Semiconductor-Ionic Nanocomposite La0.1Sr0.9MnO3−δ-Ce0.8Sm0.2O2−δ Functional Layer for High Performance Low Temperature SOFC
Abstract
:1. Introduction
2. Experimental
2.1. Materials Synthesis and Physical Characterizations
2.2. Material Characterizations
2.3. Electrochemical Measurement
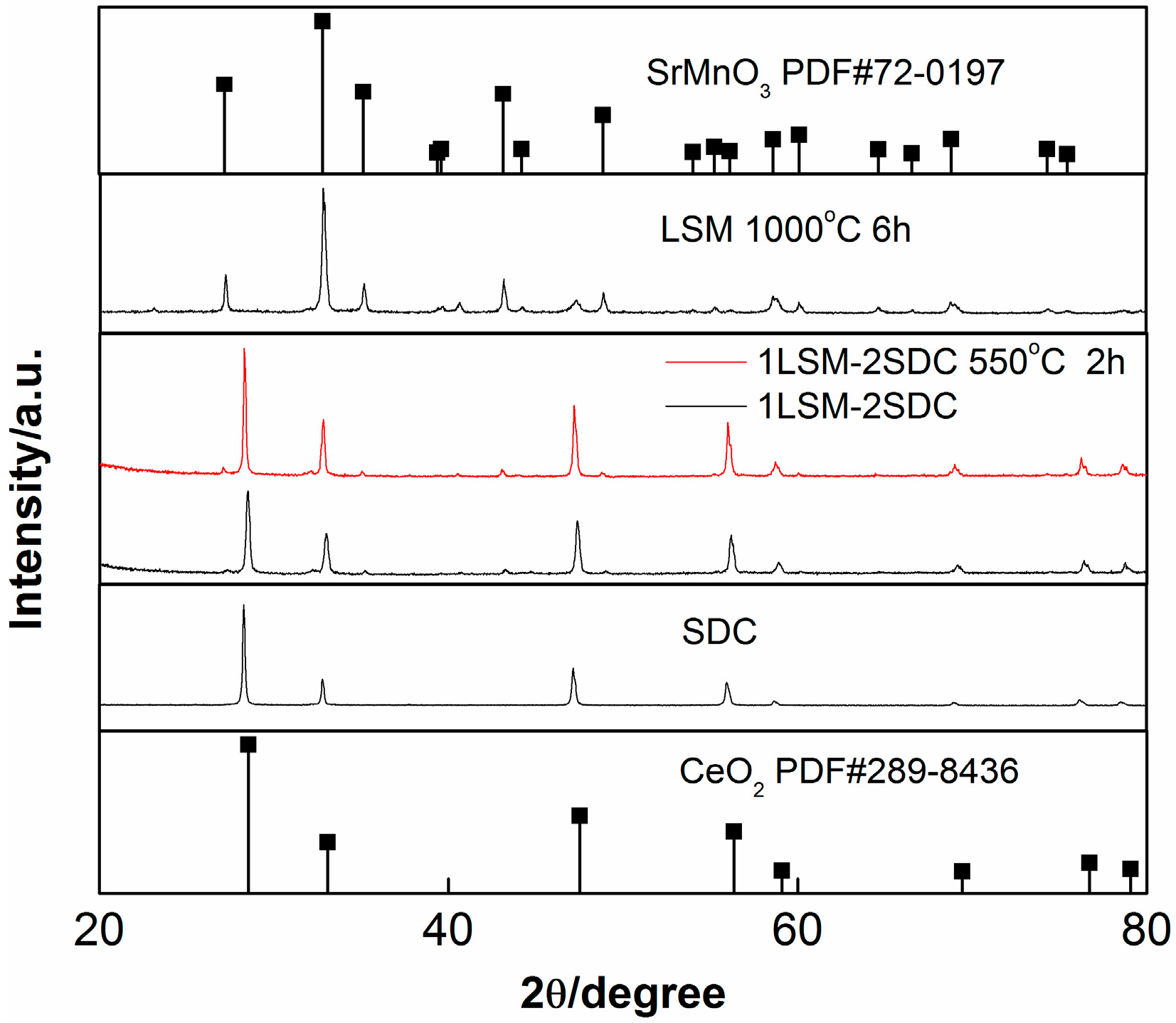
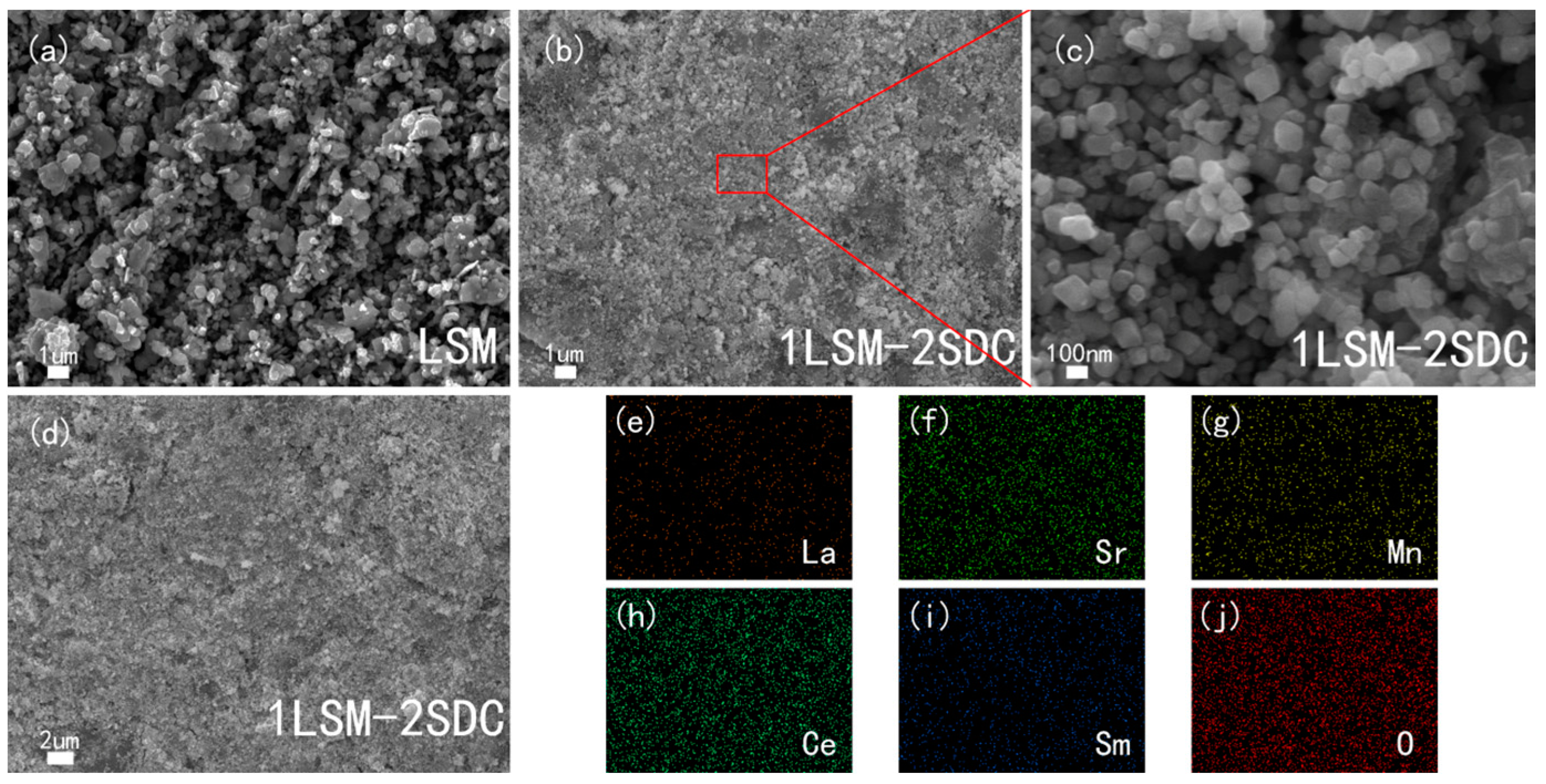
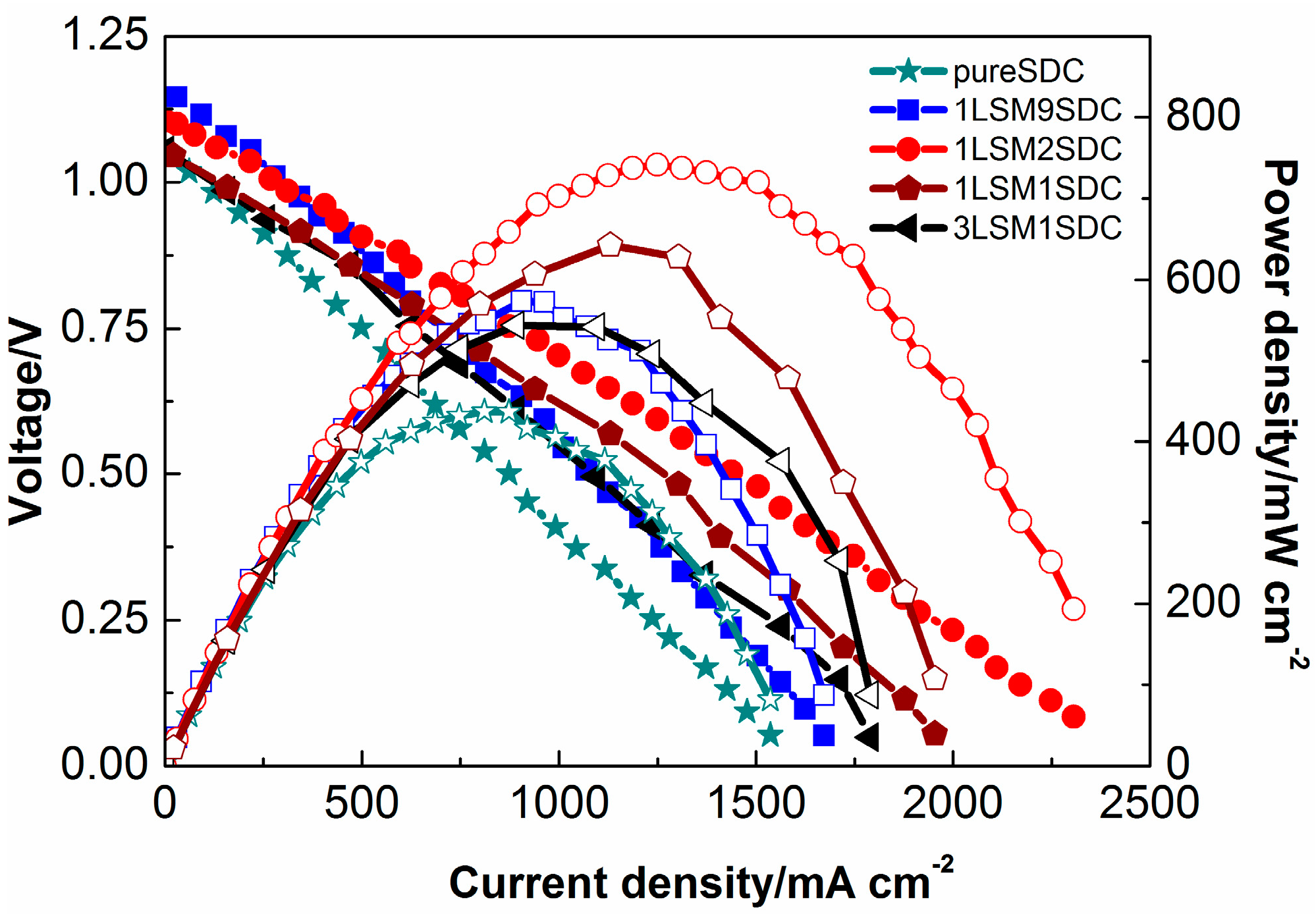
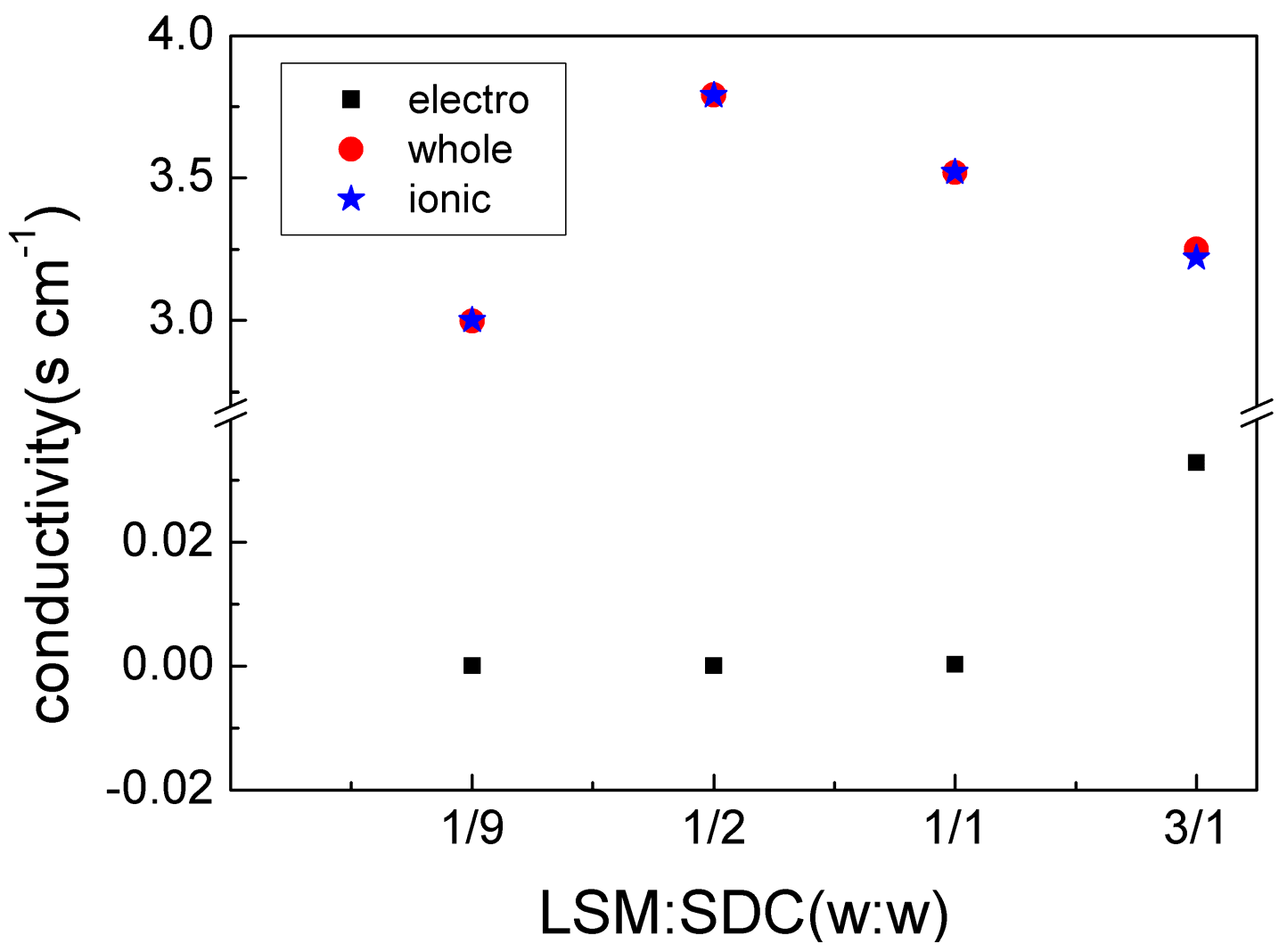
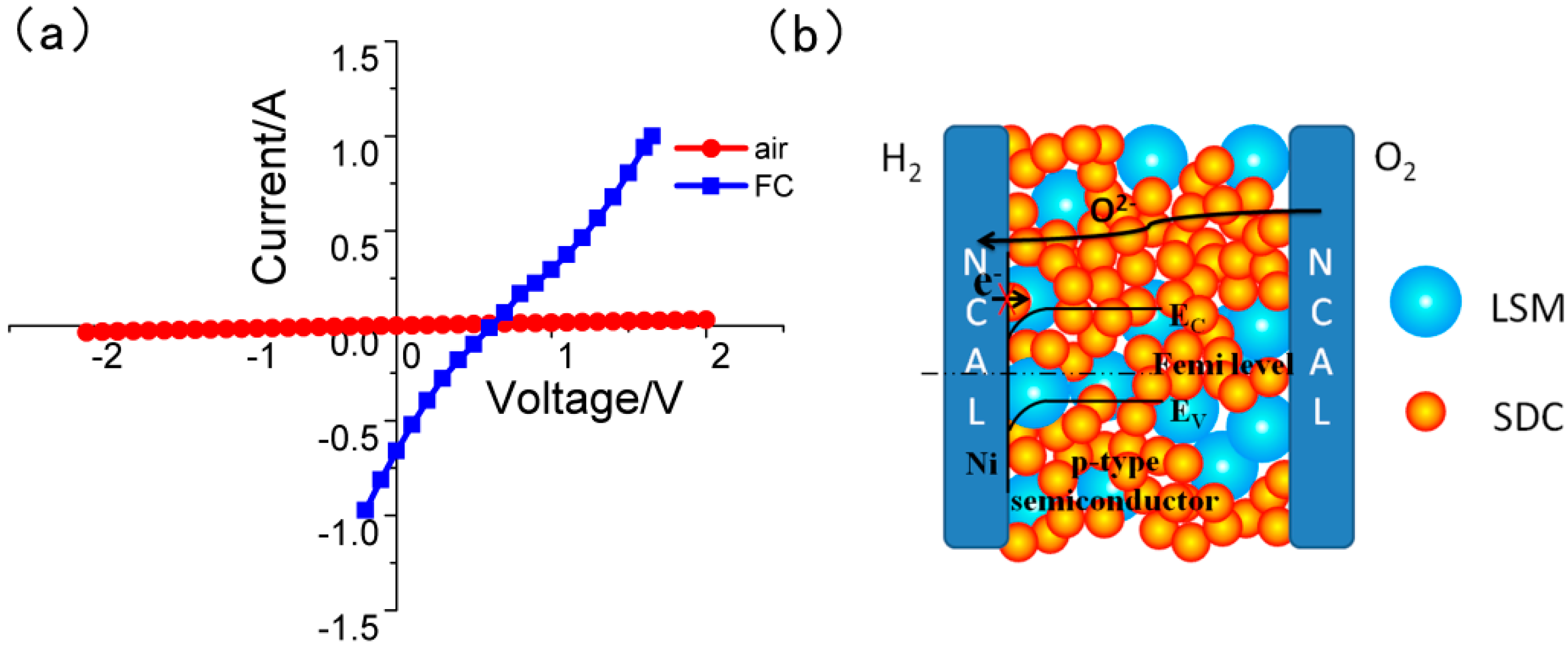
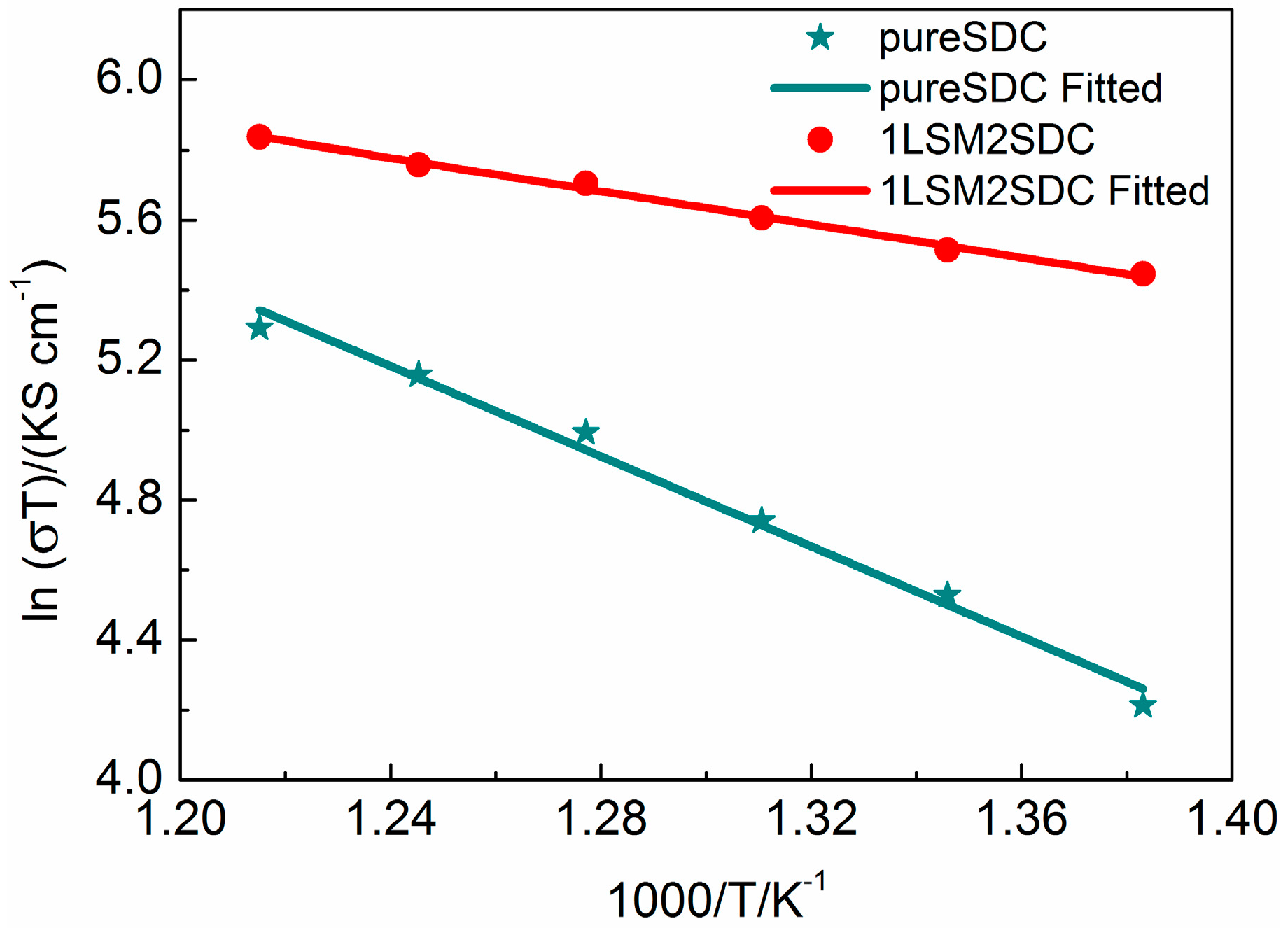
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Memorial, W.N. Uber die elektrolytische Leitund fester Korper bei sehr hohen Temperaturen. Z. Elektrochem. 1899, 6, 41–43. [Google Scholar]
- Garcia-Barriocanal, J.; Rivera-Calzada, A.; Varela, M.; Sefrioui, Z.; Iborra, E.; Leon, C.; Pennycook, S.J.; Santamaria, J. Colossal ionic conductivity at interfaces of epitaxial ZrO2:Y2O3/SrTiO3 heterostructures. Science 2008, 321, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Ormerod, R.M. Solid oxide fuel cells. Chem. Soc. Rev. 2003, 32, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Wachsman, E.D.; Lee, K.T. Lowering the temperature of solid oxide fuel cells. Science 2011, 334, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xie, F.; Wang, C.; Mao, Z. Development of solid oxide fuel cell materials for intermediate-to-low temperature operation. Int. J. Hydrogen Energy 2012, 37, 877–883. [Google Scholar] [CrossRef]
- Zhao, Y.; Xia, C.; Jia, L.; Wang, Z.; Li, H.; Yu, J.; Li, Y. Recent progress on solid oxide fuel cell: Lowering temperature and utilizing non-hydrogen fuels. Int. J. Hydrogen. Energy 2013, 38, 16498–16517. [Google Scholar] [CrossRef]
- Leonard, K.; Lee, Y.S.; Okuyama, Y.; Miyazaki, K.; Matsumoto, H. Influence of dopant levels on the hydration properties of SZCY and BZCY proton conducting ceramics for hydrogen production. Int. J. Hydrogen Energy 2017, 42, 3926–3937. [Google Scholar] [CrossRef]
- Fan, L.; He, C.; Zhu, B. Role of carbonate phase in ceria–carbonate composite for low temperature solid oxide fuel cells: A review. Int. J. Energy Res. 2017, 41, 465–481. [Google Scholar] [CrossRef]
- Goodenough, J.B. Oxide-ion conductors by design. Nature 1999, 404, 821–822. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Yamaguchi, T.; Suzuki, T.; Sumi, H.; Hamamoto, K.; Fujishiro, Y. High power density cell using nanostructured Sr-doped SmCoO3 and Sm-doped CeO2 composite powder synthesized by spray pyrolysis. J. Power Sources 2016, 302, 308–314. [Google Scholar] [CrossRef]
- Wu, Y.C.; Liao, Y.Y. Effect of Ca2+ and Sr2+ doping on the microstructure and cell performance of samaria-doped ceria electrolytes used in solid oxide fuel cells. Int. J. Hydrogen Energy 2016, 41, 13591–13602. [Google Scholar] [CrossRef]
- Sun, K.; Zhang, J.; Jiang, T.; Qiao, J.; Sun, W.; Rooney, D.; Wang, Z. Flash-Sintering and Characterization of La0.8Sr0.2Ga0.8Mg0.2O3−δ Electrolytes for Solid Oxide Fuel Cells. Electrochim. Acta 2016, 196, 487–495. [Google Scholar] [CrossRef]
- Da Silva, F.S.; De Souza, T.M. Novel materials for solid oxide fuel cell technologies: A literature review. Int. J. Hydrogen Energy 2017, 42, 26020–26036. [Google Scholar] [CrossRef]
- Yao, L.; Liu, W.; Ou, G.; Nishijima, H.; Pan, W. Enhanced ionic conductivity in magnetron-sputtered Ce0.8Sm0.2O2−δ/Al2O3 multilayers. Electrochim. Acta 2015, 158, 196–201. [Google Scholar] [CrossRef]
- Wen, K.C.; Lv, W.Q.; He, W.D. Interfacial lattice-strain effects on improving the overall performance of micro-solid oxide fuel cells. J. Mater. Chem. A 2015, 3, 20031–20050. [Google Scholar] [CrossRef]
- Wen, K.C.; Zhang, K.H.L.; Wang, W.; Lin, J.H.; Lv, W.Q.; Wang, B.; Wang, Z.M.; Dickerson, J.H.; Guo, X.; He, W.D. Physical justification for ionic conductivity enhancement at strained coherent interfaces. J. Power Sources 2015, 285, 37–42. [Google Scholar] [CrossRef]
- Baiutti, F.; Logvenov, G.; Gregori, G.; Cristiani, G.; Wang, Y.; Sigle, W.; Van Aken, P.A.; Maier, J. High-temperature superconductivity in space-charge regions of lanthanum cuprate induced by two-dimensional doping. Nat. Commun. 2015, 6, 8586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.M.; Lee, S.; Jian, J.; Zhang, W.R.; Lu, P.; Jia, Q.X.; Wang, H.Y.; Noh, T.W.; Kalinin, S.V.; MacManus-Driscoll, J.L. Strongly enhanced oxygen ion transport through samarium-doped CeO2 nanopillars in nanocomposite films. Nat. Commun. 2015, 6, 8588. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B. Solid oxide fuel cell (SOFC) technical challenges and solutions from nano-aspects. Int. J. Energy Res. 2009, 33, 1126–1137. [Google Scholar] [CrossRef]
- Fan, L.D.; Wang, C.Y.; Chen, M.M.; Zhu, B. Recent development of ceria-based (nano)composite materials for low temperature ceramic fuel cells and electrolyte-free fuel cells. J. Power Sources 2013, 234, 154–174. [Google Scholar] [CrossRef]
- Fan, L.D.; Ma, Y.; Wang, X.D.; Singh, M.; Zhu, B. Understanding the electrochemical mechanism of the core-shell ceria-LiZnO nanocomposite in a low temperature solid oxide fuel cell. J. Mater. Chem. A 2014, 2, 5399–5407. [Google Scholar] [CrossRef]
- Ma, Y.; Singh, M.; Wang, X.D.; Yang, F.; Huang, Q.A.; Zhu, B. Study on GDC-KZnAl composite electrolytes for low-temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2014, 39, 17460–17465. [Google Scholar] [CrossRef]
- Zhu, B.; Fan, L.D.; Zhao, Y.F.; Tan, W.Y.; Xiong, D.B.; Wang, H. Functional semiconductor-ionic composite GDC-KZnAl/LiNiCuZnOx for single-component fuel cell. RSC Adv. 2014, 4, 9920–9925. [Google Scholar] [CrossRef]
- Wang, X.D.; Ma, Y.; Zhu, B. State of the art ceria-carbonate composites (3C) electrolyte for advanced low temperature ceramic fuel cells (LTCFCs). Int. J. Hydrogen Energy 2012, 37, 19417–19425. [Google Scholar] [CrossRef]
- Zhu, B. Functional ceria–salt-composite materials for advanced ITSOFC applications. J. Power Sources 2003, 114, 1–9. [Google Scholar] [CrossRef]
- Zhu, B.; Yang, X.T.; Xu, J.; Zhu, Z.G.; Ji, S.J.; Sun, M.T.; Sun, J.C. Innovative low temperature SOFCs and advanced materials. J. Power Sources 2003, 118, 47–53. [Google Scholar] [CrossRef]
- Zhu, B. Nanocomposites for Advanced Fuel Cell Technology. J. Nanosci. Nanotechnol. 2011, 11, 8873–8879. [Google Scholar] [CrossRef] [PubMed]
- Bin, Z.; Sining, Y.; Lund, P.D. Semiconductor-ionic materials could play an important role in advanced fuel-to-electricity conversion. Int. J. Energy Res. 2018, 42, 3413–3415. [Google Scholar]
- Zhu, B.; Fan, L.D.; Deng, H.; He, Y.J.; Afzal, M.; Dong, W.J.; Yaqub, A.; Janjua, N.K. LiNiFe-based layered structure oxide and composite for advanced single layer fuel cells. J. Power Sources 2016, 316, 37–43. [Google Scholar] [CrossRef]
- Wang, B.Y.; Cai, Y.X.; Xia, C.; Kim, J.S.; Liu, Y.Y.; Dong, W.J.; Wang, H.; Afzal, M.; Li, J.J.; Raza, R.; et al. Semiconductor-ionic Membrane of LaSrCoFe-oxide-doped Ceria Solid Oxide Fuel Cells. Electrochim. Acta 2017, 248, 496–504. [Google Scholar] [CrossRef]
- Zhang, W.; Cai, Y.; Wang, B.; Deng, H.; Feng, C.; Dong, W.; Li, J.; Zhu, B. The fuel cells studies from ionic electrolyte Ce0.8Sm0.05Ca0.15O2-delta to the mixture layers with semiconductor Ni0.8Co0.15Al0.05LiO2-delta. Int. J. Hydrogen Energy 2016, 41, 18761–18768. [Google Scholar] [CrossRef]
- Zhang, W.; Cai, Y.; Wang, B.; Xia, C.; Dong, W.; Li, J.; Zhu, B. Mixed ionic-electronic conductor membrane based fuel cells by incorporating semiconductor Ni0.8Co0.15Al0.05LiO2−δ into the Ce0.8Sm0.2O2−δ-Na2CO3 electrolyte. Int. J. Hydrogen Energy 2016, 41, 15346–15353. [Google Scholar] [CrossRef]
- Li, X.; Shao, G.; Luo, J.; Lu, J.; Xue, M.; Hou, Y.; Deng, L. Fabrication and characterization of GDC electrolyte/electrode integral SOFC with BaO/Ni-GDC anode. Mater. Res. Bull. 2014, 50, 337–340. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Fan, L.; Cai, Y.; Xia, C.; Liu, Y.; Raza, R.; Van Aken, P.A.; Wang, H.; Zhu, B. Preparation and characterization of Sm and Ca co-doped ceria-La0.6Sr0.4Co0.2Fe0.8O3-delta semiconductor-ionic composites for electrolyte-layer-free fuel cells. J. Mater. Chem. A 2016, 4, 15426–15436. [Google Scholar] [CrossRef]
- Zhu, B.; Lund, P.D.; Raza, R.; Ma, Y.; Fan, L.; Afzal, M.; Patakangas, J.; He, Y.; Zhao, Y.; Tan, W. Schottky Junction Effect on High Performance Fuel Cells Based on Nanocomposite Materials. Adv. Energy Mater. 2015, 5, 1–6. [Google Scholar] [CrossRef]
- Zhu, B.; Huang, Y.; Fan, L.; Ma, Y.; Wang, B.; Xia, C.; Afzal, M.; Zhang, B.; Dong, W.; Wang, H.; et al. Novel fuel cell with nanocomposite functional layer designed by perovskite solar cell principle. Nano Energy 2016, 19, 156–164. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, F.; Brinkman, K.; Reifsnider, K.L.; Virkar, A.V. A study of gadolinia-doped ceria electrolyte by electrochemical impedance spectroscopy. J. Power Sources 2014, 247, 947–960. [Google Scholar] [CrossRef]
- Braun, P.; Uhlmann, C.; Weber, A.; Störmer, H.; Gerthsen, D.; Ivers-Tiffée, E. Separation of the bulk and grain boundary contributions to the total conductivity of solid lithium-ion conducting electrolytes. J. Electroceram. 2017, 38, 157–167. [Google Scholar] [CrossRef]
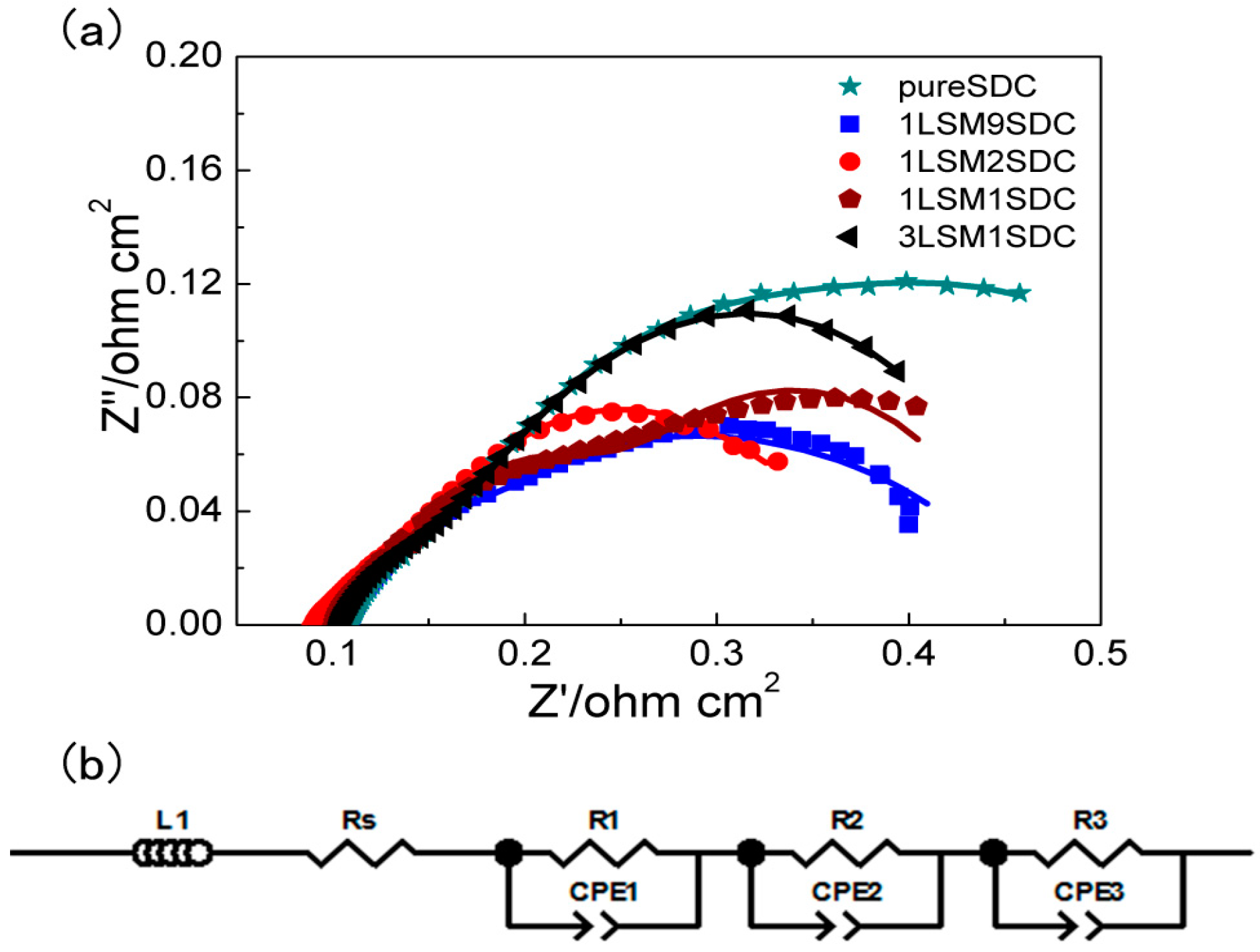
| Proportion | RΩ (Ω·cm2) | Ra (Ω·cm2) | Rb (Ω·cm2) | Rc (Ω·cm2) | Rb + Rc (Ω·cm2) |
|---|---|---|---|---|---|
| Pure SDC | 0.11 | 0.084 | 0.11 | 0.31 | 0.42 |
| 1LSM9SDC | 0.11 | 0.060 | 0.080 | 0.22 | 0.30 |
| 1LSM2SDC | 0.090 | 0.023 | 0.096 | 0.21 | 0.31 |
| 1LSM1SDC | 0.10 | 0.046 | 0.095 | 0.22 | 0.31 |
| 3LSM1SDC | 0.10 | 0.052 | 0.074 | 0.23 | 0.31 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Wang, X.; Xu, Z.; Deng, H.; Dong, W.; Wang, B.; Feng, C.; Liu, X.; Wang, H. Semiconductor-Ionic Nanocomposite La0.1Sr0.9MnO3−δ-Ce0.8Sm0.2O2−δ Functional Layer for High Performance Low Temperature SOFC. Materials 2018, 11, 1549. https://doi.org/10.3390/ma11091549
Wang Z, Wang X, Xu Z, Deng H, Dong W, Wang B, Feng C, Liu X, Wang H. Semiconductor-Ionic Nanocomposite La0.1Sr0.9MnO3−δ-Ce0.8Sm0.2O2−δ Functional Layer for High Performance Low Temperature SOFC. Materials. 2018; 11(9):1549. https://doi.org/10.3390/ma11091549
Chicago/Turabian StyleWang, Zhaoqing, Xunying Wang, Zhaoyun Xu, Hui Deng, Wenjing Dong, Baoyuan Wang, Chu Feng, Xueqi Liu, and Hao Wang. 2018. "Semiconductor-Ionic Nanocomposite La0.1Sr0.9MnO3−δ-Ce0.8Sm0.2O2−δ Functional Layer for High Performance Low Temperature SOFC" Materials 11, no. 9: 1549. https://doi.org/10.3390/ma11091549





