y.S.; Kim, U.C. Effect of Zn injection on the SCC crack growth of Alloy 600 in water at 360 °C. Presented at the International Conference on Water Chemistry of Nuclear Reactor Systems, Jeju Island, Korea, 23–26 October 2006. [Google Scholar]
- Jones, R.H.; Henager, C.H., Jr. Effect of gamma irradiation on stress corrosion behavior of austenitic stainless steel under ITER-relevant conditions. J. Nucl. Mater. 1992, 191–194, 1012–1017. [Google Scholar] [CrossRef]
- Brnic, J.; Niu, J.; Canadija, M.; Turkalj, G.; Lanc, D. Behavior of AISI 316L steel subjected to uniaxial state of stress at elevated temperatures. J. Mater. Sci. Technol. 2009, 25, 175–179. [Google Scholar]
- Wang, J.; Wang, J.; Ming, H.; Zhang, Z.; Han, E.H. Effect of temperature on corrosion behavior of Alloy 690 in high temperature hydrogenated water. J. Mater. Sci. Technol. 2017, 16, 230–242. [Google Scholar] [CrossRef]
- Lee, D.; Huang, Y.; Achenbach, J.D. A comprehensive analysis of the growth rate of stress corrosion cracks. Proc. R. Soc. A 2015, 471, 20140703. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Ming, H.; Zhang, Z.; Han, E.H. Modelling of stress-corrosion cracking by using peridynamics. Int. J. Hydrogen Energy 2016, 41, 6593–6609. [Google Scholar] [Green Version]
- Lister, D.H. Activity transport and corrosion processes in PWRs. Nucl. Energy 1993, 32, 103–114. [Google Scholar]
- Ziemniak, S.E.; Hanson, M. Corrosion behavior of NiCrFe alloy 600 in high temperature, hydrogenated water. Corros. Sci. 2006, 48, 498–521. [Google Scholar] [CrossRef]
- Katada, Y.; Nagata, N. The effect of temperature on fatigue crack growth behaviour of a low alloy pressure vessel steel in a simulated BWR environment. Corros. Sci. 1985, 25, 693–704. [Google Scholar] [CrossRef]
- Atkinson, J.D.; Yu, J. The role of dynamic strain-ageing in the environment assisted cracking observed in pressure vessel steels. Fatigue Fract. Eng. Mater. Struct. 1997, 20, 1–12. [Google Scholar] [CrossRef]
- Tang, Z.; Hu, S.; Zhang, P. Stress corrosion cracking of 316Ti in 300 °C high temperature water containing chloride ions. J. Chin. Soc. Corros. Prot. 2012, 32, 32–291. [Google Scholar] [CrossRef]
- Kim, Y.J. Characterization of the oxide film formed on type 316 stainless steel in 288 °C water in cyclic normal and hydrogen water chemistries. Corrosion 1995, 51, 849–860. [Google Scholar] [CrossRef]
- Kim, Y.J. Analysis of oxide film formed on type 304 stainless steel in 288 °C water containing oxygen, hydrogen, and hydrogen peroxide. Corrosion 1999, 55, 81–88. [Google Scholar] [CrossRef]
- Ziemniak, S.E.; Hanson, M. Zinc treatment effects on corrosion behavior of 304 stainless steel in high temperature, hydrogenated water. Corros. Sci. 2006, 48, 2525–2546. [Google Scholar] [CrossRef] [Green Version]
- Ehrnstén, U. Corrosion and stress corrosion cracking of austenitic stainless steels. Compr. Nucl. Mater. 2012, 93–104. [Google Scholar] [CrossRef]




| Element | C | Si | Mn | P | S | Cr | Ni | Mo | N | Co | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Content | 0.019 | 0.22 | 1.21 | 0.014 | 0.002 | 16.96 | 13.19 | 2.38 | 0.14 | 0.012 | Bal. |
| Samples | #1 * | #2 | #3 | #4 | #5 | #6 | #7 | #8 | #9 | #10 | #11 | #12 | #13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zn content (ppb) | 0 | 0 | 50 | 75 | 100 | 0 | 50 | 75 | 100 | 75 | 75 | 75 | 75 |
| Experiment temperature | 300 °C | 250 | 330 | 300 °C | |||||||||
| Strain rate | 9.26 × 10−7/s | 4.63 × 10−7/s | 9.26 × 10−7/s | 4.63 × 10−7/s | |||||||||
| Prefilming time, h | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 300 | 600 |
| Sample | #1 | #2 | #3 | #4 | #5 | #6 | #7 | #8 | #9 | #10 | #11 | #12 | #13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UTS, MPa | 507.3 ± 5.48 | 492.7 ± 5.32 | 495.2 ± 5.35 | 491 ± 5.30 | 484.7 ± 5.23 | 480.5 ± 5.19 | 464.6 ± 5.01 | 487.7 ± 5.26 | 475.3 ± 5.13 | 477.1 ± 5.15 | 496.1 ± 5.35 | 483.3 ± 5.22 | 496.9 ± 5.36 |
| δ, % | 55.0 ± 0.05 | 50.5 ± 0.05 | 52.8 ± 0.05 | 52.4 ± 0.05 | 51.7 ± 0.05 | 45.1 ± 0.05 | 44.3 ± 0.05 | 46.7 ± 0.05 | 43.4 ± 0.05 | 47.0 ± 0.05 | 53.3 ± 0.05 | 49.4 ± 0.05 | 54 ± 0.05 |
| Iscc, % | / | 8.2 | 4.0 | 4.7 | 6.0 | 18.0 | 19.5 | 15.1 | 21.1 | 14.5 | 3.1 | 10.2 | 1.8 |
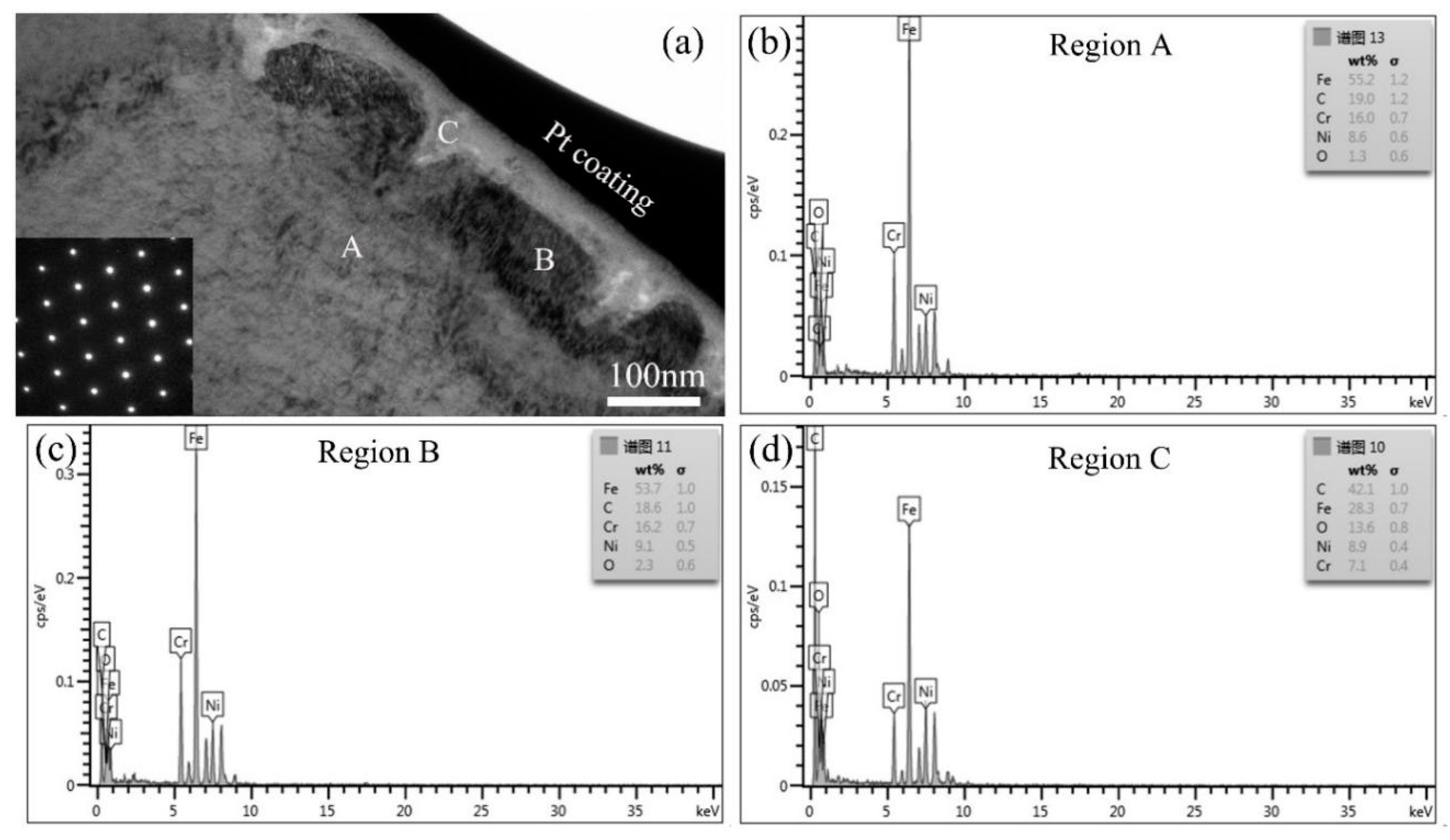
| Main Elements | Fe | Cr | Ni | C | O |
|---|---|---|---|---|---|
| Location ‘A’ | 55.2 | 16.0 | 8.6 | 19.0 | 1.3 |
| Location ‘B’ | 53.7 | 16.2 | 9.1 | 18.6 | 2.3 |
| Location ‘C’ | 28.3 | 7.1 | 8.9 | 42.1 | 13.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Wu, W.; Cong, S.; Ran, G.; Cen, D.; Li, N. Stress Corrosion Behaviors of 316LN Stainless Steel in a Simulated PWR Primary Water Environment. Materials 2018, 11, 1509. https://doi.org/10.3390/ma11091509
Huang Y, Wu W, Cong S, Ran G, Cen D, Li N. Stress Corrosion Behaviors of 316LN Stainless Steel in a Simulated PWR Primary Water Environment. Materials. 2018; 11(9):1509. https://doi.org/10.3390/ma11091509
Chicago/Turabian StyleHuang, Yong, Weisong Wu, Shuo Cong, Guang Ran, Danxia Cen, and Ning Li. 2018. "Stress Corrosion Behaviors of 316LN Stainless Steel in a Simulated PWR Primary Water Environment" Materials 11, no. 9: 1509. https://doi.org/10.3390/ma11091509
APA StyleHuang, Y., Wu, W., Cong, S., Ran, G., Cen, D., & Li, N. (2018). Stress Corrosion Behaviors of 316LN Stainless Steel in a Simulated PWR Primary Water Environment. Materials, 11(9), 1509. https://doi.org/10.3390/ma11091509




